Abstract
The paper presents the results of studies on the viscosity of the glass mass in various temperature ranges, determining the basic technological parameter, very important from the point of view of melting and forming. For this purpose, six sets based on natural raw materials such as basalt, dolomite, and amphibolite, modified with different amounts of float glass cullet, were melted. The melting process was carried out in an electric furnace at the temperature of 1450 °C for 2 h. Using the dilatometric method, high-temperature microscopy and theoretical calculation methods, the viscosity of the produced glasses was determined in various temperature ranges. Comparative analyses of the employed methods were carried out. The significance of the applied calculation methods for aluminosilicate glasses depending on the basic chemical composition of the glasses was presented. The relationship between the manner of incorporating amphoteric ions Al3+, Fe3+ and Mg2+ into the glass structure and the change in viscosity in the temperature range corresponding to the working point range at 104 [dPa·s] viscosity and the relaxation range—Tg temperature at 1013 [dPa·s] viscosity was justified. It was justified that in order to plot the viscosity curve with the correct slope in the forming range for aluminosilicate glasses, it is appropriate to use the two-point method based on the fixed viscosity points of 104 [dPa·s] and 1013 [dPa·s].
1. Introduction
Knowledge of the basic technological parameters in the production of each type of glass products has a significant impact on the entire organization of the process of melting, clarifying, forming and annealing the glass mass. One of the most important parameters having a significant impact on the quality of the manufactured products is the viscosity of the glass mass. This property determines the formation of an amorphous glass structure, which in relation to the glassy state is of great theoretical and practical importance [1]. From a technological point of view, due to the high energy consumption of the entire process of melting the batch of raw materials and then forming the glass mass, a thorough analysis of the dependence of the glass mass viscosity on the temperature becomes justified [2,3]. Owing to the type of melted glass, the viscosity of the glass mass can change in different temperature ranges. The basic chemical composition of the glass mass has a significant impact on the course of viscosity change, i.e., on the so-called viscosity curve. The chemical composition of the glasses is also the basis for choosing their forming method, e.g., float glass (soda–lime–silicate glass), mineral fibers (aluminosilicate glass), laboratory vessels (borosilicate glass) [4,5,6,7,8,9]. The high viscosity of the glass means that the process of melting and clarifying the mass must be carried out in an increased temperature range, which in turn generates higher production costs. For economic reasons, in order for the entire technological process to be conducted in optimal conditions, it is necessary, among others, to monitor the stability of the correct temperature at each stage of glass production. Due to the significant importance of the viscosity of the glass mass in the entire production cycle, many theoretical studies have been performed to analyze the impact of various factors on changes in the viscosity of glasses in specific temperature ranges [10]. Among others, models and mathematical relationships have been developed to describe viscosity as a function of temperature, e.g., the Vogel–Fulcher–Tammann (VFT) model [11,12], the Tuszynski equation [13], the M.W. Ochotin model and others [14,15]. One of the best-known and most commonly used models is the Vogel–Fulcher–Tammann (VFT) model. It is utilized to determine the viscosity of most industrial glasses in a wide temperature range, e.g., from ambient temperature to melting and homogenization temperatures [16,17,18]. The use of this model is very convenient because it is based on the transition temperature, Tg, characteristic for each type of glass, with its constant viscosity η = 1013 [dPa·s]. Taking into account the value of temperature Tg, it is possible to determine the course of the viscosity curve in any temperature range. Unfortunately, the use of this model in the case of borosilicate glasses, in which the content of alkali oxides exceeds 15%, is not possible because with the increase in their content, the properties of these glasses change in a non-linear way (borate anomaly). Similarly, in the case of aluminosilicate glasses, the Vogel–Fulcher–Tammann model will not be applicable. For this type of glass, depending on the content of various oxides, including mainly amphoteric oxides (Al2O3, MgO or Fe2O3), the properties do not change linearly either, but rather exponentially, which means that for glasses which are extremely different in terms of chemical composition, it is possible to obtain similar properties, e.g., transition temperature Tg [19,20,21].
Therefore, it is necessary to intensify research and analysis for this type of glass in terms of optimizing the process of melting the glass mass and improving the quality of the formed products. As part of this work, the viscosity of aluminosilicate glasses from the SiO2–Al2O3–Fe2O3–CaO–MgO system, melted from mineral resources such as amphibolite and basalt with the addition of dolomite and float glass cullet, was analyzed.
Experimental methods were employed to determine the viscosity curves, i.e., dilatometric analysis, by means of which transition temperature Tg was determined at a glass viscosity of 1013 [dPa·s], and the dilatometric softening point (Td) at a viscosity of 1012 [dPa·s]; in addition, the high temperature microscopy method was employed, by means of which the temperature of the hemisphere was determined at a glass viscosity of approx. 104 [dPa·s]. Ochotin and Tuszynski and Dietzel–Brückner calculation methods were also used [22,23,24].
2. Materials and Methods
The research material consisted of aluminosilicate glasses obtained from the main mineral raw materials, i.e., amphibolite (Pilawa Gorna mine) and basalt (Wilkow mine) as well as raw materials modifying glass sets, i.e., dolomite (Redziny—Lower Silesia) and float cullet. Six sets of raw materials were prepared for melting, the chemical composition and the percentage composition of which is shown in Table 1.

Table 1.
Chemical composition and percentage of individual raw materials in sets, in weight %.
The weighed sets of raw materials were homogenized and melted in a platinum crucible in an electric furnace (Czylok-Polska) at a temperature of 1450 °C for 2 h, and then poured onto a steel plate. Analysis of the basic chemical composition was carried out for the obtained glasses by means of XRF spectroscopy. The study was performed using a WDXRF Axios mAX spectrometer with a 4 kW Rh lamp from PANalytical. The dilatometric test was performed utilizing a Sadamel DA-3 linear dilatometer, in an air atmosphere, at a furnace heating rate of 10 K/min. This test was conducted on rod-shaped glass samples with a diameter of about 3 mm and length of 10 mm. Hot-stage microscopy analysis was carried out on a Hesse Instruments microscope in air atmosphere, with a furnace heating rate of 10 K/min. Crushed glass (fraction below 63 μm) was used for the measurement, from which cylinders with a diameter and height of 3 mm were formed, then placed on a corundum plate and the measurement was carried out.
3. Results and Analysis
3.1. Determination of Chemical Composition
The basic chemical composition of the obtained glasses was determined using XRF spectroscopy, and the results of the analysis are presented in Table 2.

Table 2.
Chemical composition of obtained glasses in wt%.
On the basis of the analysis of the chemical compositions of all the obtained glasses, the ranges of percentages of individual oxides were determined, which are presented in Table 3.

Table 3.
Ranges of content of individual oxides in investigated glasses in wt%.
3.2. Determination of Temperatures for Constant Viscosity Values by Experimental Methods
3.2.1. Dilatometric Test
The dilatometric method was employed to determine transition temperature Tg, at which the glass viscosity was 1013 [dPa·s]. The measurement was carried out for all the glasses, and then on the obtained dilatometric curves (Figure 1), the Tg temperature and dilatometric softening point Td at a viscosity of about 1012 [dPa·s] were determined graphically [25,26]. The test results are presented in Table 4.
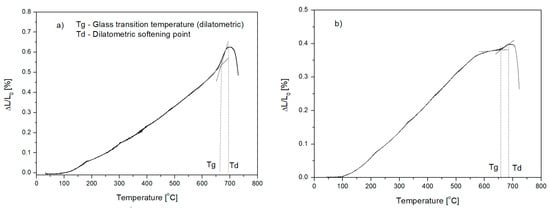
Figure 1.
Dilatometric thermogram of glasses: (a) glass from Set 1, (b) glass from Set 4; Tg—glass transition temperature, Td—dilatometric softening point.

Table 4.
Transition (Tg) and dilatometric softening (Td) temperatures of glasses in °C.
By analyzing the determined values of the transition temperatures and dilatometric softening of the examined glasses, it can be seen that the glass obtained from Set 1 had the highest temperatures, while the glass melted from Set 2 had the lowest temperatures (Table 1). Similar temperatures of Tg and Td to the temperatures of the melted glass from Set 1 were also obtained for the glasses from Sets 4 and 5. Taking into account the individual chemical compositions of all the glasses, it is difficult to unambiguously assess the effect of the additives, i.e., dolomite, glass cullet, on the viscosity of the obtained glasses. Interpretation of the determined temperatures is difficult because of the fact that the basic chemical composition of the glasses contains oxides of amphoteric metal ions, i.e., Al, Fe and Mg, which can obtain a coordination number of both 6 and 4 in the glass structure [20,27,28,29,30]. This means that they can act as modifying ions (coordination number—CN 6) or build into the network as network-forming ions (CN 4). In the case of glasses containing a high proportion of metal ions in their chemical composition, which may occur in variable coordination, each increase in the additional amount of free oxygen in the form of O2− ions in the glass mass, e.g., by adding alkaline cullet (the introduction of alkali oxides), will cause an increase in the number of amphoteric metal ions in the lower coordination. The increase in metal ions in coordination number 4 leads to the incorporation of these ions into the glass network, and thus its strengthening, which in turn raises the melt viscosity [31]. Such examples are Glasses 3 and 6 and Glasses 4 and 5, whose Tg temperature reached similar values, although the composition of Glasses 5 and 6 contained the highest content of alkali oxides (a high share of glass cullet in the set, respectively: 20% and 30%).
By analyzing the determined values of the Tg and Td temperatures, it can be concluded that the modification of amphibolite glass (Set 1 Tg = 661 °C) with the addition of 10% dolomite and 20% glass cullet (Set 2) causes a significant decrease in the viscosity of the glass mass (Tg = 600 °C), and thus easier melting of the raw set material [32]. With the modification of amphibolite glass with a smaller amount of dolomite (5%) and glass cullet in the amount of 10%, Glass 3 (Tg = 639 °C) did not have such a significant effect on reducing the viscosity of the glass as was in the case of Glass 2. The viscosity of the glass melted from Set 3 (85 wt% amphibolite, 5 wt% dolomite, 10 wt% cullet) can be compared with the viscosity of Glass 6 (Tg = 641 °C) obtained from the set: 20 wt% amphibolite, 50 wt% basalt, 30 wt% cullet. Despite significant differences in the basic chemical composition of the glasses, they had a similar transition temperature. A similar relationship can be observed in Glasses 4 (Tg = 657 °C) and 5 (Tg = 658 °C). The increase in the share of alkali oxides in Glass 5 did not cause a significant decrease in its viscosity. This fact is confirmed by the statement that the presence of alkali metal oxides (i.e., Na2O and K2O) has a significant impact on the viscosity of aluminosilicate glasses containing a large proportion of metal ions that can assume coordination 4 and 6 (e.g., Al3+, Fe3+, Mg2+) [30]. The presence of these modifiers in the glass mass increases the number of free oxygen ions, enabling the amphoteric ions to achieve tetrahedral coordination and incorporate them into the glass network, which results in an increase in the viscosity of the glass mass [20,21]. The same trend can be observed in determining the temperature for dilatometric softening point Td (η = 1012 [dPa·s]).
3.2.2. High Temperature Microscopy
On the basis of the high-temperature analysis, characteristic temperatures were determined, accompanied by a change in the shape of the sample depending on the viscosity of the glass. This study allowed the temperatures to be determined at glass viscosity η defined in [dPa·s]: deformation of logη = 6.3, sphere of logη = 5.4, hemisphere of logη = 4.1 and flow of logη = 3.4 [33,34]. The temperatures for the characteristic (constant) viscosity values of the researched glasses are presented in Table 5 and Figure 2.

Table 5.
Fixed viscosity points determined by hot stage microscopy (η in [dPa·s]).
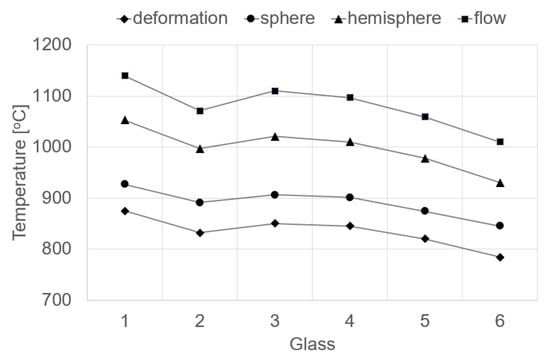
Figure 2.
Fixed viscosity points of studied glasses determined by hot stage microscopy.
The values of the characteristic glass temperatures obtained in the high temperature tests reveal a different temperature dependence than in the case of the dilatometric tests. At higher temperatures, with viscosity in the range of 103–107 [dPa·s], called the working range, the highest temperature values were obtained for the glass melted from Set 1 (100% amphibolite: 875–1140 °C), and the lowest from Set 6 (20% amphibolite, 50% dolomite, 30% cullet: 784–1010 °C). This means that in the range of high temperatures, diffusion grows and the mobility of ions in the glass mass increases, which results in a decline in its viscosity. In this case, the modifying effect of basalt and cullet on the viscosity of the glass mass is visible, consisting in lowering the temperatures in the molding range [35,36].
3.3. Calculation Methods
All the methods of calculating the viscosity of glasses are based on their basic chemical composition. The influence of individual oxides on the viscosity of the glass mass is conditioned by the way individual metal ions are incorporated into the amorphous structure of the glass. The high proportion of ions forming the glass network will significantly increase the viscosity of the glass, while the growth in the content of modifying ions will have a diverse effect on it in specific temperature ranges. In order to determine the viscosity of the studied glasses by computational methods, the following methods were selected: Vogel–Fulcher–Tammann, M.W. Ochotin, Tuszynski and Dietzel-Brückner. These methods were used to determine the transition temperature, Tg, which is a fixed point on the viscosity curve at the value of η = 1013 [dPa·s] and the working point as the temperature value at the viscosity of η = 104 [dPa·s]. The working point describes the temperature at which the glass is soft enough to be processed in glassworks by the most common methods such as blowing, pressing or drawing [1].
3.3.1. Viscosity—Vogel–Fulcher–Tammann Method
The viscosity of glasses can be determined mathematically using Equation (1) given by Vogel–Fulcher–Tammann, called the VFT equation in logarithmic form [37,38]:
where A, B, T0 are constants depending on the chemical composition of the glass. Based on Equation (1), the temperatures corresponding to the given viscosity value were calculated (Table 6).

Table 6.
Calculated temperature [°C] at specified viscosities of 1013 and 104 [dPa·s] according to Vogel–Fulcher–Tammann.
Based on the obtained results, it can be clearly stated that this method cannot be employed to determine the viscosity of aluminosilicate glasses. The calculated temperatures drastically differ from the actual ones determined by experimental methods. The temperature values obtained in the calculation method clearly indicate the need to take into account the share of amphoteric oxides (e.g., Al2O3, Fe2O3, MgO) in the theoretical calculations, together with their ability to perform both bond-forming and modifying functions. The high-temperature values obtained in the calculations also result from the fact that the share of iron oxide was not included in the VFT equation. The investigated glasses from the SiO2–Al2O3–Fe2O3–CaO–MgO system were characterized by a high content of Fe2O3 oxide (from 7.31% by weight—Set 6 to 10.3% by weight—Set 1), which excludes the use of the Vogel–Fulcher–Tammann method in determining the viscosity curves of these glasses.
3.3.2. Viscosity—M.W. Ochotin Method
The method according to Ochotin is used for glasses in which, apart from the main glass-forming oxide SiO2, there are also amphoteric oxides, i.e., Al2O3 and MgO, able to play a bond-forming role. This type of glass is mainly used in the production of fibers. This method makes it possible to calculate temperatures for characteristic viscosity values, important from the point of view of technological properties, with fairly high accuracy. These temperatures can be determined using Equation (2) [14]:
where
T = Ax + By + Cz + D
- T—temperature [°C]
- x—percentage content of Na2O
- y—percentage content of the sum of CaO and MgO
- z—percentage content of Al2O3

Table 7.
Coefficients for determining temperature [°C] at specified viscosities of 1013 and 104 [dPa·s] according to M.W. Ochotin method.
Table 7.
Coefficients for determining temperature [°C] at specified viscosities of 1013 and 104 [dPa·s] according to M.W. Ochotin method.
| Viscosity [dPa·s] | Coefficients | |||
|---|---|---|---|---|
| A | B | C | D | |
| 1013 | −7.32 | 3.49 | 5.37 | 603.4 |
| 104 | −17.49 | −9.95 | 5.9 | 1381.4 |
By means of Equation (2), the temperatures for the examined glasses were calculated at the specified viscosity values of 1013 and 104 [dPa·s]. The results are presented in Table 8.

Table 8.
Calculated temperature [°C] at specified viscosities of 1013 and 104 [dPa·s] according to M.W. Ochotin method.
Table 8.
Calculated temperature [°C] at specified viscosities of 1013 and 104 [dPa·s] according to M.W. Ochotin method.
| Viscosity [dPa·s] | Glass | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Temperature [°C] | ||||||
| 1013 | 677 | 683 | 686 | 689 | 669 | 650 |
| 104 | 1262 | 1134 | 1198 | 1175 | 1147 | 1118 |
Based on the obtained results, it can be seen that the temperature values at the viscosity of 1013 [dPa·s] are close to those obtained experimentally. Therefore, it is concluded that this method can be utilized to calculate the transition temperature Tg for the researched aluminosilicate glasses.
3.3.3. Viscosity—Tuszynski Method
In the Tuszynski method, to calculate the temperatures at the viscosities of 1013 and 104 [dPa·s], the chemical compositions of the glasses listed in Table 2 were used. The calculations were carried out using Polynomial (3) [13].
where: A1, A2, …, An—calculation factors (Table 9), x1, x2, …, xn—mass percentage of oxides present in the glass.
T = A1x1 + A2x2 + A3x3 + A4x4 + A5x5 + …+ Anxn

Table 9.
Coefficients for determining temperature [°C] at specified viscosity from 103 to 1013 [dPa·s] according to Tuszynski method [13].
The obtained results are summarized in Table 10.

Table 10.
Calculated temperature [°C] at specific viscosities of 1013 and 104 [dPa·s] according to Tuszynski method.
Also in the case of this method, the Tg temperature values obtained for the studied glasses are comparable to the experimental data, but with a tendency to underestimate them.
3.3.4. Viscosity Calculated from Polynomial for Aluminosilicate Glasses
Using the calculation method developed by the authors of this work for glasses from the SiO2–Al2O3–Fe2O3–CaO–MgO system [19,39], based on the basic chemical composition of the investigated glasses, the temperatures at specific viscosities were calculated at η = 1013 and η = 104 [dPa·s]. In the employed calculation method, the percentage content of all the oxides having a significant impact on the viscosity of the glasses was taken into account. The proposed algorithm works well for glasses whose basic oxide composition falls within the ranges presented in Table 11.

Table 11.
Main content ranges of oxides, wt% [19].
With the calculation method used, the obtained results will be close to the experimental values only when the percentage compositions of the tested glasses are close to the ranges in Table 11. In Table 3 shows the percentage ranges of oxide contents the tested glasses.
Observing the dependence of the glass properties on the basic chemical composition, a second-order computational prediction method in the form of Equation (4) was assumed.
where y is the property, b1…b21 are parameters, x1…x6 are the composition in weight % of the glasses.
y = b1x1 + b2x2 + b3x3 + b4x4 + b5x5 + b6x6 + b7x1x2 + b8x1x3 + … + b21x5x6
Table 12 presents the temperatures calculated according to the polynomial for aluminosilicate glasses at the viscosities of η = 1013 and η = 104.0 [dPa·s].

Table 12.
Calculated temperature [°C] at specified viscosities of η = 1013 and η = 104.0 [dPa·s], according to polynomial for aluminosilicate glasses.
By comparing the Tg temperature values obtained on the basis of calculations from all the selected calculation methods with the temperature values obtained from the dilatometric tests, it can be concluded that the calculation results that are the closest to the experimental ones were obtained utilizing the method developed by the authors of this article and by the Tuszynski method (Figure 3). Both of these methods take into account a high proportion of CaO oxide and amphoteric oxides, which can strengthen or modify the glass structure in various temperature ranges, both high (forming range) and low (relaxation range). Based on the analysis, it can be concluded that when using analytical methods, the appropriate calculation model should always be selected for the type of glass for which the calculations are carried out. In the case of aluminosilicate glasses, the influence of the chemical composition on the properties, both technological and functional, is quite different than in the case of soda–lime–silicate glasses. The temperatures calculated by the Vogel–Fulcher–Tammann method, Tg and working point (Table 6), completely eliminate this type of calculation for applications for aluminosilicate glasses.

Figure 3.
Comparison of Tg temperature [°C] of η = 1013 [dPa·s] determined by various methods; * own elaboration [39].
3.4. Viscosity Curve Plotting Based on Specific Temperatures at Viscosities of 1013 and 104 [dPa·s]
The relationship between viscosity and temperature is very important in the production of glass. Hence, the basic feature of glass is a constant and smooth change in viscosity over the entire temperature range. In the range where the viscosity of the glass is 1013 [dPa·s], temperature Tg is taken as the annealing temperature. This is crucial for stress relaxation after hot forming, indicating the upper temperature limit of the so-called the annealing range in which internal stresses are released within minutes. At higher temperatures, the characteristic temperature at the viscosity of 104 [dPa·s] is the working point temperature. Depending on the size of the temperature range from 103 to 107 [dPa·s], a distinction is made between “long” glasses (large intervals between the softening point and the working point temperature) and “short” glasses (small intervals between the softening point and the working point temperature).
Knowing the values of the main temperatures, Tg and the working point, it is possible to plot the viscosity curves over a wide temperature range [24].
3.4.1. One-Point Method
In the one-point method, the Tg temperature is the basis for plotting the viscosity curve. Transition temperature Tg determined by the dilatometric method was used in the work, and then, using the relationship presented in Equation (5) [24], the viscosity curves of the studied glasses were plotted (Figure 4).
where
- Tg—transition temperature at a viscosity of 1013 [dPa·s];
- T—temperature [°C];
- C1, C2—constant: C1—14.97; C2—278.
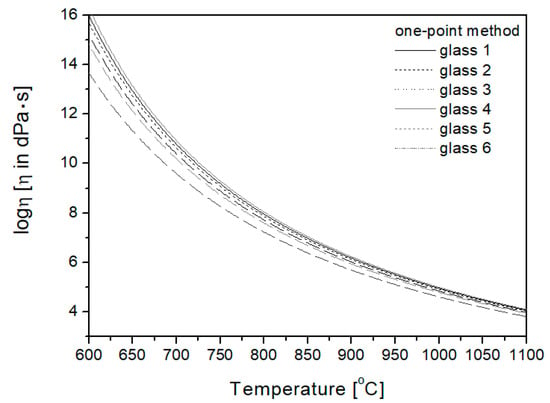
Figure 4.
Viscosity curves plotted using the one-point method.
3.4.2. Two-Point Method
In the two-point method, to determine the viscosity curve, in addition to the Tg temperature, an additional point on the curve is taken into account in the range of higher temperatures at the viscosity of 104 [dPa·s], i.e., the working point (TE) temperature. Taking this temperature into account allows more precise determination of the viscosity curve in the range in which the glass mass is characterized by greater fluidity [24].
In the viscosity calculations, temperature TE is taken into account to determine constant C2 (Equation (6)), which is then included in the calculations in Equation (5).
C2 = 0.588·(TE − Tg)
For the researched glasses, the Tg temperature was used to plot the viscosity curves by the two-point method, determined dilatometrically and the hemisphere temperature, close to the working point temperature (TE), defined by hot stage microscopy as a fixed viscosity point, for which the viscosity value is 104.1 [dPa·s]. The obtained curves are given in Figure 5.

Figure 5.
Viscosity curves plotted using the two-point method.
Taking into account the additional point (working point temperature TE) on the viscosity curve is very important for glasses differing in the so-called technological length during forming. By comparing the viscosity curves of Glasses 1 and 6, determined by the one- and two-point method, it can be seen that there are changes in the inclination of the curves for the investigated glasses. For Glass 1, compared to Glass 6, there is a smaller difference between the transition temperature Tg and the working point temperature (Figure 6). In the case of Glass 6, the curve determined by the two-point method has a greater slope and a smaller difference between Tg and TE T = 350 °C) than in the case of Glass 1 (ΔT = 450 °C). This means that Glass 6 is technologically “shorter” than Glass 1, which was not noticeable on the curves determined by the one-point method.
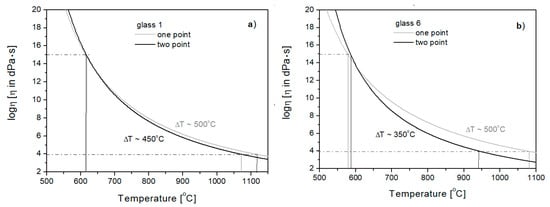
Figure 6.
Viscosity curves of Glasses 1 (a) and 6 (b) determined by one- and two-point methods.
Comparing the viscosity curves in the range of from 103 to 107 [dPa·s] (Figure 7), plotted for Glasses 1 and 6 using the one- and two-point methods with fixed viscosity points determined experimentally (by hot stage microscopy—HSM), it can be seen that all the fixed viscosity points better fit into the surroundings of the curves drawn using the two-point method. This means that for aluminosilicate glasses, in order to actually present the course of viscosity changes in terms of greater liquidity of the glass mass (forming range), it is necessary to include temperature TE (η = 104 [dPa·s]) in the calculations.
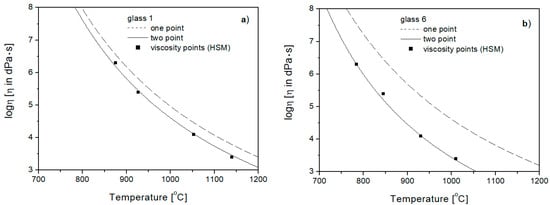
Figure 7.
Viscosity curves of Glasses 1 (a) and 6 (b), in range of from 103 to 107 [dPa·s], determined by one- and two-point methods with fixed viscosity points.
4. Conclusions
Based on the obtained research results and the conducted analysis, it was found that:
- Based on the analytical methods used to assess the viscosity of the glass mass in a wide temperature range, it was found that when selecting the appropriate calculation model, the basic chemical composition of the glass should always be taken into account, with particular emphasis on the share of amphoteric elements.
- It was justified that in order to plot the viscosity curve with the correct slope in the forming range, for aluminosilicate glasses it is appropriate to use the two-point method, based on fixed viscosity points for viscosities of 104 [dPa·s] (working point) and 1013 [dPa·s] (transition temperature).
- Amphoteric metal ions (e.g., Al3+, Fe3+ and Mg2+) have a significant impact on the viscosity of the glass mass, and thus on the quality of the manufactured products. The way they are embedded in the glass structure can cause a modifying effect (coordination number 6) or a binding effect (coordination number 4). An increase in the content of metal ions in coordination number 4 results in the incorporation of these ions into the glass network, and thus its strengthening, which in turn causes an increase in the viscosity of the melt.
- In the range of high temperatures, with at viscosity in the range of from η = 103 to 107 [dPa·s], the modifying effect of the addition of basalt and cullet was found, manifested by a decline in the viscosity of the glass mass, accompanied by a drop in the TE temperature in the working range.
- In the process of producing aluminosilicate glass, a thorough analysis of the temperature parameters characterizing the raw material sets is of great importance.
Author Contributions
Conceptualization, M.L. and A.Z.; methodology, M.L., A.Z. and A.N.; software, A.Z.; validation, M.L., A.Z. and A.N.; formal analysis, A.Z., M.L.; data curation, A.Z.; writing—original draft preparation, A.Z., A.N.; writing—review and editing, A.Z., M.L.; visualization, A.Z.; supervision, M.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Resources available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scholze, H. Glass: Nature, Structure, and Properties; Springer: New York, NY, USA, 1991. [Google Scholar] [CrossRef]
- Cantini, A.; Leoni, L.; Ferraro, S.; De Carlo, F.; Martini, C.; Martini, F.; Salvio, M. Technological Energy Efficiency Improvements in Glass-Production Industries and Their Future Perspectives in Italy. Processes 2022, 10, 2653. [Google Scholar] [CrossRef]
- Seo, K.; Edgar, T.F.; Baldea, M. Optimal demand response operation of electric boosting glass furnaces. Appl. Energy 2020, 269, 115077. [Google Scholar] [CrossRef]
- Galewicz, M.; Wasylak, J. Możliwości obniżenia temperatury topienia szkła opakowaniowego. Szkło i Ceramika 2008, 59, 11–15. [Google Scholar]
- Shao, Z.F.; Bao, Y.W.; Jia, Y.N.; Rao, C.D.; Sui, M.; Wang, Y.F.; Xiang, Z.K. Analysis of influence of high temperature homogenizing on UV transmittance of quartz glass. Key Eng. Mater. 2016, 680, 285–288. [Google Scholar] [CrossRef]
- Kim, Y.; Morita, K. Thermal conductivity of molten B2O3, B2O3-SiO2, Na2O-B2O3, and Na2O-SiO2 systems. J. Am. Ceram. Soc. 2015, 98, 1588–1595. [Google Scholar] [CrossRef]
- Liu, C.K.; Lee, R.Y.; Lin, K.F. Effects of lanthanum-to-calcium ratio on the thermal and crystalline properties of BaO-Al2O3-B2O3-SiO2 based glass sealants for solid oxide fuel cells. J. Ceram. Soc. Jpn. 2015, 123, 239–244. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Zhang, X.; Liu, W.; Lv, X.; Bai, C.; Wang, L. Relationship between structure and viscosity of CaO–SiO2–Al2O3–MgO–TiO2 slag. J. Non-Cryst. Solids 2014, 402, 214–222. [Google Scholar] [CrossRef]
- Brosh, E.; Pelton, A.D.; Decterov, S.A. A model to calculate the viscosity of silicate melts: Part IV: Alkali-free borosilicate melts. Int. J. Mater. Res. 2012, 103, 494–501. [Google Scholar] [CrossRef]
- Gan, L.; Xin, J.; Zhou, Y. Accurate Viscosity Calculation for Melts in SiO2–Al2O3–CaO–MgO Systems. ISIJ Int. 2017, 57, 1303–1312. [Google Scholar] [CrossRef]
- Gan, L.; Lai, C.A. General Viscosity Model for Molten Blast Furnace Slag. Metall. Mater. Trans. B 2014, 45, 875–888. [Google Scholar] [CrossRef]
- Shelby, J.E. Introduction to Glass Science and Technology, 2nd ed.; RSC: New York, NY, USA, 2005; pp. 120–122. [Google Scholar]
- Ciecińska, M.; Dorosz, D.; Greiner-Wrona, E.; Gruszka, B.; Kucharski, J.; Lisiecki, M.; Łączka, M. Technologia Szkła, Właściwości Fizykochemiczne, Część I; Polskie Towarzystwo Ceramiczne: Kraków, Poland, 2002. [Google Scholar]
- Ochotin, M.W. Calculation of Viscosity of Commercial Silicate Classes by the Use of Nomographs. Steklo i Keram 1954, 11, 7–11. [Google Scholar]
- Jebsten-Marwedel, H.; Bruckner, R. Glastechnische Fabrikationsfehler; Springer: New York, NY, USA, 1980. [Google Scholar]
- Mauro, J.C.; Yue, Y.; Ellison, A.J.; Guptac, P.K.; Allan, D.C. Viscosity of glass-forming liquids. Proc. Natl. Acad. Sci. USA 2009, 106, 19780–19784. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Chen, M.; Zhang, W.; Zhao, Z.; Evans, T.; Zhao, B. Evaluation of Existing Viscosity Data and Models and Developments of New Viscosity Model for Fully Liquid Slag in the SiO2-Al2O3-CaO-MgO System. Metall. Mater. Trans. B 2016, 47, 2861–2874. [Google Scholar] [CrossRef]
- Grundy, A.N.; Liu, H.; Jung, I.-H.; Decterov, S.A.; Pelton, A.D. A model to calculate the viscosity of silicate melts: Part I: Viscosity of binary SiO2–MeOx systems (Me = Na, K, Ca, Mg, Al). Int. J. Mater. Res. 2008, 99, 1185–1194. [Google Scholar] [CrossRef]
- Zawada, A.; Bieniarz, P.; Kolan, C.; Hessenkemper, H. Modelling Selected Properties of Glasses Based on Slag from a Waste. Inciner. Plant. Glass Tech. Eur. J. Glass Sci. Tech. Part A 2013, 54, 72–76. [Google Scholar]
- Zawada, A.; Lubasa, M.; Przerada, I. Mössbauer spectroscopy and FT-IR to describe coordination of amphoteric ions in structure of glasses from the SiO2–Na2O–MgO–CaO–Al2O3–Fe2O3 system. J. Mol. Struct. 2023, 1285, 135368. [Google Scholar] [CrossRef]
- Karlsson, S. Viscosity of alumina doped soda lime silicate glasses—Observation of anomaly in the linear increase as Al2O3 replaces SiO2. J. Non-Cryst. Solids 2021, 573, 121149. [Google Scholar] [CrossRef]
- Clarke, J.B.; Hastie, J.W.; Kihlborg, L.H.E.; Metselaar, R.; Thackeray, M.M. Definitions of terms relating to phase transitions of the solid state (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 577–594. [Google Scholar] [CrossRef]
- Hutchinson, J.M. Determination of the glass transition temperature. J. Therm. Anal. Calorim. 2009, 98, 579. [Google Scholar] [CrossRef]
- Dietzel, A.; Brückner, R. Ein Fixpunkt der Zähigkeit im Verarbeitungsbereich der Gläser. Schnellbestimmung des Viskositäts-, Temperatur- Verlaufes. Glastech. Berichte 1957, 3, 73–79. [Google Scholar]
- Mazurin, O.V.; Gankin, Y.V. Glass transition temperature: Problems of measurements and analysis of the existing data. In Proceedings of the International Congress on Glass, Strasbourg, France, 1–6 July 2007. [Google Scholar]
- Le Bourhis, E. Glass—Mechanics and Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Zawada, A.; Lubasa, M.; Przerada, I.; Sitarz, M.; Adamczyk-Habrajska, M. The Effect of the Reducing Melting Atmosphere on Coordination Moieties in Aluminosilicate Glasses. J. Mol. Struct. 2020, 1218, 128474. [Google Scholar] [CrossRef]
- Yuan, F.; Zhao, Z.; Zhang, Y.; Wu, T. Effect of Al2O3 content on the viscosity and structure of CaO–SiO2–Cr2O3–Al2O3 slags. Int. J. Miner. Metall. Mater. 2022, 29, 1522–1531. [Google Scholar] [CrossRef]
- Lü, J.; Jin, Z.; Yang, H.; Tong, L.-L.; Chen, G.-B.; Xiao, F.-X. Effect of the CaO/SiO2 mass ratio and FeO content on the viscosity of CaO–SiO2–“FeO”–12wt%ZnO–3wt%Al2O3 slags. Int. J. Miner. Metall. Mater. 2017, 24, 756–767. [Google Scholar] [CrossRef]
- Richet, P. Encyclopedia of Glass Science, Technology, History, and Culture; Wiley: Hoboken, NJ, USA, 2021; pp. 538–539. [Google Scholar]
- Gao, Y.; Wang, S.; Hong, C. Effects of basicity and MgO content on the viscosity of the SiO2-CaO-MgO-9wt%Al2O3 slag system. Int. J. Miner. Metall. Mater. 2014, 21, 353–362. [Google Scholar] [CrossRef]
- Nowak, A.; Caban, A.; Lubas, M.; Iwaszko, J. Właściwości modyfikowanego szkła amfibolitowego. Mater. Ceram./Ceram. Mater. 2020, 72, 161–170. [Google Scholar]
- Pascual, M.J.; Pascual, L.; Durán, A. Determination of the viscosity temperature curve for glasses on the basis of fixed viscosity points determined by hot stage microscopy. Phys. Chem. Glasses 2001, 42, 61–66. [Google Scholar]
- Stábile, F.M.; Piccico, M.; Serra, M.F.; Rafti, M.; Súarez, G.; Rendtorff, N.M. Viscosity and Thermal Evolution of Density and Wetting Angle of a Commercial Glaze by Means of Hot Stage Microscopy. Procedia Mater. Sci. 2015, 9, 563–570. [Google Scholar] [CrossRef]
- Wasylak, J.; Czarnacki, K. Wpływ stłuczki szklanej z recyklingu na właściwości produkowanych opakowań szklanych w aspekcie nowych metod uszlachetniania powierzchni. Prace Instytutu Ceramiki i Materiałów Budowlanych 2012, 11, 9–19. [Google Scholar]
- Reben, M.; Wasylak, J.; Lisiecki, M.; Kuciński, G.; Kosmal, M. Surowce odpadowe jako nukleatory krystalizacji stłuczki kineskopowej. Mater. Ceram./Ceram. Mater. 2012, 64, 405–410. [Google Scholar]
- Teschner, R. Glasviskosität. In Glasfasern; Springer Vieweg: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Gao, Q.; Jian, Z. Fragility and Vogel-Fulcher-Tammann parameters near Glass transition temmperature. Mater. Chem. Phys. 2020, 252, 123252. [Google Scholar] [CrossRef]
- Zawada, A.; Przerada, I.; Lubas, M.; Sitarz, M.; Leśniak, M. Application of Statistical Methods in Predicting the Properties of Glass-Ceramic Materials Obtained from Inorganic Solid Waste. Materials 2021, 14, 2651. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).