Wettability, Adsorption and Adhesion in Polymer (PMMA)—Commercially Available Mouthrinse System
Abstract
:1. Introduction
2. Theoretical Background
2.1. Adsorptive and Thermodynamic Surface Characteristics of Surfactant Solutions at Liquid-Vapor (LV) Interface
2.2. Surface Wettability Energetics
2.3. Surfactant Adsorption at Polymer Surfaces—Adhesional Tension
3. Materials and Methods
4. Results and Discussion
4.1. Surface Adsorptive Parameters of Mouthrinse Solutions at Air/Water Interface
4.2. Surface Thermodynamics Characteristics
4.3. Wettability Energetics from the Contact Angles Analysis
4.4. Wettability Characteristics vs. Bacterial Adhesion
4.5. Mouthrinse Dissolution Effect on PMMA Wettability
4.6. Mouthrinse Surfactants Partitioning between LV and SL Interfaces
4.7. Penetration Coefficient and Marangoni Spreading Speed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perdok, J.F.; van der Mei, H.C.; Busscher, H.J. Physicochemical Properties of Commercially Available Mouthrinses. J. Dent. 1990, 18, 147–150. [Google Scholar] [CrossRef]
- O’Brien, W.J. Dental Materials and Their Selection, 3rd ed.; Quintessence Publishing Co.: Batavia, IL, USA, 2002; ISBN 0-86715-406-3. [Google Scholar]
- Dai, Q.; Huang, W.; Wang, X.; Khonsari, M.M. Directional Interfacial Motion of Liquids: Fundamentals, Evaluations, and Manipulation Strategies. Tribol. Int. 2021, 154, 106749. [Google Scholar] [CrossRef]
- Radzki, D.; Wilhelm-Węglarz, M.; Pruska, K.; Kusiak, A.; Ordyniec-Kwaśnica, I. A Fresh Look at Mouthwashes—What Is Inside and What Is It For? Int. J. Environ. Res. Public Health 2022, 19, 3926. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley and Sons: New York, NY, USA, 1997; ISBN 0471148733. [Google Scholar]
- Chibowski, E. Surface Free Energy of a Solid from Contact Angle Hysteresis. Adv. Colloid Interface Sci. 2003, 103, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Du, Y.; Zhang, L.; Ma, W.; Yan, F.; Zhang, L.; Zhao, S. Wettability of a Polymethylmethacrylate Surface by Extended Anionic Surfactants: Effect of Branched Chains. Molecules 2021, 26, 863. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, Y.W.; Wilson, M.; Lewis, M.; Williams, D.; Senna, P.M.; Del-Bel-Cury, A.A.; Silva, W.J. da Salivary Pellicles Equalise Surfaces’ Charges and Modulate the Virulence of Candida Albicans Biofilm. Arch. Oral Biol. 2016, 66, 129–140. [Google Scholar] [CrossRef]
- Rüttermann, S.; Trellenkamp, T.; Bergmann, N.; Raab, W.H.-M.; Ritter, H.; Janda, R. A New Approach to Influence Contact Angle and Surface Free Energy of Resin-Based Dental Restorative Materials. Acta Biomater. 2011, 7, 1160–1165. [Google Scholar] [CrossRef]
- Qureshi, S.; Milić, L.; Petrović, B.; Vejin, M.; Kojić, S.; Jarić, S.; Stojanović, G. The Measurement of Contact Angle, PH, and Conductivity of Artificial Saliva and Mouthwashes on Enamel, Glass-Ionomer, and Composite Dental Materials. Materials 2022, 15, 4533. [Google Scholar] [CrossRef]
- Pogorzelski, S.; Watrobska-Swietlikowska, D.; Sznitowska, M. Surface Tensometry Studies on Formulations of Surfactants with Preservatives as a Tool for Antimicrobial Drug Protection Characterization. J. Biophys. Chem. 2012, 03, 324–333. [Google Scholar] [CrossRef]
- Schwarz, G.; Wackerbauer, G.; Taylor, S.E. Partitioning of a Nearly Insoluble Lipid Monolayer into Its Aqueous Subphase. Colloids Surf. A Physicochem. Eng. Asp. 1996, 111, 39–47. [Google Scholar] [CrossRef]
- Mazurek, A.Z.; Pogorzelski, S.J.; Kogut, A.D. A Novel Approach for Structure Quantification of Fatty Acids Films on Rain Water. Atmos. Environ. 2006, 40, 4076–4087. [Google Scholar] [CrossRef]
- Cini, R.; Loglio, G.; Ficalbi, A. Temperature Dependence of the Surface Tension of Water by the Equilibrium Ring Method. J. Colloid Interface Sci. 1972, 41, 287–297. [Google Scholar] [CrossRef]
- Boniewicz-Szmyt, K.; Pogorzelski, S.J. Thermoelastic Surface Properties of Seawater in Coastal Areas of the Baltic Sea. Oceanologia 2016, 58, 25–38. [Google Scholar] [CrossRef]
- Gindl, M.; Sinn, G.; Gindl, W.; Reiterer, A.; Tschegg, S. A Comparison of Different Methods to Calculate the Surface Free Energy of Wood Using Contact Angle Measurements. Colloids Surf. A Physicochem. Eng. Asp. 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Pogorzelski, S.J.; Berezowski, Z.; Rochowski, P.; Szurkowski, J. A Novel Methodology Based on Contact Angle Hysteresis Approach for Surface Changes Monitoring in Model PMMA-Corega Tabs System. Appl. Surf. Sci. 2012, 258, 3652–3658. [Google Scholar] [CrossRef]
- Rodríguez-Valverde, M.; Cabrerizo-Vílchez, M.; Rosales-López, P.; Páez-Dueñas, A.; Hidalgo-Álvarez, R. Contact Angle Measurements on Two (Wood and Stone) Non-Ideal Surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2002, 206, 485–495. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Z.-H.; Hu, S.-S.; Li, S.-M.; Ma, D.-S.; Zhou, X.-Y.; Han, L.; Zhang, L.; Zhang, L. Wettability of a Polymethylmethacrylate Surface in the Presence of Benzyl-Substituted Alkyl Betaines. J. Mol. Liq. 2019, 277, 571–576. [Google Scholar] [CrossRef]
- Lucassen-Reynders, E.H. Contact Angles and Adsorption on Solids. J. Phys. Chem. 1963, 67, 969–972. [Google Scholar] [CrossRef]
- Jańczuk, B.; Białlopiotrowicz, T. Surface Free-Energy Components of Liquids and Low Energy Solids and Contact Angles. J. Colloid Interface Sci. 1989, 127, 189–204. [Google Scholar] [CrossRef]
- Pogorzelski, S.J.; Rochowski, P.; Szurkowski, J. Pinus Sylvestris L. Needle Surface Wettability Parameters as Indicators of Atmospheric Environment Pollution Impacts: Novel Contact Angle Hysteresis Methodology. Appl. Surf. Sci. 2014, 292, 857–866. [Google Scholar] [CrossRef]
- Li, D.; Xie, M.; Neumann, A.W. Vapour Adsorption and Contact Angles on Hydrophobic Solid Surfaces. Colloid Polym. Sci. 1993, 271, 573–580. [Google Scholar] [CrossRef]
- Pogorzelski, S.; Boniewicz-Szmyt, K.; Grzegorczyk, M.; Rochowski, P. Wettability of Metal Surfaces Affected by Paint Layer Covering. Materials 2022, 15, 1830. [Google Scholar] [CrossRef]
- Makkonen, L. A Thermodynamic Model of Contact Angle Hysteresis. J. Chem. Phys. 2017, 147, 064703. [Google Scholar] [CrossRef] [PubMed]
- Lemanska, J. Adsorptive and Thermoelastic Surface Properties of Denture Cleansers and Mouthrinses for Oral Cavity Hygiene. Master’s Thesis, University of Gdansk, Gdańsk, Poland, 2020. [Google Scholar]
- Butt, H.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces, 1st ed.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Yamabe, T.; Moroi, Y.; Abe, Y.; Takahasi, T. Micelle Formation and Surface Adsorption of N-(1,1-Dihydroperfluoroalkyl)-N,N,N-Trimethylammonium Chloride. Langmuir 2000, 16, 9754–9758. [Google Scholar] [CrossRef]
- Gelbart, W.M.; Ben-Shaul, A.; Roux, D. Micelles, Membranes, Microemulsions, and Monolayers; Gelbart, W.M., Ben-Shaul, A., Roux, D., Eds.; Partially Ordered Systems; Springer: New York, NY, USA, 1994; ISBN 978-1-4613-8391-8. [Google Scholar]
- Chibowski, E.J. Surface Free Energy and Wettability of Silyl Layers on Silicon Determined from Contact Angle Hysteresis. Adv. Colloid Interface Sci. 2005, 113, 121–131. [Google Scholar] [CrossRef]
- Johnson, B.A.; Kreuter, J.; Zografi, G. Effects of Surfactants and Polymers on Advancing and Receding Contact Angles. Colloids Surf. 1986, 17, 325–342. [Google Scholar] [CrossRef]
- Law, K.-Y. Contact Angle Hysteresis on Smooth/Flat and Rough Surfaces. Interpretation, Mechanism, and Origin. Acc. Mater. Res. 2022, 3, 1–7. [Google Scholar] [CrossRef]
- Samuel, B.; Zhao, H.; Law, K.-Y. Study of Wetting and Adhesion Interactions between Water and Various Polymer and Superhydrophobic Surfaces. J. Phys. Chem. C 2011, 115, 14852–14861. [Google Scholar] [CrossRef]
- Svitova, T.; Hill, R.; Radke, C. Adsorption Layer Structures and Spreading Behavior of Aqueous Non-Ionic Surfactants on Graphite. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 183–185, 607–620. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Krawczyk, J.; Jańczuk, B. Wettability, Adhesion, Adsorption and Interface Tension in the Polymer/Surfactant Aqueous Solution System. I. Critical Surface Tension of Polymer Wetting and Its Surface Tension. Colloids Surf. A Physicochem. Eng. Asp. 2012, 402, 132–138. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface Characteristics Influencing Bacterial Adhesion to Polymeric Substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Krawczyk, J.; Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Wettability of Polymers by Aqueous Solution of Binary Surfactants Mixture with Regard to Adhesion in Polymer–Solution System II. Critical Surface Tension of Polymers Wetting and Work of Adhesion. Int. J. Adhes. Adhes. 2013, 45, 106–111. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Effect of Polysorbates on Solids Wettability and Their Adsorption Properties. Colloids Interfaces 2018, 2, 26. [Google Scholar] [CrossRef]
- Zdziennicka, A. The Wettability of Polytetrafluoroethylene and Polymethylmethacrylate by Aqueous Solutions of Triton X-100 and Propanol Mixtures. Appl. Surf. Sci. 2009, 255, 3801–3810. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Jańczuk, B. Behavior of Cationic Surfactants and Short-Chain Alcohols in Mixed Surface Layers at Water–Air and Polymer–Water Interfaces with Regard to Polymer Wettability. J. Colloid Interface Sci. 2010, 350, 568–576. [Google Scholar] [CrossRef]
- Schäffer, E.; Wong, P. Contact Line Dynamics near the Pinning Threshold: A Capillary Rise and Fall Experiment. Phys. Rev. E 2000, 61, 5257–5277. [Google Scholar] [CrossRef]
- Bracke, M.; Voeght, F.; Joos, P. The Kinetics of Wetting: The Dynamic Contact Angle. In Trends in Colloid and Interface Science III; Steinkopff: Darmstadt, Germany, 2000; Volume 61, pp. 142–149. [Google Scholar]
- Berg, S. Marangoni-Driven Spreading along Liquid-Liquid Interfaces. Phys. Fluids 2009, 21, 032105. [Google Scholar] [CrossRef]
- Pratap, V.; Moumen, N.; Subramanian, R.S. Thermocapillary Motion of a Liquid Drop on a Horizontal Solid Surface. Langmuir 2008, 24, 5185–5193. [Google Scholar] [CrossRef]
- Mao, Z.; Lu, P.; Zhang, G.; Yang, C. Numerical Simulation of the Marangoni Effect with Interphase Mass Transfer Between Two Planar Liquid Layers. Chin. J. Chem. Eng. 2008, 16, 161–170. [Google Scholar] [CrossRef]
- Boniewicz-Szmyt, K.; Pogorzelski, S.J. Influence of Surfactant Concentration and Temperature Gradients on Spreading of Crude-Oil at Sea*. Front. Mar. Sci. 2018, 5, 388. [Google Scholar] [CrossRef]
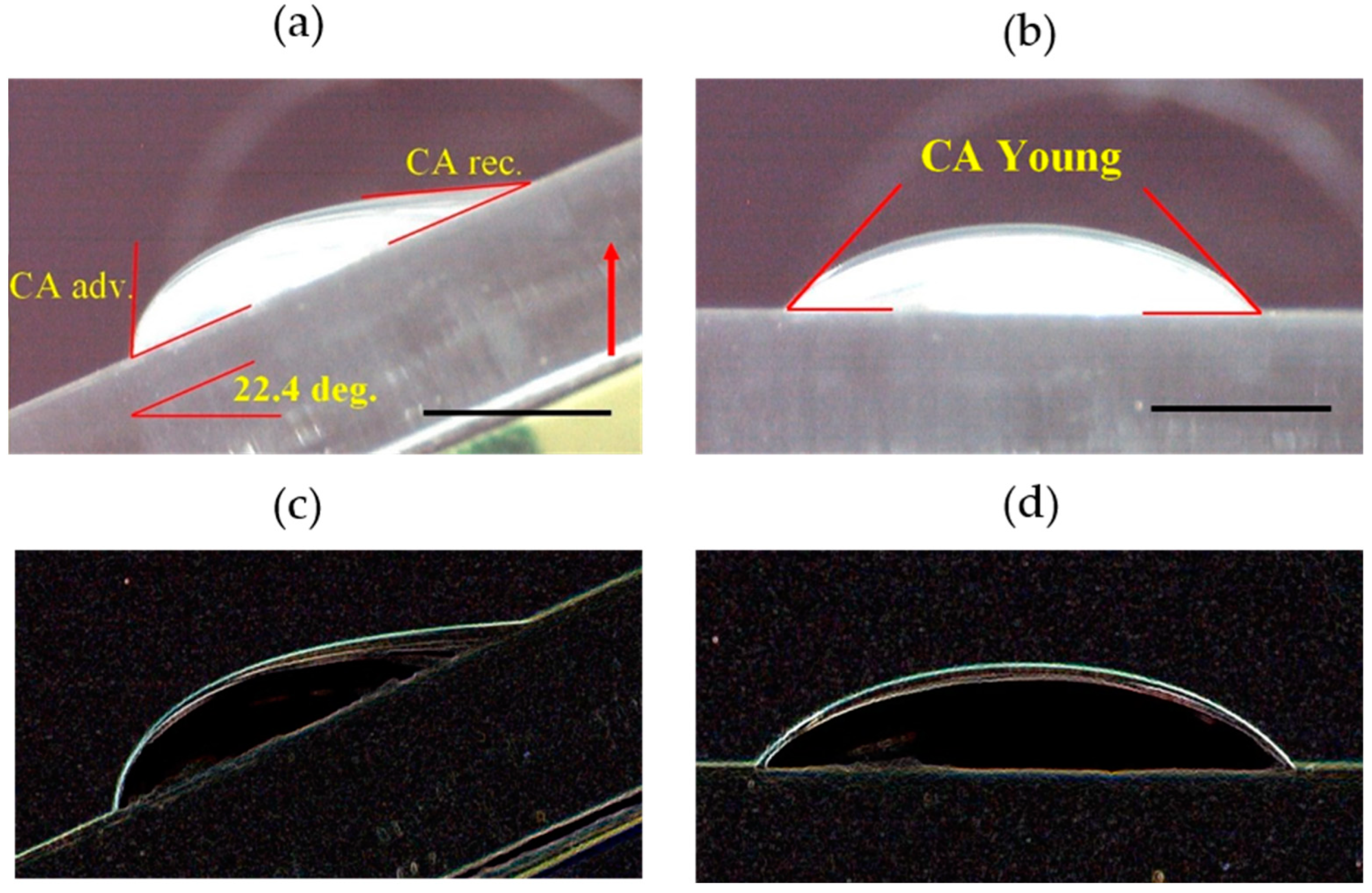

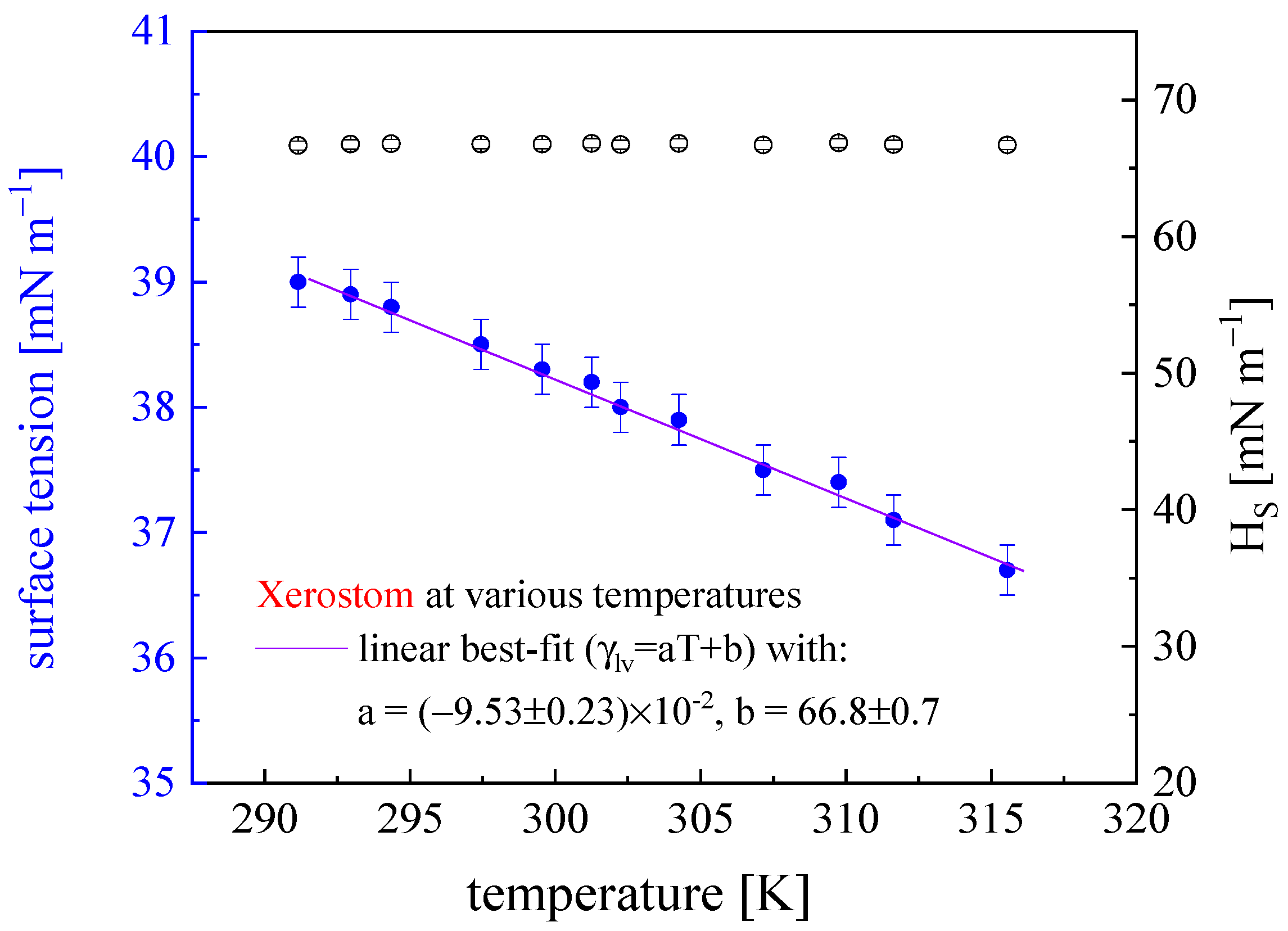
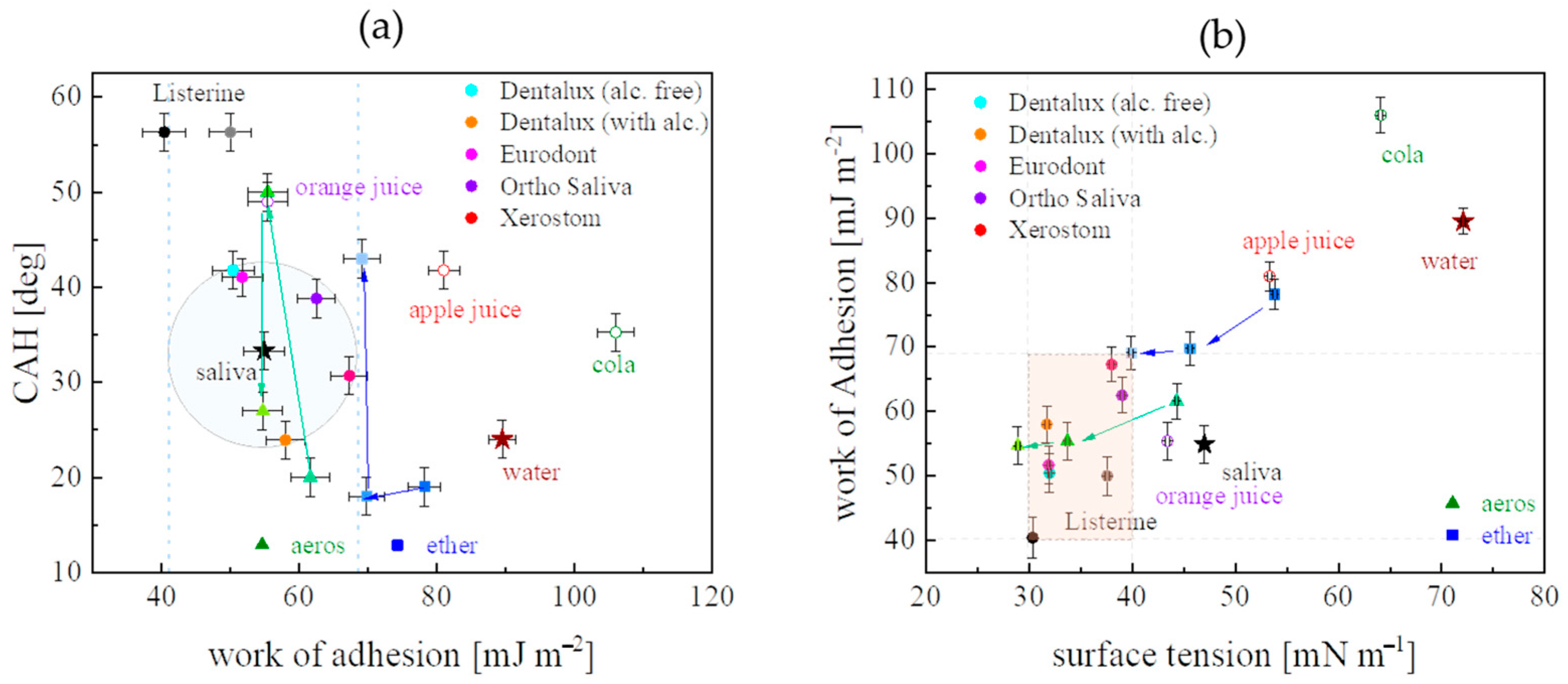


| Liquid | ΓLV [mN m−1] (0.2) | ρ [103 kg m−3] (0.03) | μ [mPa s] (0.05) | pH [-] (0.1) | Product Source |
|---|---|---|---|---|---|
| Xerostom | 38.7 | 0.92 | 1.03 | 7.2 | Bio Cosmetics, Madrid, Spain |
| Ortho Salvia Dental | 39.9 | 0.97 | 1.12 | 7.3 | Atos, Warsaw, Poland |
| Listerine Cool Mint | 37.6 | 0.98 | 1.32 | 4.3 | Johnson & Johnson Consumer Health, France |
| Dentalux (alcohol free) | 29.7 | 0.96 | 1.27 | 6.0 | Cosmolux, Pulheim, Germany |
| Dentalux (+alcohol) | 31.7 | 0.93 | 1.10 | 4.9 | Cosmolux, Pulheim, Germany |
| Eurodont | 33.7 | 0.92 | 1.05 | 5.6 | Maxim Markenprodukte, Pulheim, Germany |
| Coca Cola | 64.1 | 0.91 | 1.01 | 3.8 | Lidl Market |
| Apple Juice | 54.1 | 1.04 | 1.12 | 3.6 | Lidl Market |
| Orange Juice | 61.1 | 1.19 | 1.21 | 4.5 | Lidl Market |
| Pure water | 72.5 | 0.89 | 0.86 | 6.8 | |
| Saliva | 53.6 | 1.12 | 1.02 | 6.8 | 20-year old healthy volunteer |
| Liquid | cmc [c/c0] | γcmc [mN m−1] | Γmax [×106 mol cm−2] | Amolec nm2 molec.−1 | |σ| [mN m−1] |
|---|---|---|---|---|---|
| Xerostom | 0.06 (0.01) | 40.9 (0.1) | 0.42 (0.09) | 392.21 (10.15) | 210 (16) |
| Ortho Salvia Dental | 0.03 (0.01) | 42.7 (0.2) | 0.24 (0.08) | 839.53 (15.23) | 380 (18) |
| Listerine Cool Mint | 0.12 (0.02) | 40.0 (0.2) | 0.94 (0.09) | 170.24 (4.45) | 230 (19) |
| Dentalux (alcohol free) | 0.03 (0.01) | 31.2 (0.2) | 0.50 (0.08) | 331.87 (6.23) | 810 (32) |
| Dentalux (+alcohol) | 0.03 (0.01) | 30.9 (0.2) | 0.61 (0.07) | 310.61 (6.10) | 850 (24) |
| Eurodont | 0.03 (0.01) | 32.4 (0.2) | 0.91 (0.06) | 178.46 (3.78) | 1120 (67) |
| Saliva | 0.31 (0.08) | 53.7 (0.2) | 1.35 (0.08) | 12.634 (1.87) | 40 (5) |
| Liquid | ΓLV [mN m−1] | TSs [mN m−1] | Hs [mN m−1] |
|---|---|---|---|
| Xerostom | 38.7 (0.2) | 28.1 (0.2) | 66.8 (0.4) |
| Ortho Salvia Dental | 39.9 (0.2) | 38.4 (0.2) | 78.3 (0.4) |
| Listerine Cool Mint | 37.6 (0.2) | 41.3 (0.2) | 78.9 (0.4) |
| Dentalux (alcohol free) | 29.7 (0.2) | 67.9 (0.2) | 97.6 (0.4) |
| Dentalux (+alcohol) | 31.7 (0.2) | 62.0 (0.2) | 93.7 (0.4) |
| Eurodont | 33.7 (0.2) | 37.4 (0.2) | 71.1 (0.4) |
| Saliva | 53.6 (0.2) | 31.9 (0.2) | 85.6 (0.4) |
| Pure Water | 72.5 (0.2) | 46.3 (0.2) | 118.8 (0.4) |
| Coca Cola | 64.1 (0.2) | 52.7 (0.2) | 116.8 (0.4) |
| Orange Juice | 61.1 (0.2) | 38.0 (0.2) | 99.1 (0.4) |
| Liquid | Θ [°] | ΘA [°] | ΘR [°] | CAH [°] | Π [mN m−1] |
|---|---|---|---|---|---|
| Xerostom | 41.7 (1) | 42.2 (1) | 12.1 (1) | 30.7 (2) | 9.2 (0.4) |
| Ortho Salvia Dental | 43.1 (1) | 55.6 (1) | 16.7 (1) | 38.9 (2) | 15.7 (0.6) |
| Listerine Cool Mint | 46.1 (1) | 65.2 (1) | 18.7 (1) | 46.5 (2) | 19.8 (0.7) |
| Dentalux (alcohol free) | 39.2 (1) | 54.9 (1) | 13.1 (1) | 41.8 (2) | 12.7 (0.5) |
| Dentalux (+alcohol) | 31.9 (1) | 43.9 (1) | 10.0 (1) | 23.9 (2) | 4.9 (0.3) |
| Eurodont | 30.2 (1) | 55.7 (1) | 22.3 (1) | 33.4 (2) | 12.3 (0.5) |
| Coca Cola | 36.5 (1) | 49.2 (1) | 13.9 (1) | 35.3 (2) | 20.3 (0.8) |
| Apple Juice | 54.2 (1) | 58.8 (1) | 16.9 (1) | 41.9 (2) | 23.3 (0.9) |
| Orange Juice | 68.8 (1) | 74.0 (1) | 25.0 (1) | 49.0 (2) | 27.4 (1.1) |
| Water | 64.7 (1) | 76.0 (1) | 52.0 (1) | 24.0 (2) | 26.9 (1.0) |
| Saliva | 72.7 (1) | 88.6 (1) | 55.3 (1) | 33.3 (2) | 29.2 (1.2) |
| Liquid | WA [mJ m−2] | ΓSV [mJ m−2] | ΓSVd [mJ m−2] | ΓSVp [mJ m−2] | ΓLV cosΘA [mN m−1] |
| Xerostom | 67.3 (1.2) | 31.5 (1.5) | 29.3 (1.3) | 2.2 (0.2) | 28.7 (0.2) |
| Ortho Salvia Dental | 62.5 (1.1) | 27.8 (1.5) | 24.5 (1.2) | 3.3 (0.3) | 22.6 (0.2) |
| Listerine Cool Mint | 53.3 (1.1) | 22.5 (1.4) | 18.9 (1.1) | 3.6 (0.3) | 15.6 (0.2) |
| Dentalux (alcohol free) | 50.4 (1.1) | 22.4 (1.3) | 19.9 (1.1) | 2.5 (0.2) | 18.4 (0.2) |
| Dentalux (+alcohol) | 58.0 (1.2) | 27.8 (1.5) | 26.5 (1.4) | 1.3 (0.1) | 26.3 (0.2) |
| Eurodont | 52.7 (1.1) | 23.6 (1.4) | 20.6 (1.1) | 3.0 (0.3) | 18.9 (0.2) |
| Coca Cola | 106.1 (1.6) | 48.4 (2.0) | 43.8 (1.5) | 4.5 (0.5) | 41.9 (0.2) |
| Apple Juice | 81.0 (1.4) | 35.4 (1.8) | 30.7 (1.3) | 4.6 (0.5) | 27.6 (0.2) |
| Orange Juice | 55.4 (1.1) | 22.2 (1.5) | 17.7 (1.0) | 4.5 (0.5) | 11.9 (0.2) |
| Water | 89.5 (1.4) | 38.9 (2.0) | 27.8 (1.3) | 11.1 (0.7) | 10.2 (0.2) |
| Saliva | 54.9 (1.1) | 21.7 (1.5) | 14.1 (1.0) | 7.6 (0.5) | 1.3 (0.2) |
| c/c0 (2%) | ΘA [°] (1°) | ΘR [°] (1°) | Π [mN m−1] | WA [mJ m−2] | ΓSV [mJ m−2] | ΓSVd [mJ m−2] | ΓSVp [mJ m−2] | ΓLV cosΘA [mN m−1] |
|---|---|---|---|---|---|---|---|---|
| 1 | 55.8 | 22.2 | 12.3 (0.5) | 52.7 (1.1) | 23.6 (1.4) | 20.6 (1.1) | 3.0 (0.3) | 18.9 (0.2) |
| 0.25 | 47.9 | 19.3 | 8.5 (0.3) | 51.8 (1.1) | 23.8 (1.4) | 21.6 (1.1) | 2.3 (0.3) | 21.0 (0.2) |
| 0.125 | 55.9 | 24.4 | 11.1 (0.5) | 49.6 (1.0) | 22.3 (1.4) | 19.4 (1.0) | 2.9 (0.4) | 17.8 (0.2) |
| 0.063 | 62.9 | 30.4 | 13.1 (0.5) | 46.9 (1.0) | 20.6 (1.3) | 17.1 (0.8) | 3.5 (0.5) | 14.6 (0.2) |
| 0.032 | 64.8 | 32.3 | 13.6 (0.5) | 46.2 (0.9) | 20.1 (1.3) | 16.5 (0.8) | 3.7 (0.5) | 13.8 (0.2) |
| 0.016 | 70.2 | 34.0 | 16.2 (0.6) | 44.4 (0.8) | 18.8 (1.2) | 14.9 (0.6) | 3.9 (0.6) | 11.3 (0.1) |
| 0.0078 | 73.0 | 50.9 | 12.0 (0.5) | 45.7 (0.9) | 20.2 (1.3) | 14.8 (0.6) | 5.4 (0.7) | 10.3 (0.1) |
| 0.0039 | 82.4 | 37.3 | 27.7 (0.8) | 47.4 (0.9) | 18.3 (1.2) | 13.4 (0.5) | 4.9 (0.7) | 5.5 (0.1) |
| 0.00195 | 83.9 | 56.3 | 22.6 (0.7) | 55.8 (1.3) | 23.2 (1.4) | 15.4 (0.7) | 7.8 (0.7) | 5.4 (0.1) |
| 0.00098 | 84.0 | 63.5 | 19.8 (0.6) | 64.2 (1.5) | 27.8 (1.5) | 17.7 (0.8) | 10.1 (0.7) | 6.1 (0.1) |
| 0.00048 | 88.2 | 64.3 | 25.2 (0.8) | 64.7 (1.5) | 27.1 (1.5) | 16.7 (0.8) | 10.4 (0.7) | 2.0 (0.1) |
| 0.00024 | 96.5 | 69.0 | 32.0 (0.9) | 60.3 (1.4) | 23.8 (1.4) | 13.4 (0.5) | 10.5 (0.9) | −7.7 (0.1) |
| Liquid | ΓSL/ΓLV | Remarks, Data Source |
|---|---|---|
| Mouthrinses | ||
| Xerostom | −1.23 (0.14) | c < cmc |
| Ortho Salvia Dental | −0.20 (0.08) | c < cmc |
| Listerine Cool Mint | −1.37 (0.19) | c < cmc |
| Dentalux (alcohol free) | −1.21 (0.17) | c < cmc |
| Dentalux (+alcohol) | −1.14 (0.24) | c < cmc |
| Eurodont | −1.49 (0.23) c < cmc | −1.81 (0.31) c > cmc |
| Monocomponent model surfactant water solution | ||
| AOT (dioctyl sodium sulfosuccinate), anionic, double alkyl chains | −0.85 (0.12) | c < cmc [32] |
| Tween 20 (polyethylene glycol sorbitan monooleate) | −0.76 (0.15) | c < cmc [39] |
| SDS (sodium dodecyl sulphate), anionic | −0.31 (0.09) | c < cmc [39] |
| CTAB (cetyltrimethylammonium bromide), cationic | −0. 34 (0.12) | c < cmc [40,41] |
| Triton TX-100, nonionic | −0.17 (0.06) | c < cmc [36,41] |
| Zwitterionic and cationic gemini surfactants (extended surfactants) | −0.31 (0.01) | (0.34) (0.03) |
| c < cmc | c > cmc | |
| [20] | [7] |
| Liquid | Xerostom | Ortho Salvia Dental | Listerine Cool Mint | Dentalux (Alcohol Free) | Dentalux (+Alcohol) | Eurodont |
|---|---|---|---|---|---|---|
| PC [10−2 m s−1] | 9.8 (0.5) | 8.9 (0.5) | 6.5 (0.3) | 7.4 (0.4) | 7.8 (0.4) | 7.4 (0.4) |
| US [10−2 m s−1] | 0.21 (0.1) | 0.23 (0.1) | 0.20 (0.1) | 0.46 (0.2) | 0.32 (0.1) | 0.28 (0.1) |
| Liquid | Coca Cola | Apple Juice | Orange Juice | Pure water | Saliva | - |
| PC [10−2 m s−1] | 7.2 (0.4) | 5.8 (0.3) | 6.2 (0.3) | 9.8 (0.5) | 4.3 (0.2) | - |
| US [10−2 m s−1] | 0.41 (0.2) | 0.64 (0.3) | 0.43 (0.2) | 0.80 (0.4) | 0.39 (0.2) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogorzelski, S.; Janowicz, P.; Dorywalski, K.; Boniewicz-Szmyt, K.; Rochowski, P. Wettability, Adsorption and Adhesion in Polymer (PMMA)—Commercially Available Mouthrinse System. Materials 2023, 16, 5753. https://doi.org/10.3390/ma16175753
Pogorzelski S, Janowicz P, Dorywalski K, Boniewicz-Szmyt K, Rochowski P. Wettability, Adsorption and Adhesion in Polymer (PMMA)—Commercially Available Mouthrinse System. Materials. 2023; 16(17):5753. https://doi.org/10.3390/ma16175753
Chicago/Turabian StylePogorzelski, Stanislaw, Paulina Janowicz, Krzysztof Dorywalski, Katarzyna Boniewicz-Szmyt, and Pawel Rochowski. 2023. "Wettability, Adsorption and Adhesion in Polymer (PMMA)—Commercially Available Mouthrinse System" Materials 16, no. 17: 5753. https://doi.org/10.3390/ma16175753
APA StylePogorzelski, S., Janowicz, P., Dorywalski, K., Boniewicz-Szmyt, K., & Rochowski, P. (2023). Wettability, Adsorption and Adhesion in Polymer (PMMA)—Commercially Available Mouthrinse System. Materials, 16(17), 5753. https://doi.org/10.3390/ma16175753






