New Materials Used for the Development of Anion-Selective Electrodes—A Review
Abstract
:1. Introduction
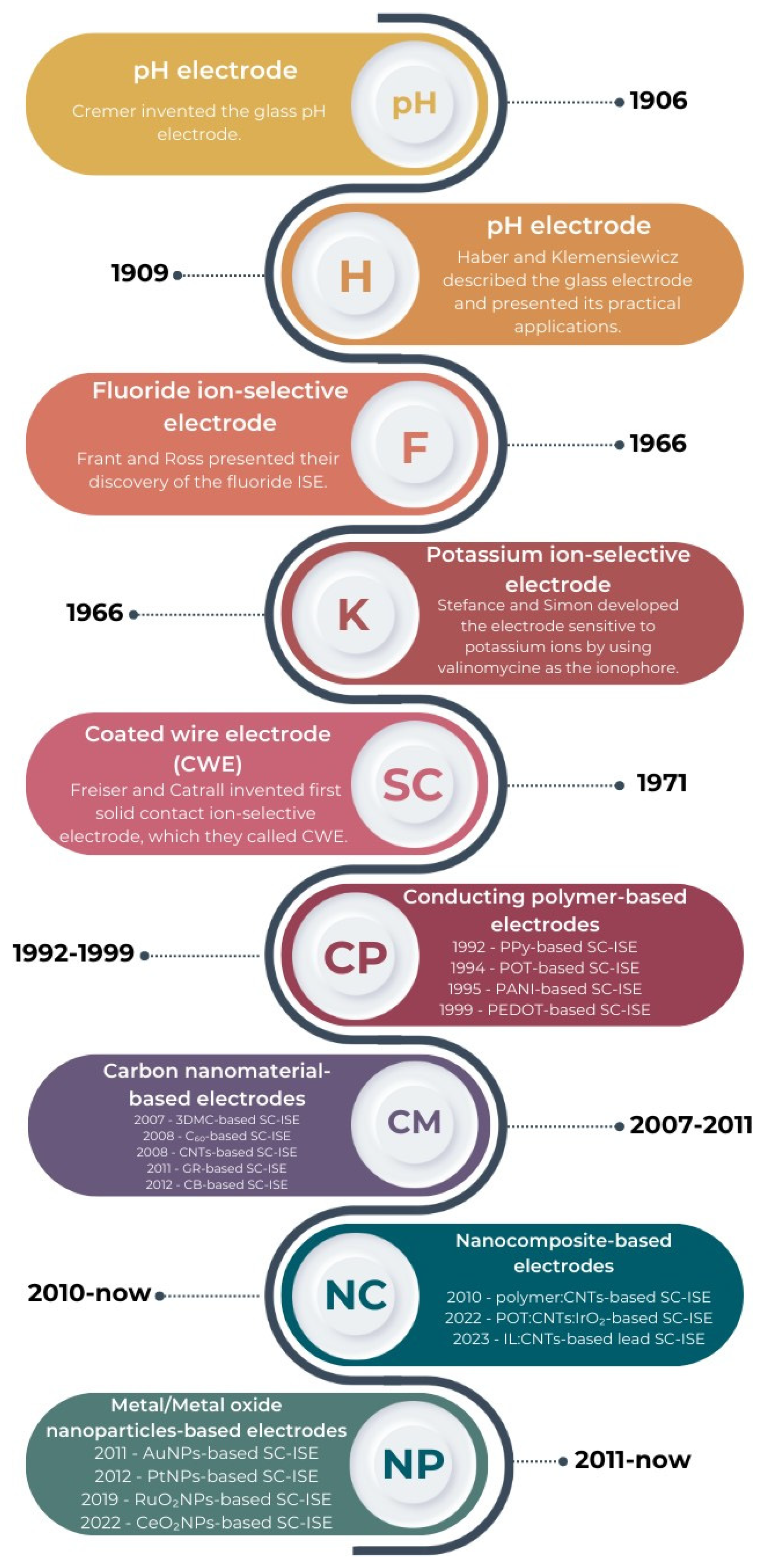
2. Constructional Variants of Anionic Ion-Selective Electrodes
- -
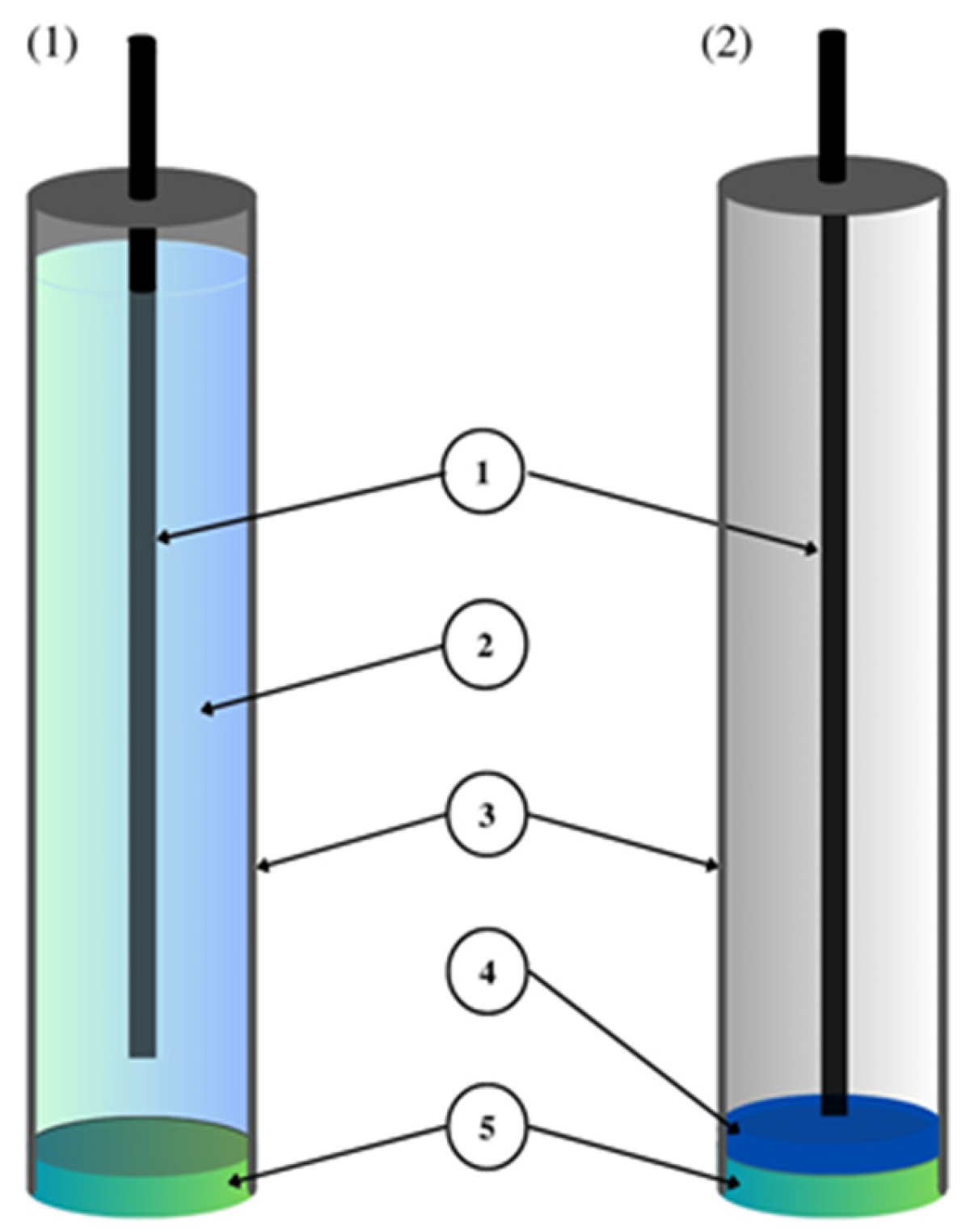
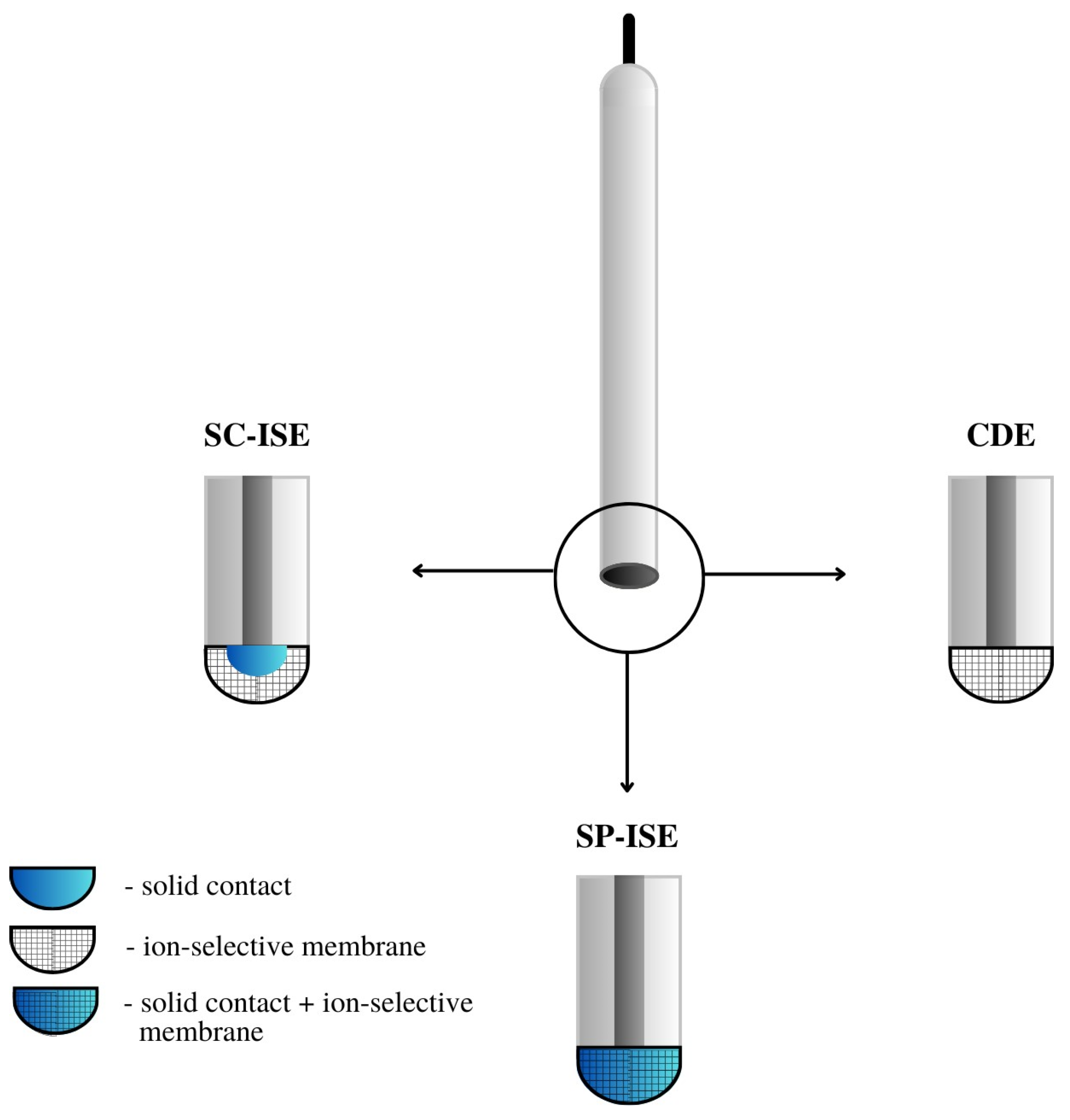
- CWE, CDE—a coated wire/coated disc electrode, which was originally a platinum wire coated with a layer of PVC membrane. CWEs/CDEs are created by applying, for example, a polymer membrane or crystal membrane on a wire/disc used as the inner electrode. Such sensors are now rarely used. In publications, they are studied as a comparative system to assess the effectiveness of solid contact or modification of the membrane composition.
- -
- SC-ISE—solid contact ISE is a type of electrode in which an intermediate layer of material, generally called solid contact, is introduced between the ion-selective membrane and the inner electrode. Conductive polymers, carbon nanomaterials, metal and metal oxide nanoparticles and composite materials are most often used as intermediate layers.
- -
- SP-ISE—a single-piece ion-selective electrode with a membrane in which the modifying material is dispersed or dissolved. An ion-selective electrode is created by depositing a membrane cocktail of conductive polymers or other conductive materials, e.g., carbon nanotubes, onto a surface that is usually a glassy carbon electrode [17,18]. Carbon paste ion-selective electrodes can also be included in this group of electrodes in which the active component of the membrane is mixed with graphite powder and binder material, and the mixture is packed in the sensor holder with an internal electrode placed inside (often a copper wire). In order to improve their work, the composition of the paste electrode membrane is also modified by the addition of various materials, which are often carbon nanomaterials. A comparison of the construction of different ASS-ISEs electrodes is shown in Figure 3.
3. Research Methods Used to Assess New ISEs
4. Ion-Selective Electrodes Sensitive to Nitrates Ions
5. Ion-Selective Electrodes Sensitive to Fluoride Ions
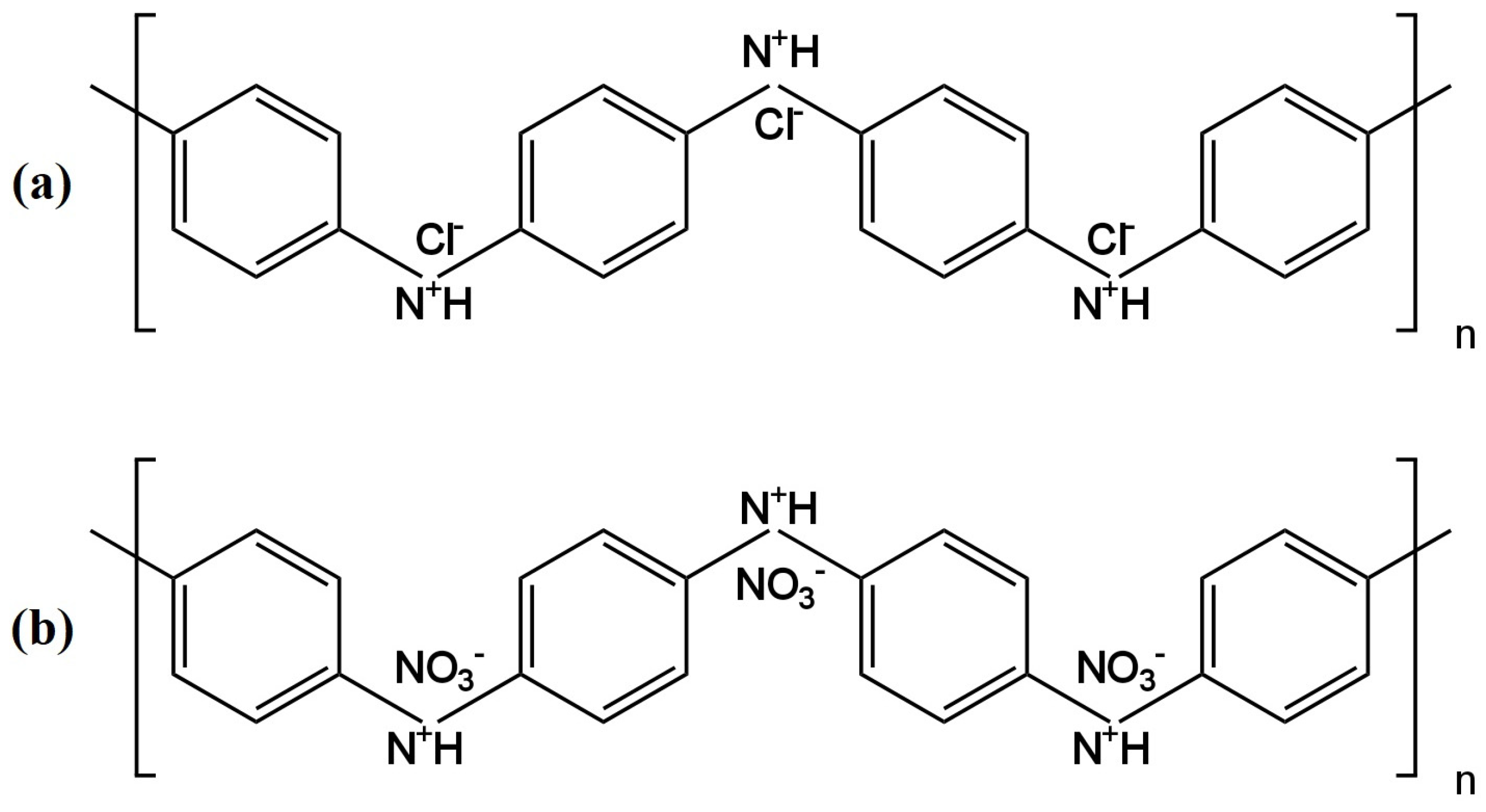
6. Ion-Selective Electrodes Sensitive to Chloride and Perchloride Ions
7. Bromide Ion-Selective Electrodes
8. Iodide Ion-Selective Electrodes
9. Ion-Selective Electrodes Sensitive to S2−, SO32− and SO42− Ions
10. Ion-Selective Electrodes Sensitive to Phosphates
11. Ion-Selective Electrodes Sensitive to Tiocyanate Ions
12. Ion-Selective Electrodes Sensitive to Other Ions
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morf, W.E. The Principles of Ion-Selective Electrodes and of Membrane Transport; Elsevier: New York, NY, USA, 1981. [Google Scholar]
- Głąb, S.; Maj-Żurawska, M.; Hulanicki, A. Ion-Selective Electrodes|Glass Electrodes. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Sohail, M.; De Marco, R. ELECTRODES|Ion-Selective Electrodes. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Paczosa-Bator, B.; Cabaj, L.; Piech, R.; Skupień, K. Platinum Nanoparticles Intermediate Layer in Solid-State Selective Electrodes. Analyst 2012, 137, 5272. [Google Scholar] [CrossRef]
- Schwarz, J.; Trommer, K.; Mertig, M. Solid-Contact Ion-Selective Electrodes Based on Graphite Paste for Potentiometric Nitrate and Ammonium Determinations. Am. J. Anal. Chem. 2018, 9, 591–601. [Google Scholar] [CrossRef]
- Wardak, C.; Morawska, K.; Paczosa-Bator, B.; Grabarczyk, M. Improved Lead Sensing Using a Solid-Contact Ion-Selective Electrode with Polymeric Membrane Modified with Carbon Nanofibers and Ionic Liquid Nanocomposite. Materials 2023, 16, 1003. [Google Scholar] [CrossRef]
- Wardak, C.; Pietrzak, K.; Morawska, K.; Grabarczyk, M. Ion-Selective Electrodes with Solid Contact Based on Composite Materials: A Review. Sensors 2023, 23, 5839. [Google Scholar] [CrossRef] [PubMed]
- Wardak, C. Solid Contact Cadmium Ion-Selective Electrode Based on Ionic Liquid and Carbon Nanotubes. Sens. Actuators B Chem. 2015, 209, 131–137. [Google Scholar] [CrossRef]
- Wardak, C.; Pietrzak, K.; Morawska, K. Nanocomposite of Copper Oxide Nanoparticles and Multi-Walled Carbon Nanotubes as a Solid Contact of a Copper-Sensitive Ion-Selective Electrode: Intermediate Layer or Membrane Component–Comparative Studies. Appl. Nanosci. 2023. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Y.; Zhang, W.; Wang, Y.; Li, G. Solid-Contact Ion-Selective Electrodes for Histamine Determination. Sensors 2021, 21, 6658. [Google Scholar] [CrossRef]
- Bobacka, J. Conducting Polymer-Based Solid-State Ion-Selective Electrodes. Electroanalysis 2006, 18, 7–18. [Google Scholar] [CrossRef]
- Lenik, J.; Wesoły, M.; Ciosek, P.; Wróblewski, W. Evaluation of Taste Masking Effect of Diclofenac Using Sweeteners and Cyclodextrin by a Potentiometric Electronic Tongue. J. Electroanal. Chem. 2016, 780, 153–159. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential Drifts of Solid-Contacted Ion-Selective Electrodes Due to Zero-Current Ion Fluxes Through the Sensor Membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- Suman, S.; Singh, R. Anion Selective Electrodes: A Brief Compilation. Microchem. J. 2019, 149, 104045. [Google Scholar] [CrossRef]
- Bobacka, J.; Lindfors, T.; McCarrick, M.; Ivaska, A.; Lewenstam, A. Single-Piece All-Solid-State Ion-Selective Electrode. Anal. Chem. 1995, 67, 3819–3823. [Google Scholar] [CrossRef]
- Wardak, C.; Grabarczyk, M. Single-Piece All-Solid-State Co(II) Ion-Selective Electrode for Cobalt Monitoring in Real Samples. Int. Agrophys 2019, 1, 17–24. [Google Scholar] [CrossRef]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Lindner, E.; Umezawa, Y. Performance Evaluation Criteria for Preparation and Measurement of Macro- and Microfabricated Ion-Selective Electrodes (IUPAC Technical Report). Pure Appl. Chem. 2008, 80, 85–104. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J.; Lewenstam, A.; Ivaska, A. Equilibrium Potential of Potentiometric Ion Sensors under Steady-State Current by Using Current-Reversal Chronopotentiometry. J. Electroanal. Chem. 2001, 509, 27–30. [Google Scholar] [CrossRef]
- Paré, F.; Visús, A.; Gabriel, G.; Baeza, M. Novel Nitrate Ion-Selective Microsensor Fabricated by Means of Direct Ink Writing. Chemosensors 2023, 11, 174. [Google Scholar] [CrossRef]
- Hjort, R.G.; Soares, R.R.A.; Li, J.; Jing, D.; Hartfiel, L.; Chen, B.; Van Belle, B.; Soupir, M.; Smith, E.; McLamore, E.; et al. Hydrophobic Laser-Induced Graphene Potentiometric Ion-Selective Electrodes for Nitrate Sensing. Microchim. Acta 2022, 189, 122. [Google Scholar] [CrossRef]
- Ali, A.; Wang, X.; Chen, Y.; Jiao, Y.; Mahal, N.K.; Moru, S.; Castellano, M.J.; Schnable, J.C.; Schnable, P.S.; Dong, L. Continuous Monitoring of Soil Nitrate Using a Miniature Sensor with Poly(3-Octyl-Thiophene) and Molybdenum Disulfide Nanocomposite. ACS Appl. Mater. Interfaces 2019, 11, 29195–29206. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Wardak, C.; Malinowski, S. Application of Polyaniline Nanofibers for the Construction of Nitrate All-Solid-State Ion-Selective Electrodes. Appl. Nanosci. 2021, 11, 2823–2835. [Google Scholar] [CrossRef]
- Zhang, Z.; Papautsky, I. Miniature Ion-selective Electrodes with Mesoporous Carbon Black as Solid Contact. Electroanalysis 2021, 33, 2143–2151. [Google Scholar] [CrossRef]
- Thuy, N.T.D.; Wang, X.; Zhao, G.; Liang, T.; Zou, Z. A Co3O4 Nanoparticle-Modified Screen-Printed Electrode Sensor for the Detection of Nitrate Ions in Aquaponic Systems. Sensors 2022, 22, 9730. [Google Scholar] [CrossRef] [PubMed]
- Fozia; Zhao, G.; Nie, Y.; Jiang, J.; Chen, Q.; Wang, C.; Xu, X.; Ying, M.; Hu, Z.; Xu, H. Preparation of Nitrate Bilayer Membrane Ion-Selective Electrode Modified by Pericarpium Granati-Derived Biochar and Its Application in Practical Samples. Electrocatalysis 2023, 14, 534–545. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Z.; Liu, P. An All-Solid-State NO3− Ion-Selective Electrode with Gold Nanoparticles Solid Contact Layer and Molecularly Imprinted Polymer Membrane. PLoS ONE 2020, 15, e0240173. [Google Scholar] [CrossRef]
- Baumbauer, C.L.; Goodrich, P.J.; Payne, M.E.; Anthony, T.; Beckstoffer, C.; Toor, A.; Silver, W.; Arias, A.C. Printed Potentiometric Nitrate Sensors for Use in Soil. Sensors 2022, 22, 4095. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Meng, Z.; Qin, Y.; Jiang, D.; Xi, K.; Wang, P. Thiol-Functionalized Reduced Graphene Oxide as Self-Assembled Ion-to-Electron Transducer for Durable Solid-Contact Ion-Selective Electrodes. Talanta 2020, 208, 120374. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, Y.; Linthicum, W.; Liu, F.; Beringhs, A.O.; Dang, Y.; Xu, Z.; Chang, S.-Y.; Ling, J.; Huey, B.D.; et al. Toward Long-Term Accurate and Continuous Monitoring of Nitrate in Wastewater Using Poly(Tetrafluoroethylene) (PTFE)–Solid-State Ion-Selective Electrodes (S-ISEs). ACS Sens. 2020, 5, 3182–3193. [Google Scholar] [CrossRef]
- Pietrzak, K.; Wardak, C.; Łyszczek, R. Solid Contact Nitrate Ion-selective Electrode Based on Cobalt(II) Complex with 4,7-Diphenyl-1,10-phenanthroline. Electroanalysis 2020, 32, 724–731. [Google Scholar] [CrossRef]
- Pietrzak, K.; Wardak, C. Comparative Study of Nitrate All Solid State Ion-Selective Electrode Based on Multiwalled Carbon Nanotubes-Ionic Liquid Nanocomposite. Sens. Actuators B Chem. 2021, 348, 130720. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Lee, J.-W.; Park, D.J.; Lee, J.-Y.; Myung, N.V.; Kwon, S.H.; Lee, K.H. Highly Stable Potentiometric Sensor with Reduced Graphene Oxide Aerogel as a Solid Contact for Detection of Nitrate and Calcium Ions. J. Electroanal. Chem. 2021, 897, 115553. [Google Scholar] [CrossRef]
- Ivanova, A.; Mikhelson, K. Electrochemical Properties of Nitrate-Selective Electrodes: The Dependence of Resistance on the Solution Concentration. Sensors 2018, 18, 2062. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Eldin, A.G.; Amr, A.E.-G.E.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Improved Solid-Contact Nitrate Ion Selective Electrodes Based on Multi-Walled Carbon Nanotubes (MWCNTs) as an Ion-to-Electron Transducer. Sensors 2019, 19, 3891. [Google Scholar] [CrossRef]
- Fukao, Y.; Kitazumi, Y.; Kano, K.; Shirai, O. Construction of Nitrate-Selective Electrodes and Monitoring of Nitrates in Hydroponic Solutions. Anal. Sci. 2018, 34, 1373–1377. [Google Scholar] [CrossRef]
- Pięk, M.; Piech, R.; Paczosa-Bator, B. TTF-TCNQ Solid Contact Layer in All-Solid-State Ion-Selective Electrodes for Potassium or Nitrate Determination. J. Electrochem. Soc. 2018, 165, B60–B65. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, L.; Kong, M.; Qiu, L.; Lü, P.; Wu, P.; Yang, Y.; Chen, K. Toxic Effects of Fluoride on Organisms. Life Sci. 2018, 198, 18–24. [Google Scholar] [CrossRef]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T.; et al. Toxicity of Fluoride: Critical Evaluation of Evidence for Human Developmental Neurotoxicity in Epidemiological Studies, Animal Experiments and in Vitro Analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef]
- Yamada, T.; Kanda, K.; Yanagida, Y.; Mayanagi, G.; Washio, J.; Takahashi, N. Fluoride Ion Sensor Based on LaF3 Nanocrystals Prepared by Low-Temperature Process. J. Ceram. Soc. Jpn. 2023, 131, 22127. [Google Scholar] [CrossRef]
- Yamada, T.; Kanda, K.; Yanagida, Y.; Mayanagi, G.; Washio, J.; Takahashi, N. All-solid-state Fluoride Ion-selective Electrode Using LaF 3 Single Crystal with Poly(3,4-ethylenedioxythiophene) as Solid Contact Layer. Electroanalysis 2023, 35, e202200103. [Google Scholar] [CrossRef]
- Radić, J.; Bralić, M.; Kolar, M.; Genorio, B.; Prkić, A.; Mitar, I. Development of the New Fluoride Ion-Selective Electrode Modified with FexOy Nanoparticles. Molecules 2020, 25, 5213. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Sandhu, S.S.; Teymourian, H.; Yin, L.; Tostado, N.; Raushel, F.M.; Harvey, S.P.; Moores, L.C.; Wang, J. Textile-Based Wearable Solid-Contact Flexible Fluoride Sensor: Toward Biodetection of G-Type Nerve Agents. Biosens. Bioelectron. 2021, 182, 113172. [Google Scholar] [CrossRef]
- Biyareh, M.N.; Rezvani, A.R.; Dashtian, K.; Montazerozohori, M.; Ghaedi, M.; Masoudi Asl, A.; White, J. Potentiometric Ion-Selective Electrode Based on a New Single Crystal Cadmium(II) Schiff Base Complex for Detection of Fluoride Ion: Central Composite Design Optimization. IEEE Sens. J. 2019, 19, 413–425. [Google Scholar] [CrossRef]
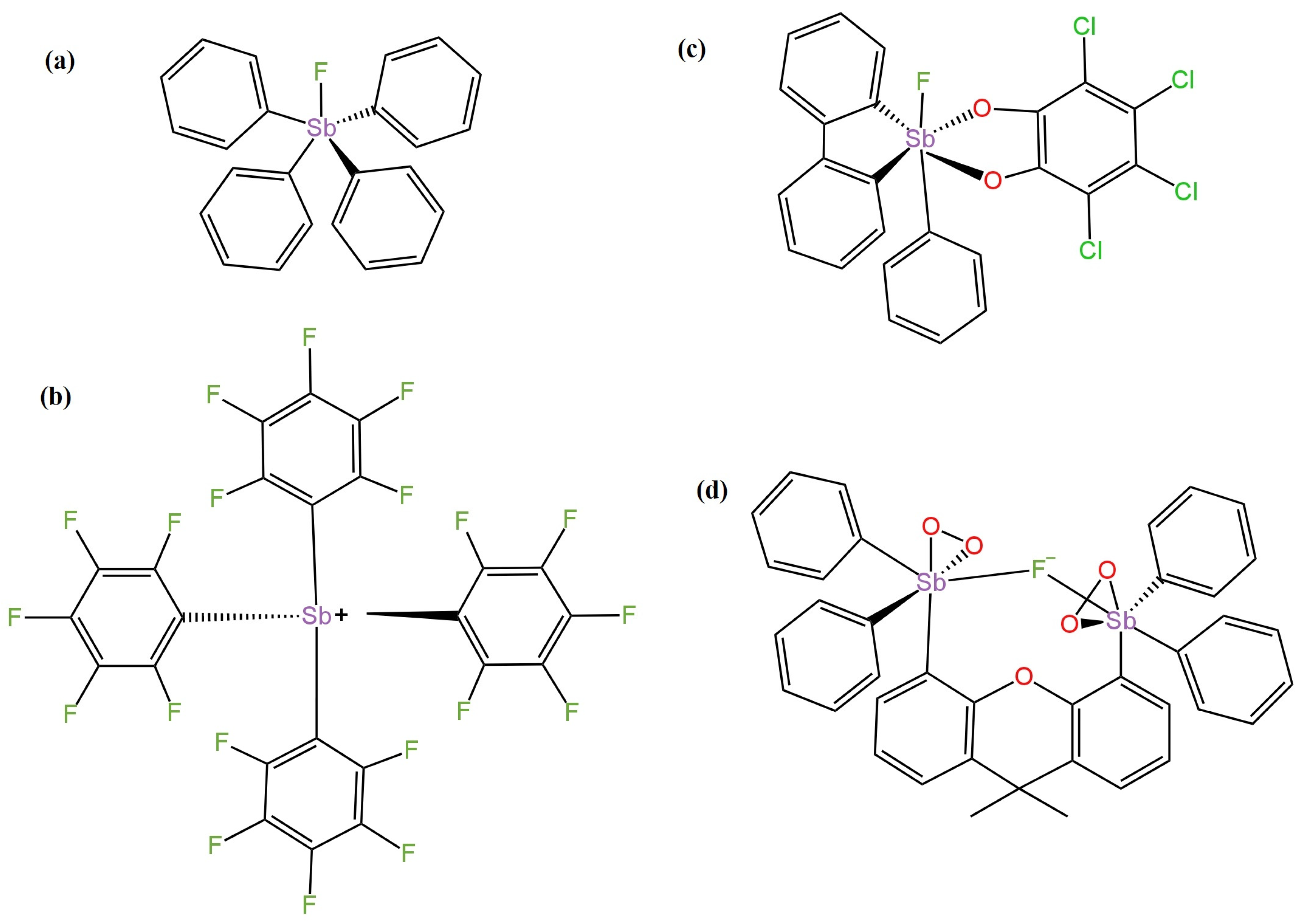
- Li, L.; Zhang, Y.; Li, Y.; Duan, Y.; Qian, Y.; Zhang, P.; Guo, Q.; Ding, J. Polymeric Membrane Fluoride-Selective Electrodes Using Lewis Acidic Organo-Antimony(V) Compounds as Ionophores. ACS Sens. 2020, 5, 3465–3473. [Google Scholar] [CrossRef] [PubMed]
- Ke, X. Micro-Fabricated Electrochemical Chloride Ion Sensors: From the Present to the Future. Talanta 2020, 211, 120734. [Google Scholar] [CrossRef]
- de Graaf, D.B.; Abbas, Y.; Gerrit Bomer, J.; Olthuis, W.; van den Berg, A. Sensor–Actuator System for Dynamic Chloride Ion Determination. Anal. Chim. Acta 2015, 888, 44–51. [Google Scholar] [CrossRef]
- Kosaka, K.; Asami, M.; Kunikane, S. Perchlorate: Origin and Occurrence in Drinking Water. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Srinivasan, A.; Viraraghavan, T. Perchlorate: Health Effects and Technologies for Its Removal from Water Resources. Int. J. Environ. Res. Public Health 2009, 6, 1418–1442. [Google Scholar] [CrossRef]
- Pietrzak, K.; Morawska, K.; Malinowski, S.; Wardak, C. Chloride Ion-Selective Electrode with Solid-Contact Based on Polyaniline Nanofibers and Multiwalled Carbon Nanotubes Nanocomposite. Membranes 2022, 12, 1150. [Google Scholar] [CrossRef]
- Kalayci, S. Analysis of Halogens in Wastewater with a New Prepared Ion Selective Electrode. Monatshefte Chem.-Chem. Mon. 2022, 153, 1137–1141. [Google Scholar] [CrossRef]
- Pięk, M.; Paczosa-Bator, B.; Smajdor, J.; Piech, R. Molecular Organic Materials Intermediate Layers Modified with Carbon Black in Potentiometric Sensors for Chloride Determination. Electrochim. Acta 2018, 283, 1753–1762. [Google Scholar] [CrossRef]
- Alizadeh, T.; Rafiei, F.; Akhoundian, M. A Novel Chloride Selective Potentiometric Sensor Based on Graphitic Carbon Nitride/Silver Chloride (g-C3N4/AgCl) Composite as the Sensing Element. Talanta 2022, 237, 122895. [Google Scholar] [CrossRef]
- Liao, C.; Zhong, L.; Tang, Y.; Sun, Z.; Lin, K.; Xu, L.; Lyu, Y.; He, D.; He, Y.; Ma, Y.; et al. Solid-Contact Potentiometric Anion Sensing Based on Classic Silver/Silver Insoluble Salts Electrodes without Ion-Selective Membrane. Membranes 2021, 11, 959. [Google Scholar] [CrossRef]
- Prkić, A. New Sensor Based on AgCl Containing Iron Oxide or Zinc Oxide Nanoparticles for Chloride Determination. Int. J. Electrochem. Sci. 2019, 14, 861–874. [Google Scholar] [CrossRef]
- Babaei, M.; Alizadech, N. Highly Selective Perchlorate Coated-Wire Electrode (CWE) Based on an Electrosynthesized Dixanthylinum Dye and Its Application in Water Samples. Chem. Rev. Lett. 2022, 5, 241–249. [Google Scholar] [CrossRef]
- Itterheimová, P.; Bobacka, J.; Šindelář, V.; Lubal, P. Perchlorate Solid-Contact Ion-Selective Electrode Based on Dodecabenzylbambus [6]Uril. Chemosensors 2022, 10, 115. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Galal Eldin, A.; Amr, A.E.-G.E.; Al-Omar, M.A.; Kamel, A.H. Single-Walled Carbon Nanotubes (SWCNTs) as Solid-Contact in All-Solid-State Perchlorate ISEs: Applications to Fireworks and Propellants Analysis. Sensors 2019, 19, 2697. [Google Scholar] [CrossRef]
- Pavelka, S. Metabolism of Bromide and Its Interference with the Metabolism of Iodine. Physiol. Res. 2004, 53 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.; Narvencar, K.P.S.; Sindhoora, K.V. The Neurological Effects of Methyl Bromide Intoxication. J. Neurol. Sci. 2013, 335, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Sharma, S.; Iftikhar, H. Unexplained Bromide Toxicity Presenting as Hyperchloremia and a Negative Anion Gap. Cureus 2023, 15, e36218. [Google Scholar] [CrossRef] [PubMed]
- Vlascici, D.; Plesu, N.; Fagadar-Cosma, G.; Lascu, A.; Petric, M.; Crisan, M.; Belean, A.; Fagadar-Cosma, E. Potentiometric Sensors for Iodide and Bromide Based on Pt(II)-Porphyrin. Sensors 2018, 18, 2297. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kaur, J.; Badru, R.; Kaushal, S.; Singh, P.P. BGO/AlFu MOF Core Shell Nano-Composite Based Bromide Ion-Selective Electrode. J. Environ. Chem. Eng. 2020, 8, 104375. [Google Scholar] [CrossRef]
- Kou, L. Detection of Bromide Ions in Water Samples with a Nanomolar Detection Limit Using a Potentiometric Ion-Selective Electrode. Int. J. Electrochem. Sci. 2019, 14, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandi, M.; Chandhru, M.; Rani, S.K.; Vasimalai, N. Highly Selective Detection of Iodide in Biological, Food, and Environmental Samples Using Polymer-Capped Silver Nanoparticles: Preparation of a Paper-Based Testing Kit for On-Site Monitoring. ACS Omega 2019, 4, 11372–11379. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Cerqueira, C.; Ovesen, L.; Rasmussen, L.B.; Perrild, H.; Andersen, S.; Pedersen, I.B.; Carlé, A. Iodine Intake as a Determinant of Thyroid Disorders in Populations. Best. Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 13–27. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef]
- Prkić, A. Development of a New Potentiometric Sensor Based on Home Made Iodide ISE Enriched with ZnO Nanoparticles and Its Application for Determination of Penicillamine. Int. J. Electrochem. Sci. 2018, 13, 10894–10903. [Google Scholar] [CrossRef]
- Shvedene, N.V.; Abashev, M.N.; Arakelyan, S.A.; Otkidach, K.N.; Tomilova, L.G.; Pletnev, I.V. Highly Selective Solid-State Sensor for Iodide Based on the Combined Use of Platinum (IV) Phthalocyanine and Solidified Pyridinium Ionic Liquid. J. Solid. State Electrochem. 2019, 23, 543–552. [Google Scholar] [CrossRef]
- Seah, G.E.K.K.; Tan, A.Y.X.; Neo, Z.H.; Lim, J.Y.C.; Goh, S.S. Halogen Bonding Ionophore for Potentiometric Iodide Sensing. Anal. Chem. 2021, 93, 15543–15549. [Google Scholar] [CrossRef]
- Dordevic, D.; Capikova, J.; Dordevic, S.; Tremlová, B.; Gajdács, M.; Kushkevych, I. Sulfur Content in Foods and Beverages and Its Role in Human and Animal Metabolism: A Scoping Review of Recent Studies. Heliyon 2023, 9, e15452. [Google Scholar] [CrossRef]
- Fatima, U.; Okla, M.K.; Mohsin, M.; Naz, R.; Soufan, W.; Al-Ghamdi, A.A.; Ahmad, A. A Non-Invasive Tool for Real-Time Measurement of Sulfate in Living Cells. Int. J. Mol. Sci. 2020, 21, 2572. [Google Scholar] [CrossRef] [PubMed]
- Abdullad, Z.K.; Al-Samarrai, S.Y. Modified Solid Ion-Selective Electrode for Potentiometric Determination of Sulfide in Oil Refineries Water. Sci. Rev. Eng. Environ. Stud. SREES 2021, 30, 98–105. [Google Scholar] [CrossRef]
- Matveichuk, Y.; Rakhman’ko, E.; Akayeu, Y. Chemically Modified (Poly)Vinyl Chloride with Built-in Neutral Carrier Function as a New Material for Ion Selective Electrodes. Chem. Pap. 2018, 72, 1315–1323. [Google Scholar] [CrossRef]
- Ye, X.; Qi, P.; Sun, Y.; Zhang, D.; Zeng, Y. A High Flexibility All-Solid Contact Sulfide Selective Electrode Using a Graphene Transducer. Anal. Methods 2020, 12, 3151–3155. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; Amr, A.E.-G.E.; Kamel, A.H.; Al-Omar, M.A.; Sayed, A.Y.A. Integrated All-Solid-State Sulfite Sensors Modified with Two Different Ion-to-Electron Transducers: Rapid Assessment of Sulfite in Beverages. RSC Adv. 2021, 11, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Ghassab, N.; Soleymanpour, A.; Shafaatian, B. Development of an Ultrasensitive Chemically Modified Carbon Paste Electrode for Selective Determination Trace Amount of Sulfate Ion. Measurement 2022, 205, 112231. [Google Scholar] [CrossRef]
- Matveichuk, Y.V.; Rakhman’ko, E.M.; Okaev, E.B. Effect of the Nature of a Quaternary Ammonium Salt and the Addition of a Neutral Carrier on Analytical Characteristics of Sulfate-Selective Electrodes. J. Anal. Chem. 2018, 73, 374–382. [Google Scholar] [CrossRef]
- Forano, C.; Farhat, H.; Mousty, C. Recent Trends in Electrochemical Detection of Phosphate in Actual Waters. Curr. Opin. Electrochem. 2018, 11, 55–61. [Google Scholar] [CrossRef]
- Barus, C.; Romanytsia, I.; Striebig, N.; Garçon, V. Toward an in Situ Phosphate Sensor in Seawater Using Square Wave Voltammetry. Talanta 2016, 160, 417–424. [Google Scholar] [CrossRef]
- Zeitoun, R.; Biswas, A. Review—Potentiometric Determination of Phosphate Using Cobalt: A Review. J. Electrochem. Soc. 2020, 167, 127507. [Google Scholar] [CrossRef]
- Ding, X.; Behbahani, M.; Gruden, C.; Seo, Y. Characterization and Evaluation of Phosphate Microsensors to Monitor Internal Phosphorus Loading in Lake Erie Sediments. J. Environ. Manag. 2015, 160, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Oh, J.S.; Kim, D.Y.; Kim, D.G.; Kim, Y.I.; Heo, J.; Lee, H.-K. Ion-Selective Electrode Based on a Novel Biomimetic Nicotinamide Compound for Phosphate Ion Sensor. Polymers 2022, 14, 3392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Fozia; Wen, H.; Dai, Z.; Nie, Y.; Jiang, J.; Xu, X.; Ying, M.; Hu, Z.; Xu, H. Preparation of a Phosphate Ion-Selective Electrode Using One-Step Process Optimized with Response Surface Method and Its Application in Real Sample Detections. Electrocatalysis 2022, 13, 641–652. [Google Scholar] [CrossRef]
- Alizadeh, T.; Atayi, K. Synthesis of Nano-Sized Hydrogen Phosphate-Imprinted Polymer in Acetonitrile/Water Mixture and Its Use as a Recognition Element of Hydrogen Phosphate Selective All-Solid State Potentiometric Electrode. J. Mol. Recognit. 2018, 31, e2678. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, S. A New Phosphate Selective Electrode and Its Application in Some Foods. Int. J. Electrochem. Sci. 2021, 16, 210949. [Google Scholar] [CrossRef]
- Topcu, C.; Coldur, F.; Caglar, B.; Ozdokur, K.V.; Cubuk, O. Solid-state Electrochemical Sensor Based on a Cross-linked Copper(II)-doped Copolymer and Carbon Nanotube Material for Selective and Sensitive Detection of Monohydrogen Phosphate. Electroanalysis 2022, 34, 474–484. [Google Scholar] [CrossRef]
- Wu, J. An All-Solid-State Phosphate Ion-Selective Electrode Using BiPO4 as a Sensitive Membrane. Int. J. Electrochem. Sci. 2021, 16, 210641. [Google Scholar] [CrossRef]
- Xu, K.; Kitazumi, Y.; Kano, K.; Sasaki, T.; Shirai, O. Fabrication of a Phosphate Ion Selective Electrode Based on Modified Molybdenum Metal. Anal. Sci. 2020, 36, 201–205. [Google Scholar] [CrossRef]
- Bralić, M. Preparation of Phosphate Ion-Selective Membrane Based on Silver Salts Mixed with PTFE or Carbon Nanotubes. Int. J. Electrochem. Sci. 2018, 13, 1390–1399. [Google Scholar] [CrossRef]
- Birlik Özkütük, E.; Yıldız, B.; Gündüz, M.; Hür, E. Phosphate-imprinted Polymer as an Efficient Modifier for the Design of Ion-selective Electrode. J. Chem. Technol. Biotechnol. 2021, 96, 2604–2609. [Google Scholar] [CrossRef]
- King, L.; Wang, Q.; Xia, L.; Wang, P.; Jiang, G.; Li, W.; Huang, Y.; Liang, X.; Peng, X.; Li, Y.; et al. Environmental Exposure to Perchlorate, Nitrate and Thiocyanate, and Thyroid Function in Chinese Adults: A Community-Based Cross-Sectional Study. Environ. Int. 2023, 171, 107713. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, P.-C.; Wan, Y.-Q.; Wu, F.-Y. Colorimetric Detection of Thiocyanate Based on Anti-Aggregation of Gold Nanoparticles in the Presence of Cetyltrimethyl Ammonium Bromide. Sens. Actuators B Chem. 2016, 222, 790–796. [Google Scholar] [CrossRef]
- Calderón, R.; Jara, C.; Albornoz, F.; Palma, P.; Arancibia-Miranda, N.; Karthikraj, R.; Manquian-Cerda, K.; Mejias, P. Exploring the Destiny and Distribution of Thiocyanate in the Water-Soil-Plant System and the Potential Impacts on Human Health. Sci. Total Environ. 2022, 835, 155502. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Pedersen, I.B.; Carlé, A.; Andersen, S.; Knudsen, N.; Karmisholt, J. The Relationship between Thiocyanate and Iodine. In Comprehensive Handbook of Iodine; Elsevier: Amsterdam, The Netherlands, 2009; pp. 275–281. [Google Scholar]
- Matveychuk, Y.V. A Thiocyanate-Selective Electrode and Its Analytical Applications. J. Anal. Chem. 2020, 75, 662–668. [Google Scholar] [CrossRef]
- Urbanowicz, M.; Sadowska, K.; Pijanowska, D.G.; Pomećko, R.; Bocheńska, M. Potentiometric Solid-Contact Ion-Selective Electrode for Determination of Thiocyanate in Human Saliva. Sensors 2020, 20, 2817. [Google Scholar] [CrossRef] [PubMed]
- Gyeom Kwon, N. Development of Thiocyanate-Selective Membrane Electrodes by the Sol–Gel Method. Int. J. Electrochem. Sci. 2018, 13, 9481–9492. [Google Scholar] [CrossRef]
- Khan, A.A.; Jamil, B.; Shaheen, S. Electrochemical Sensing Studies of AsO4−3 Selective Poly(Methyl Methacrylate)-Zinc Oxide Fibrous Anion Exchanger. Adv. Polym. Technol. 2018, 37, 566–574. [Google Scholar] [CrossRef]
- Uner Bahar, D. A Novel Borate Ion Selective Electrode Based on Carbon Nanotube-Silver Borate. Int. J. Electrochem. Sci. 2020, 15, 899–914. [Google Scholar] [CrossRef]
- Martin, K.; Kadam, S.A.; Mattinen, U.; Bobacka, J.; Leito, I. Solid-contact Acetate-selective Electrode Based on a 1,3-bis(Carbazolyl)Urea-ionophore. Electroanalysis 2019, 31, 1061–1066. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Wu, J.; Ai, M.; Cai, W.; Ye, Y.; Tao, C.; Zhang, P.; Jin, Q. Fabrication of an All-Solid-State Carbonate Ion-Selective Electrode with Carbon Film as Transducer and Its Preliminary Application in Deep-Sea Hydrothermal Field Exploration. Chemosensors 2021, 9, 236. [Google Scholar] [CrossRef]
- Wu, R.; Chen, X.-G.; Tao, C.; Huang, Y.; Ye, Y.; Wang, Q.; Zhou, Y.; Jin, Q.; Cai, W. An All-Solid-State Silicate Ion-Selective Electrode Using PbSiO3 as a Sensitive Membrane. Sensors 2019, 19, 525. [Google Scholar] [CrossRef] [PubMed]
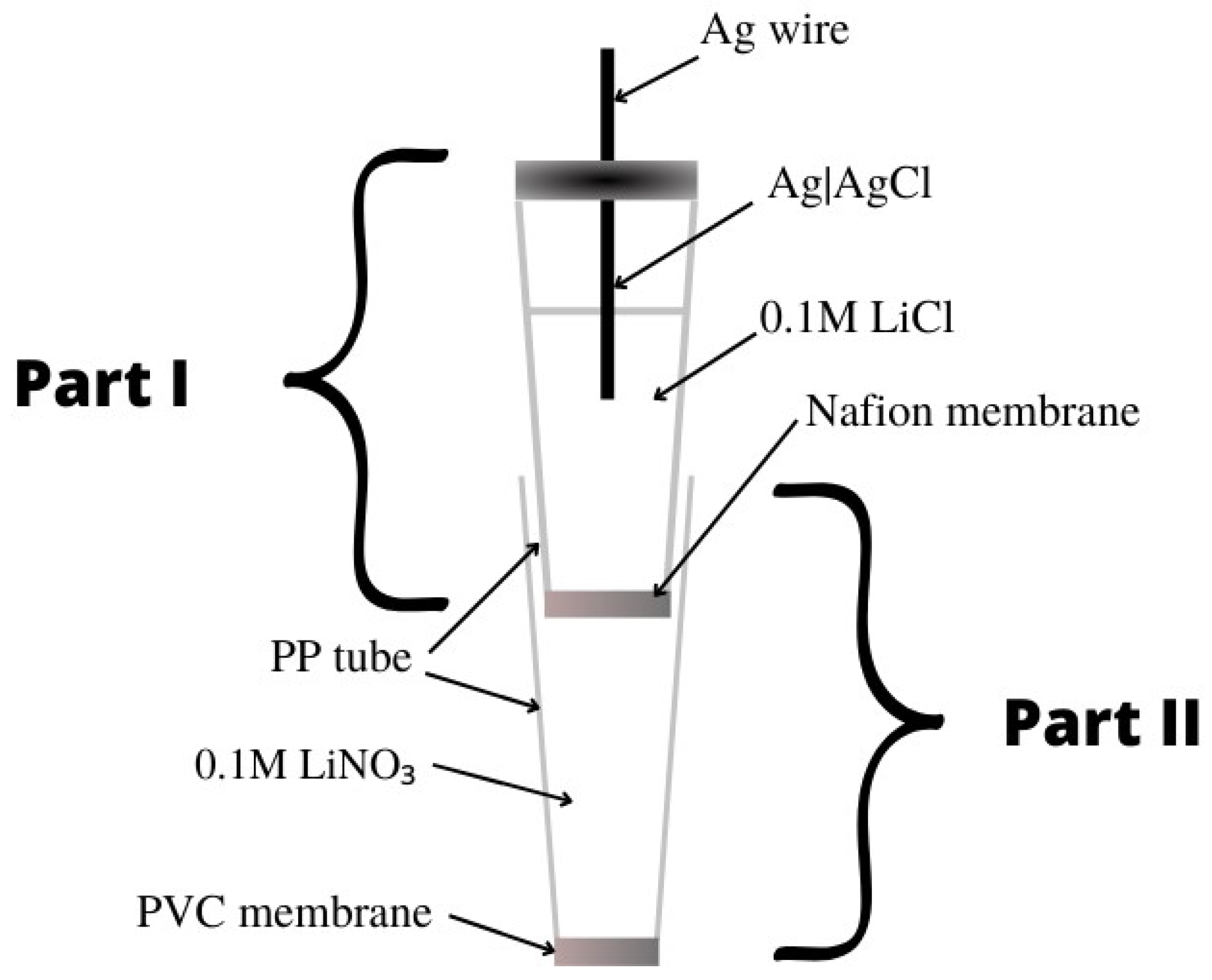
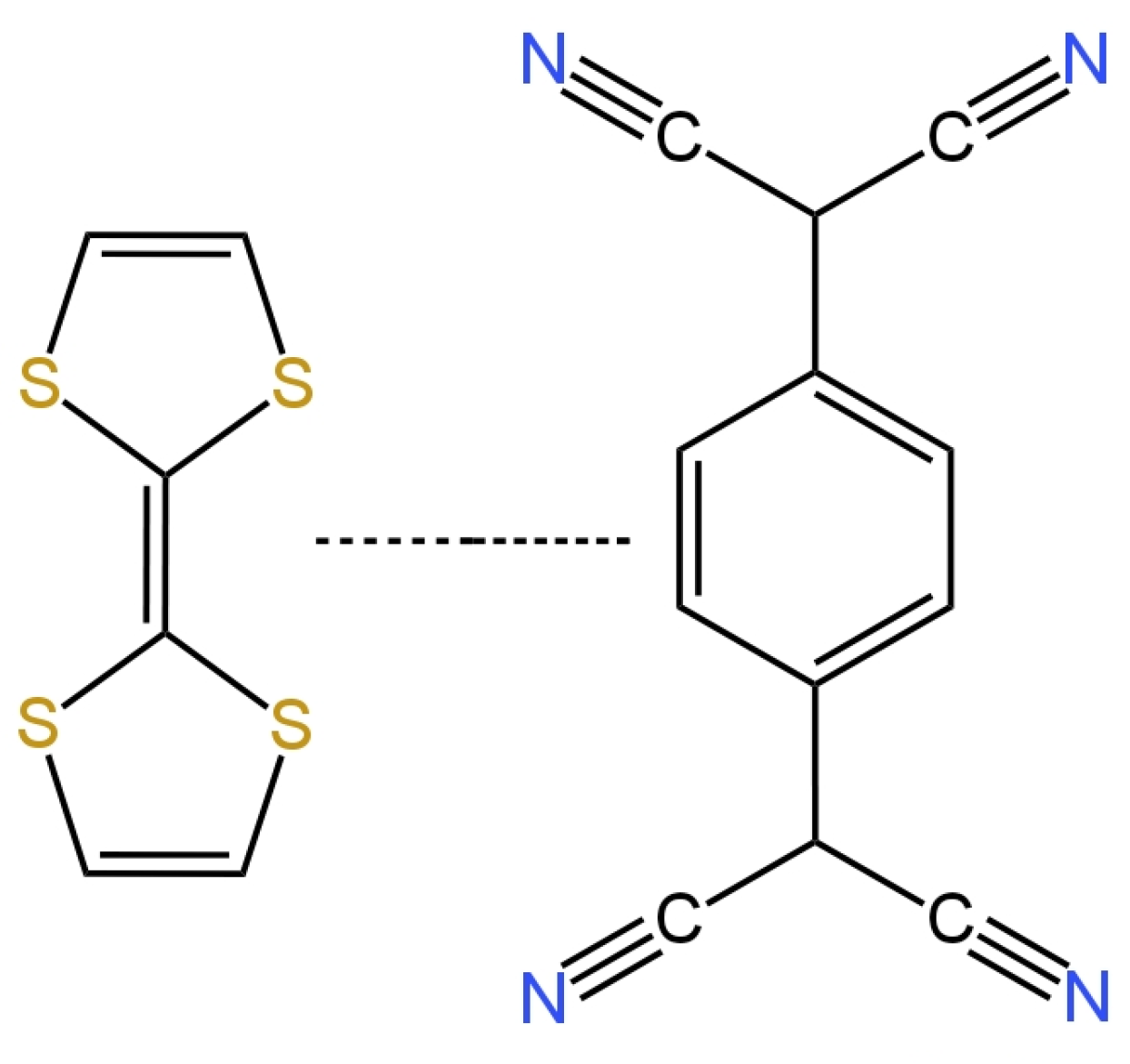
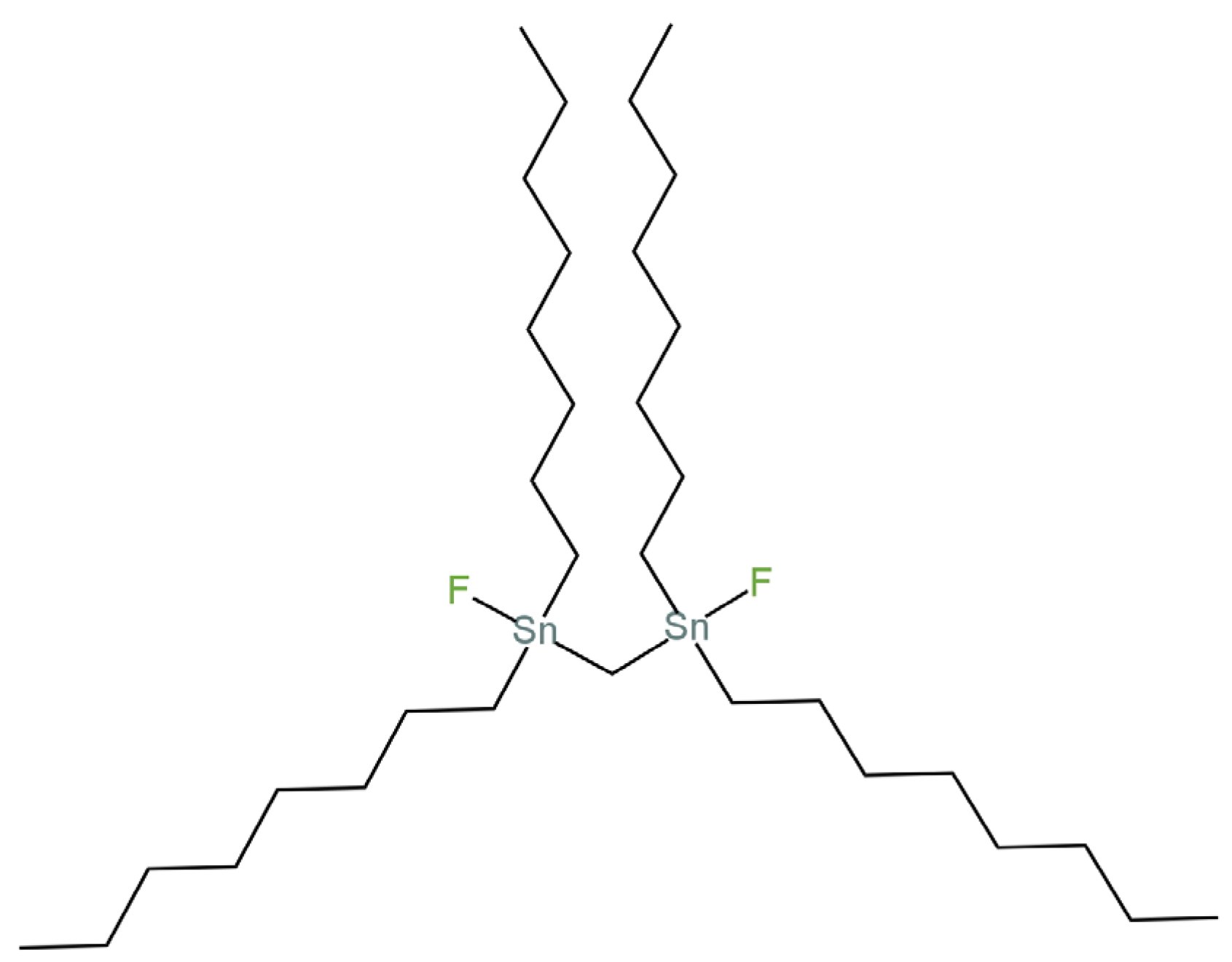
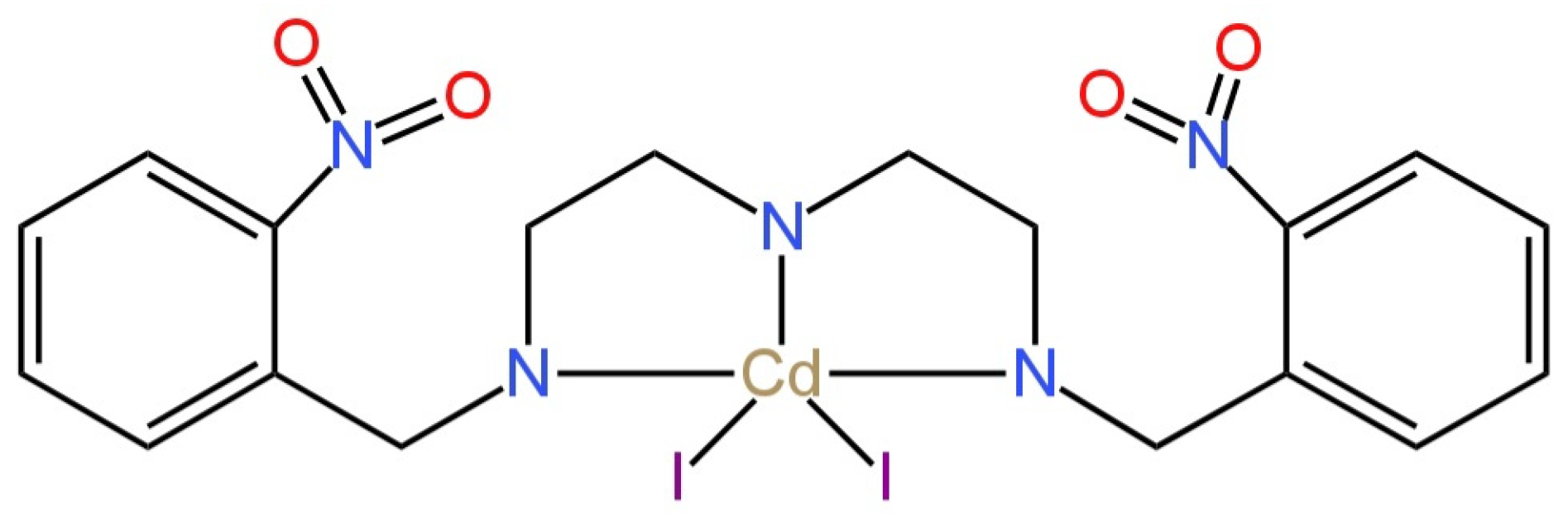
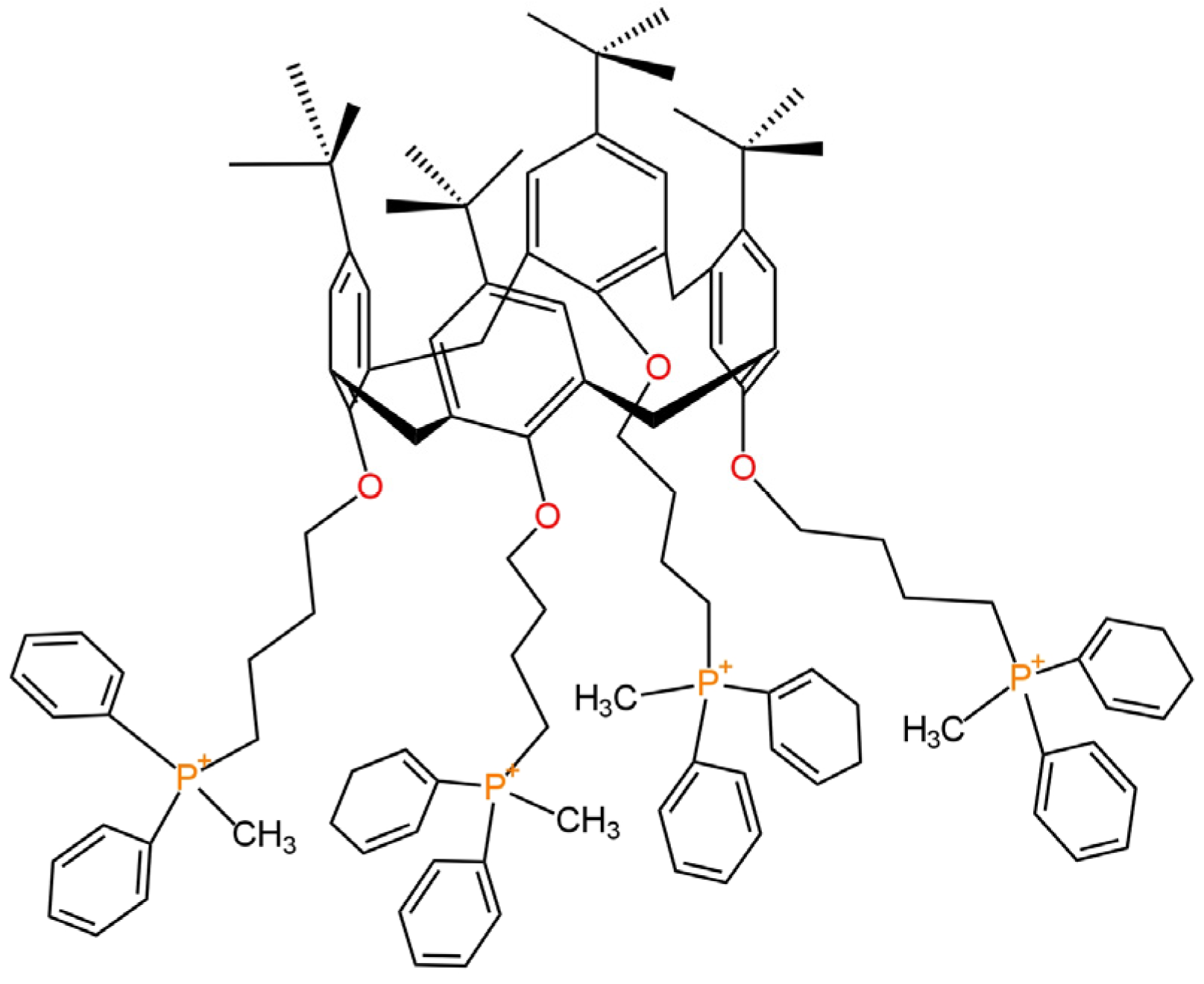
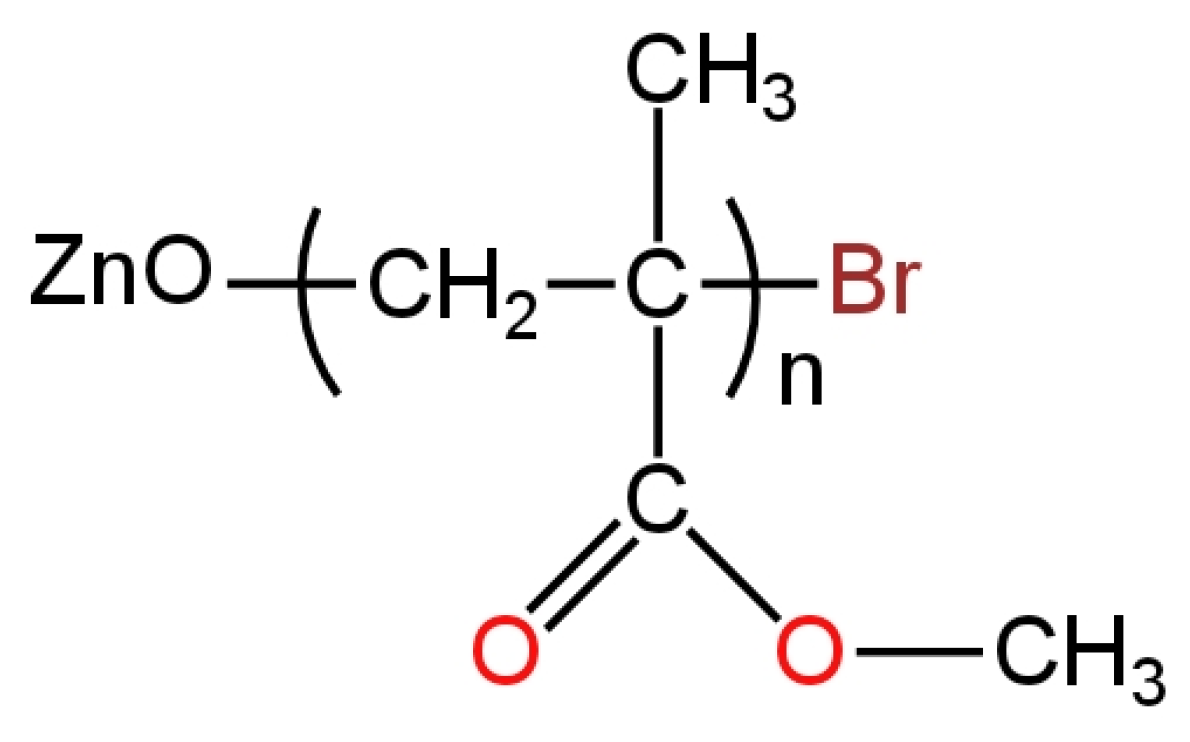
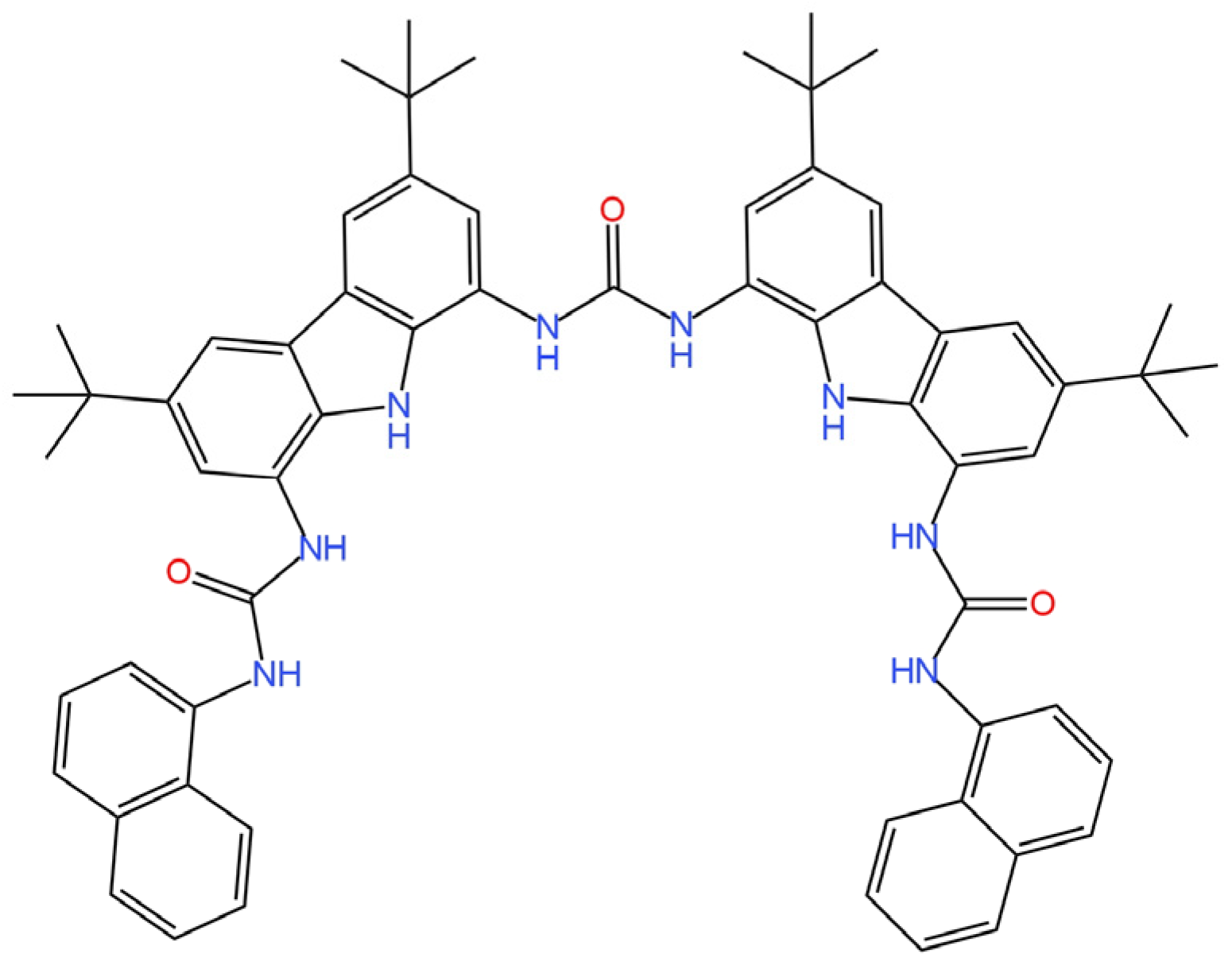
| E. No | Type of Contact | Ionophore/Ion Carrier | Intermediate/Transducer Layer | Type of Internal Electrode | Slope [mV/decade] | Range of Linearity [M] | Limit of Detection [M] | pH Range | Application/Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SC | TDMAN | Graphite | GrE | −55.4 ± 0.7 | 2.9 × 10−4–1.7 × 10−1 | 2.04 × 10−4 | 4.0–11.0 | Industrial and environmental. | [23] |
| 2 | SC | TDMAN | LIG | - | −58.2 ± 4.2 | 5.0 × 10−4–1.0 × 10−1 | 6.01 × 10−6 | 6.0–8.0 | Agricul-ture and surface water. | [24] |
| 3 | SC | TDMAN | Co3O4NPs | SPCE | −56.8 | 1.0 × 10−7–1.0 × 10−2 | 1.04 × 10−8 | 3.0–8.0 | Aquaculture, river, domestic and tap water. | [28] |
| 4 | SC | TDMAN | PANINFs-Cl | GCE | −56.8 | 1.0 × 10−6–1.0 × 10−1 | 3.16 × 10−7 | 4.0–12.5 | Environmental samples. | [26] |
| 5 | SC | TDMAN | PANINFs-NO3 | −57.8 | 1.0 × 10−6–1.0 × 10−1 | 1.12 × 10−6 | 4.0–11.5 | |||
| 6 | SC | TDMAN | MCB | Ag wire | −54.8 | 5.0 × 10−5–1.0 × 10−1 | 2.5 × 10−6 | - | - | [27] |
| 7 | SC | TDMAN | POT-MoS2 | AuE | −64.0 | 7.1 × 10−4–1.0 × 10−1 | 9.2 × 10−5 | - | Soil. | [25] |
| 8 | SC | PPy-NO3− | PGCP | GCE | −50.9 | 1.0 × 10−5–5.0 × 10−1 | 4.64 × 10−6 | 3.5–9.5 | Environmental and clinical laboratories. | [29] |
| 9 | SC | PPy-NO3− | AuNPs | GCE | −50.4 | 5.3 × 10−5–1.0 × 10−1 | 5.25 × 10−5 | - | Water samples and aqueous solutions of fertilizers. | [30] |
| 10 | SC | Nitrate ionophore VI | - | PAuE | −54.1 | 5.0 × 10−5–1.0 × 10−1 | - | - | Field soils. | [31] |
| 11 | SC | Nitrate ionophore VI | TRGO | AuE | −60.0 ± 0.5 | 4.0 × 10−5–1.0 × 10−1 | 4.0 × 10−6 | 2.0–10.0 | Blood. | [32] |
| 12 | SC | Nitrate ionophore VI | PTFE | SPCE | −58.0 | - | - | 4.0–11.0 | Wastewater. | [33] |
| 13 | SC | Co(Bphen)2(NO3)2(H2O)2 | MWCNTs-THTDPCl | GCE | −57.1 | 1.0 × 10−6–1.0 × 10−1 | 5.0 × 10−7 | 6.0–8.0 | - | [35] |
| 14 | SC | Co(Bphen)2(NO3)2(H2O)2 | Ag/AgCl/Cl− | Ag|AgCl | −56.3 | 1.0 × 10−5–1.0 × 10−1 | 3.98 × 10−6 | 5.4–10.6 | Mineral, tap and river water. | [34] |
| 15 | SC | TDANO3 | rGOA | SPCE | −59.1 | 1.0 × 10−6–1.0 × 10−1 | 7.59 × 10−7 | - | Plant sap e.g., perilla leaf. | [36] |
| 16 | LC | TDANO3 | 0.01 M KNO3 and 0.001 M KCl | Ag|AgCl | −53.7 ± 0.4 | 1.0 × 10−5–1.0 × 10−1 | 1.3 × 10−6 | - | - | [37] |
| 17 | SC | Nit+/NO3− | MWCNTs | GCE | −55.1 ± 1.0 | 8.0 × 10−8–1.0 × 10−2 | 2.8 × 10−8 | 3.5–10.0 | Environmental samples. | [38] |
| 18 | LC | THANO3 | 0.1 M LiCl and 0.1 M LiNO3 | Ag|AgCl | −53.3 ± 1.0 | 1.0 × 10−5–1.0 × 10−1 | 1.0 × 10−6 | - | Hydroponic solutions. | [39] |
| 19 | SC | Nitrate ionophore V | TTF-TCNQ | GCD | −58.5 | 1.0 × 10−5–1.0 × 10−1 | 1.6 × 10−6 | - | Aqueous samples. | [40] |
| E. No | Type of Contact | Ionophore/Ion Carrier | Intermediate/Transducer Layer | Type of Internal Electrode | Slope [mV/decade] | Range of Linearity [M] | Limit of Detection [M] | pH Range | Application/Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SC | Eu-doped LaF3 nanocrystals | - | TiE | −56 ± 13 | 1.0 × 10−5–1.0 × 10−1 | 1.0 × 10−6 | - | - | [43] |
| 2 | SC | LaF3 single crystal | PEDOT | AgE | −56.0 ± 0.9 | 1.0 × 10−5–1.0 × 10−1 | 2.0 × 10−5 | 5.0–11.0 | - | [44] |
| 3 | SC | Ag paste | AgE | −62.8 ± 3.8 | 1.0 × 10−5–1.0 × 10−1 | 1.0 × 10−2 | - | |||
| 4 | LC | PBS, 0.01 M Na2HPO3 and 0.02 M KH2PO3 | Ag|AgCl | −38.6 ± 9.1 | 1.0 × 10−5–1.0 × 10−1 | - | - | |||
| 5 | SC | LaF3 single crystal | FexOy NPs | SSDE | −52.9–−57.3 | 6.3 × 10−6–1.0 × 10−1 | 3.6 × 10−7 | 4.0–7.0 | - | [45] |
| 6 | LC | LaF3 single crystal | KCl + HCl + 0.1 M AgNO3 | Ag|AgCl | −50.8–−52.7 | 3.9 × 10−7–1.0 × 10−1 | 7.4 × 10−8 | 4.0–7.0 | ||
| 7 | SC | Bis(fluorodioctylstannyl)methane | MWCNTs-COOH | SPCE | −59.2 | - | 1.7 × 10−9 | - | - | [46] |
| 8 | LC | tetrakis-(pentafluorophenyl)stibonium | 0.2 M Gly/H3PO4 buffer and 0.001 M NaF | Ag|AgCl | −59.3 | 1.0 × 10−5–4.0 × 10−2 | 5.0 × 10−6 | 3.0 | Tap water samples. | [48] |
| 9 | LC | [Ph4Sb]+ | −58.2 | 1.0 × 10−5–4.0 × 10−2 | 2.0 × 10−5 | - | ||||
| 10 | LC | tetrachloro-substituted organoantimony(V) | −54.6 | 1.0 × 10−5–4.0 × 10−2 | 2.0 × 10−4 | - | ||||
| 11 | LC | Organo antimony(V) compound | −57.8 | 1.0 × 10−5–4.0 × 10−2 | 3.0 × 10−5 | - | ||||
| 12 | SC | CdLI2 | - | CPE | −58.9 | 1.5 × 10−6–5.5 × 10−3 | 1.2 × 10−7 | 5.0–7.0 | River water samples. | [47] |
| E. No | Type of Contact | Ionophore/Ion Carrier | Intermediate/Transducer Layer | Type of Internal Electrode | Slope [mV/decade] | Range of Linearity [M] | Limit of Detection [M] | pH Range | Application/Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SC | Chloride ionophore(III) | MWCNTs | GCE | −59.6 | 5.0 × 10−6–1.0 × 10−1 | 2.6 × 10−6 | 4.0–9.0 | Inspection of the efficiency of water desalination. | [53] |
| 2 | PANINFs-Cl | −60.3 | 2.8 × 10−6 | |||||||
| 3 | PANINFs-MWCNTs | −61.2 | 2.7 × 10−6 | |||||||
| 4 | LC | TDMACl | 0.5 M NaCl | Ag|AgCl | −55.0 ± 2 | 1.0 × 10−5–1.0 × 10−1 | 1.0 × 10−6 | 2.0–8.0 | Wastewater samples. | [54] |
| 5 | SC | TDMACl | - | GCD | −57.1 ± 0.66 | 1.0 × 10−4–1.0 × 10−1 | 1.6 × 10−5 | - | Water samples. | [55] |
| 6 | TTF | −58.2 ± 0.27 | 1.0 × 10−5–1.0 × 10−1 | 5.0 × 10−6 | ||||||
| 7 | TTF-TCNQ | −58.3 ± 0.16 | 1.0 × 10−5–1.0 × 10−1 | 4.0 × 10−6 | ||||||
| 8 | TTFCl | −58.4 ± 0.14 | 1.0 × 10−5–1.0 × 10−1 | 4.0 × 10−6 | ||||||
| 9 | CB | −59.6 ± 0.11 | 1.0 × 10−5–1.0 × 10−1 | 2.5 × 10−6 | ||||||
| 10 | CB-TTF | −58.5 ± 0.13 | 1.0 × 10−5–1.0 × 10−1 | 3.2 × 10−6 | ||||||
| 11 | CB-TTF-TCNQ | −58.7 ± 0.10 | 1.0 × 10−5–1.0 × 10−1 | 2.5 × 10−6 | ||||||
| 12 | CB-TTFCl | −59.1 ± 0.09 | 1.0 × 10−5–1.0 × 10−1 | 2.0 × 10−6 | ||||||
| 13 | SC | g-C3N4/AgCl | - | CPE | −55.4 ± 0.3 | 1.0 × 10−6–1.0 × 10−1 | 4.0 × 10−7 | - | Aqueous samples. | [56] |
| 14 | SC with ISM | Chloride ionophore(I) | - | Ag|AgCl | −61.7 ± 2.4 | 1.0 × 10−5–1.0 × 10−1 | 1.1 × 10−5 | - | Sweat. | [57] |
| 15 | SC | AgCl:Ag2S:PTFE | FexOy NPs | multi-purpose solid state electrode made from stainless steel | −44.4 | 2.0 × 10−6–1.0 × 10−1 | 1.42 × 10−6 | - | - | [58] |
| 16 | ZnO NPs | −40.5 | 3.6 × 10−6–1.0 × 10−1 | 1.0 × 10−6 | - |
| E. No | Type of Contact | Ionophore/Ion Carrier | Intermediate/Transducer Layer | Type of Internal Electrode | Slope [mV/decade] | Range of Linearity [M] | Limit of Detection [M] | pH Range | Application/Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SC | Dixanthylium dye | - | Pt wire | −57.4 | 1.0 × 10−6–6.1 × 10−2 | 5.0 × 10−7 | 1.5–11.0 | Aqueous samples. | [59] |
| 2 | SC | Bn12BU [6] | PEDOT | GCE | −59.9 ± 1.1 | 1.0 × 10−6–1.0 × 10−1 | 1.0 × 10−6 | - | Real water samples. | [60] |
| 3 | SC | InIII-porphyrin | SWCNTs | GCE | −56.0 ± 1.1 | 1.1 × 10−6–1.0 × 10−2 | 1.8 × 10−7 | - | Fireworks and propellants. | [61] |
| E. No | Type of Contact | Ionophore/Ion Carrier | Intermediate/Transducer Layer | Type of Internal Electrode | Slope [mV/decade] | Range of Linearity [M] | Limit of Detection [M] | pH Range | Application/Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LC | TDMABr | 0.5 M NaBr | Ag|AgCl | −57.4 ± 0.3 | 1.0 × 10−6–1.0 × 10−1 | 1.2 × 10−6 | 1.0–11.0 | Water samples e.g., tea samples. | [54] |
| 2 | LC | BGO/AlFu-MOF | 0.05 M KBr | GE | −54.5 ± 0.2 | 1.0 × 10−7–1.0 × 10−1 | 7.1 × 10−8 | 4.0–9.0 | Environmental samples. | [66] |
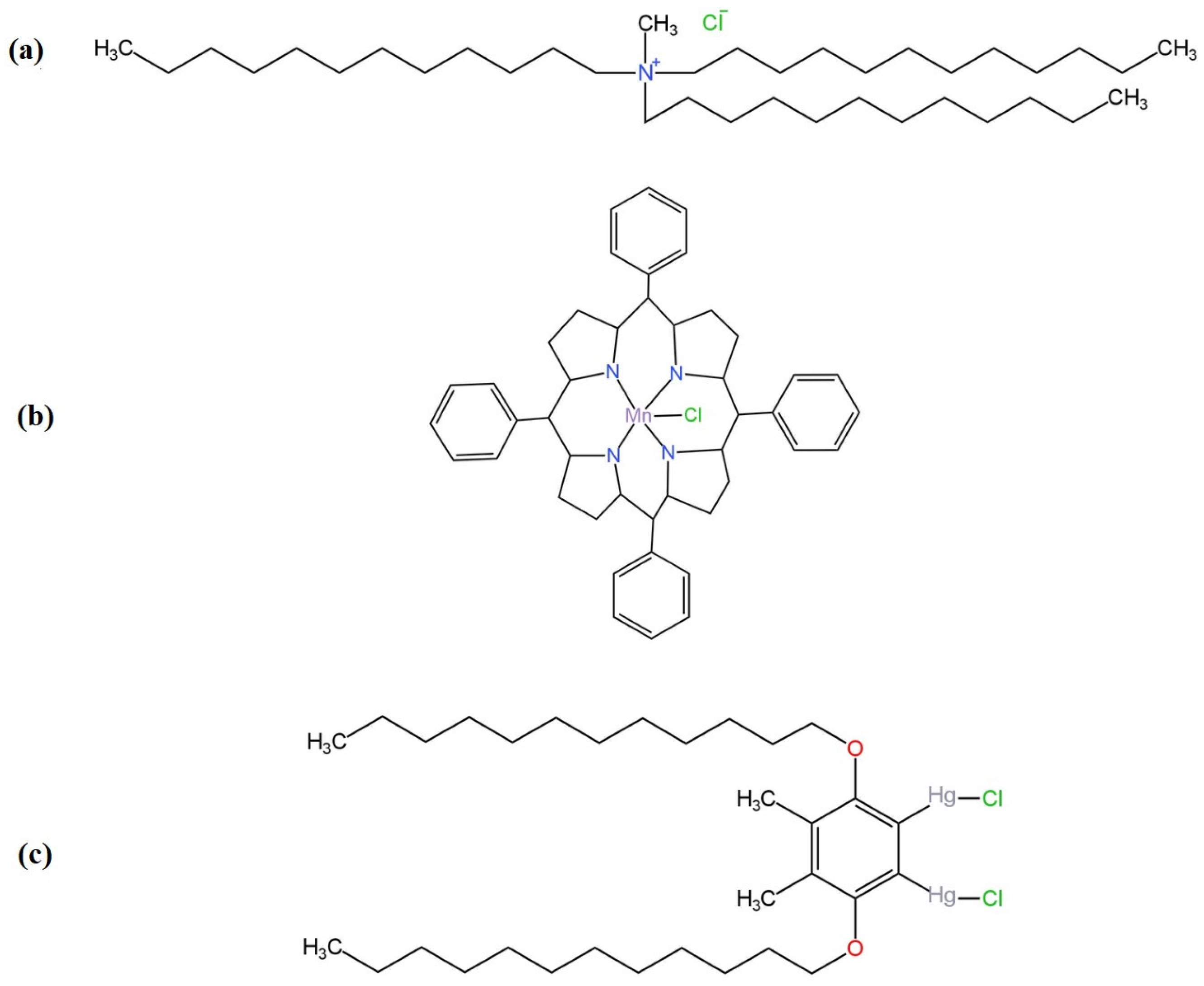
| 3 | SC | Mesotetraphenylporphyrin manganese(III)-chloride complex | POT | GCE | - | 1.0 × 10−6–1.0 × 10−2 | 2.0 × 10−9 | - | Water samples. | [67] |
| 4 | 4,5-dimethyl-3,6-dioctyloxy-o-phenylene-bis(mercurytrifluoroacetate) | |||||||||
| 5 | LC | PtTMeOPP | 0.01 M KCl | Ag|AgCl | −64.4 ± 0.4 | 1.0 × 10−5–1.0 × 10−1 | 8.0 × 10−6 | 6.0–12.0 | Pharmaceutical samples. | [65] |
| E. No | Type of Contact | Ionophore/Ion Carrier | Intermediate/Transducer Layer | Type of Internal Electrode | Slope [mV/decade] | Range of Linearity [M] | Limit of Detection [M] | pH Range | Application/Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LC | TDMAI | 0.1 M KI and 0.1 M KCl | Ag|AgCl | −54 ± 1 | 1.0 × 10−5–1.0 × 10−1 | 1.3 × 10−6 | 2.0–8.0 | Wastewater samples. | [54] |
| 2 | SC | XB1 | PANI | SPE | −54 ± 1 | 1.0 × 10−5–1.0 × 10−1 | 1.3 × 10−6 | - | - | [73] |
| 3 | HB2 | −51.9 | 1.0 × 10−6–1.0 × 10−1 | 1.3 × 10−6 | ||||||
| 4 | LC | PctPtCl2 | 0.001M KI and 0.1 KCl | Ag|AgCl | −54.9 | 1.0 × 10−6–1.0 × 10−1 | 1.0 × 10−6 | - | Medicaments such as “Iodomarine 100” and other pharmaceuticals. | [72] |
| 5 | CPCl + PctPtCl2 | −26 ± 3 | 1.0 × 10−3–1.0 × 10−1 | 1.8 × 10−4 | ||||||
| 6 | CPBr + PctPtCl2 | −45 ± 1 | 1.0 × 10−4–1.0 × 10−1 | 2.1 × 10−5 | ||||||
| 7 | CPBr | −54 ± 1 | 1.0 × 10−4–1.0 × 10−1 | 3.5 × 10−5 | ||||||
| 8 | SC | CPCl + PctPtCl2 | graphite | SPPE | −46 ± 2 | 1.0 × 10−3–1.0 × 10−1 | 3.0 × 10−4 | |||
| 9 | CPBr + PctPtCl2 | −51 ± 1 | 1.0 × 10−4–1.0 × 10−1 | 5.3 × 10−5 | ||||||
| 10 | CPBr | −54 ± 1 | 1.0 × 10−4–1.0 × 10−1 | 1.9 × 10−5 | ||||||
| 11 | DCImI + PctPtCl2 | −50 ± 1 | 1.0 × 10−3–1.0 × 10−1 | 1.0 × 10−4 | ||||||
| 12 | LC | AgCl:Ag2S:PTFE + ZnO NPs | - | - | −57 ± 2 | 1.0 × 10−4–1.0 × 10−1 | 1.8 × 10−5 | - | Penicillamine in real samples. | [71] |
| 13 | LC | PtTMeOPP | 0.01M KCl | Ag|AgCl | −57.4 ± 0.3 | 2.5 × 10−6–1.0 × 10−2 | 2.2 × 10−6 | 3.0–12.0 | Pharmaceutical such as potassium iodide tablets. | [65] |
| E. No | Ion | Type of Contact | Ionophore/Ion Carrier | Intermediate/Transducer Layer | Type of Internal Electrode | Slope [mV/decade] | Range of Linearity [M] | Limit of Detection [M] | pH Range | Application/Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S2− | LC | Ag2S | 10−6 M Na2S | Ag|AgCl | −28.2 | 1.0 × 10−6–1.0 × 10−1 | 2.3 × 10−7 | 6.0–12.0 | Industrial water, e.g., petroleum industries. | [76] |
| 2 | S2− | SC | Ag2S | RGSs | Ag wire | - | 5.0 × 10−7–1.0 × 10−3 | 1.8 × 10−7 | - | Sea and tap water. | [78] |
| 3 | S2− | LC | TFAB-PVC | 0.01 M Na2S and 0.001 M KCl | - | −27.1 | 1.0 × 10−6–1.0 × 10−1 | 6.0 × 10−7 | - | Water samples. | [77] |
| 4 | TFABAHE | −27.1 | 1.0 × 10−6–1.0 × 10−1 | 3.8 × 10−7 | |||||||
| 5 | SO32− | SC | CoPC | MWCNTs-COOH | SPCE | −29.8 ± 0.4 | 2.0 × 10−6–2.3 × 10−3 | 1.1 × 10−6 | 5.0–7.2 | Various samples. | [79] |
| 6 | SO32− | SC | PANINFs | −26.5 ± 0.6 | 5.0 × 10−6–2.3 × 10−3 | 1.5 × 10−6 | 4.8–7.7 | ||||
| 7 | SO42− | SC | Shiff base complex with nickel | - | CPE | −29.7 | 7.5 × 10−9–1.5 × 10−3 | 5.0 × 10−9 | 3.0–9.0 | Water and blood sam-ples. | [80] |
| 8 | SO42− | LC | (oxyethyl)3TM | 0.01 M Na2SO4 and 0.001 M KCl | - | −27.0 | − | 6.7 × 10−7 | - | Water samples. | [81] |
| 9 | SO42− | LC | TFAB-PVC | 0.01 M Na2S and 0.001 M KCl | - | −25.7 | 1.0 × 10−6–1.0 × 10−2 | 1.0 × 10−6 | - | Water samples. | [77] |
| 10 | SO42− | TFABAHE | - | −26.5 | 1.0 × 10−6–1.0 × 10−2 | 7.0 × 10−7 | - |
| E. No | Ion | Type of Contact | Ionophore/Ion Carrier | Intermediate/Transducer Layer | Type of Internal Electrode | Slope [mV/decade] | Range of Linearity [M] | Limit of Detection [M] | pH Range | Application/Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H2PO4− | LC | Bis-meta-NICO-PF6 | - | - | −53.3 | 1.0 × 10−6–1.0 × 10−2 | 0.9 × 10−6 | - | Environmental and other real samples. | [86] |
| 2 | H2PO4− | SC | Co-PPY-OMC | - | GCE | −31.6 | 1.0 × 10−5–5.0 × 10−2 | 6.8 × 10−6 | 3.0–5.0 | Water samples for example in human urine or wastewater. | [87] |
| 3 | H2PO4− | SC | nano-IIP | - | CPE | −30.6 ± 0.5 | 1.0 × 10−5–1.0 × 10−1 | 4.0 × 10−6 | 9.0–12.0 | Water samples. | [88] |
| 4 | HPO42− | SC | Ba3PO4 + Cu2S + Ag2S pellet | - | Cu wire | −57.0 | 1.0 × 10−6–1.0 × 10−1 | 2.4 × 10−7 | 7.0–9.0 | Food samples e.g., meat, vegetables and fruits. | [89] |
| 5 | HPO42− | SC | Cu(II)-DCP | MWCNTs + graphite | Cu wire | −30.7 ± 0.4 | 1.0 × 10−6–1.0 × 10−1 | 6.5 × 10−7 | - | Water samples. | [90] |
| 6 | HPO42− | SC | BiPO4 | Bi particles | Pt wire | −30.3 | 1.0 × 10−6–1.0 × 10−1 | 7.7 × 10−7 | 5.0–9.0 | Drinking water. | [91] |
| 7 | HPO42− | SC | MoO2 + PMo12O403− | - | Mo wire | −27.8 ± 0.5 | 1.0 × 10−5–1.0 × 10−1 | 1.0 × 10−6 | 8.0–9.5 | Wastewater, nutrient solution and Coca-Cola. | [92] |
| 8 | HPO42− | SC | Ag3PO4 + Ag2S | PTFE | SSD | −21.0 | 1.0 × 10−5–1.0 × 10−1 | 5.3 × 10−6 | 3.0–7.0 | Solution of pH range 3–7. | [93] |
| 9 | MWCNTs | Cu wire | −32.6 | 1.0 × 10−5–1.0 × 10−1 | 5.5 × 10−6 | ||||||
| 10 | HPO42− | TFAB-PVC | 0.01 M Na2S and 0.001 M KCl | - | −27.5 | 1.0 × 10−7–1.0 × 10−2 | 7.0 × 10−7 | - | Water samples | [77] | |
| 11 | TFABAHE | −28.7 | 1.0 × 10−7–1.0 × 10−2 | 5.0 × 10−7 | |||||||
| 12 | PO43− | LC | IIP-1 (chitosan-La(III)-PO43−) | 0.001 M KCl + 0.001 M Na3PO4 | Cu wire | −3.2 | 1.0 × 10−6–1.0 × 10−2 | 7.6 × 10−6 | 5.0–7.0 | Household wastewater. | [94] |
| 13 | PO43− | LC | IIP-2 (chitosan-La(III)-AAPTS-PO43−) | −1.9 | 1.0 × 10−6–1.0 × 10−2 | 5.1 × 10−6 | |||||
| 14 | PO43− | LC | IIP-3 (AAPTS-La(III)-PO43−) | −3.7 | 1.0 × 10−6–1.0 × 10−2 | 2.5 × 10−6 |
| E. No | Type of Contact | Ionophore/Ion Carrier | Intermediate/Transducer Layer | Type of Internal Electrode | Slope [mV/decade] | Range of Linearity [M] | Limit of Detection [M] | pH Range | Application/Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LC | TL | 0.01 M KSCN | - | −53.9 | 1.0 × 10−6–1.0 × 10−1 | 5.6 × 10−6 | 0.5–12.5 | Human saliva. | [99] |
| 2 | LC | Tetrakis-(4-diphenylmethylphosphonium-butoxy)-tetrakis-p-tert-butylcalix [4]arene tetrathiocyanate | 0.001 M KCl | Ag|AgCl | −55.5 ± 2.1 | 1.0 × 10−5–1.0 × 10−1 | 6.3 × 10−6 | - | Saliva and other medical measurements. | [100] |
| 3 | SC | - | GCE | −59.9 ± 0.3 | 1.0 × 10−6–1.0 × 10−1 | 1.6 × 10−6 | ||||
| 4 | SC | - | Au rods | −53.3 ± 0.3 | 1.0 × 10−6–1.0 × 10−1 | 3.2 × 10−6 | ||||
| 5 | LC | Aliquat336-SCN | 0.01 M NaSCN and 0.1 M NaCl | Ag|AgCl | −56.3 | 3.2 × 10−5–5.0 × 10−1 | 6.3 × 10−6 | - | Human saliva. | [101] |
| E. No | Ion | Type of Contact | Ionophore/Ion Carrier | Intermediate/Transducer Layer | Type of Internal Electrode | Slope [mV/decade] | Range of Linearity [M] | Limit of Detection [M] | pH Range | Application/Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AsO43− | LC | PMMA-ZnO | 0.05 M Na3AsO4 | SCE | −28.6 | 1.0 × 10−9–1.0 × 10−1 | 1.0 × 10−9 | 4.0–7.0 | Water solutions. | [102] |
| 2 | BO33− | SC | Ag2B4O7 | MWCNTs | Cu wire | −34.0 ± 1.0 | 1.0 × 10−4–1.0 × 10−1 | 5.6 × 10−5 | 5.0–8.0 | Rock, soil. | [103] |
| 3 | CH3COO− | SC | 1,3-bis(carbazolyl)urea | PEDOT | GCE | −51.3 | 3.2 × 10−5–7.9 × 10−2 | 1 × 10−5 | 6.0–8.0 | Aqueous samples. | [104] |
| 4 | CO32− | SC | Carbonate ionophore VII | Carbon film | Ni wire | −30.4 | 1.0 × 10−5–1.0 × 10−1 | 2.8 × 10−6 | - | Exploration of deep-sea hydrothermal activity. | [105] |
| 5 | SiO32− | SC | PbSiO3 | PbSiO3 | Ag wire coated by the Pb film | −31.3 | 1.0 × 10−5–1.0 × 10−1 | - | - | Aqueous samples with low-chloride content. | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardak, C.; Morawska, K.; Pietrzak, K. New Materials Used for the Development of Anion-Selective Electrodes—A Review. Materials 2023, 16, 5779. https://doi.org/10.3390/ma16175779
Wardak C, Morawska K, Pietrzak K. New Materials Used for the Development of Anion-Selective Electrodes—A Review. Materials. 2023; 16(17):5779. https://doi.org/10.3390/ma16175779
Chicago/Turabian StyleWardak, Cecylia, Klaudia Morawska, and Karolina Pietrzak. 2023. "New Materials Used for the Development of Anion-Selective Electrodes—A Review" Materials 16, no. 17: 5779. https://doi.org/10.3390/ma16175779
APA StyleWardak, C., Morawska, K., & Pietrzak, K. (2023). New Materials Used for the Development of Anion-Selective Electrodes—A Review. Materials, 16(17), 5779. https://doi.org/10.3390/ma16175779





