α-Zirconium(IV) Phosphate: Static Study of 225Ac Sorption in an Acidic Environment and Its Kinetic Sorption Study Using natEu as a Model System for 225Ac
Abstract
1. Introduction
2. Materials and Methods
2.1. α-ZrP Preparation
2.2. Kinetic Study of natEu Sorption on the Surface of α-ZrP
2.3. Batch Experiments of 225Ac Sorption on the Surface of α-ZrP
3. Results and Discussion
3.1. Kinetic Study of natEu Sorption on the Surface of α-ZrP
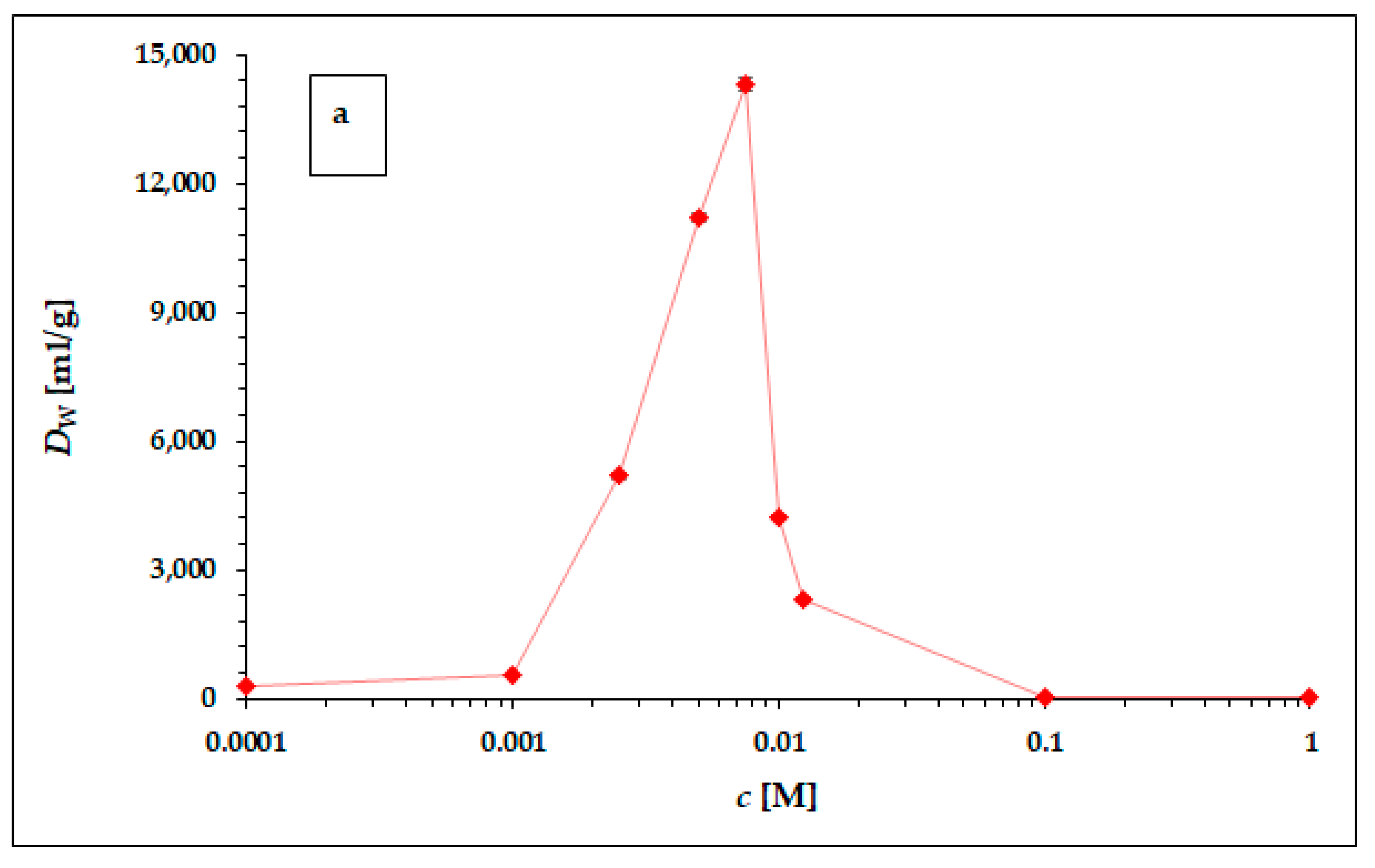
3.2. Batch Experiment of 225Ac Sorption on the Surface of α-ZrP
- (a)
- In the range of hydrochloric and nitric acid concentrations higher than 0.01 M, i.e., at pH < 2, the cationic sorption capacity of α-ZrP decreases significantly, as shown not only by our results from the evaluation of the corresponding titration curve [32], but also by data in the literature devoted to the properties of α-ZrP, e.g., [39].
- (b)
- Increasing the acid concentration increases the ionic strength, which generally leads to a decrease in the equilibrium constants, eventually also of Dw.
- (c)
- The difference between the hydrochloric and nitric acid environments is probably due to the generally higher complexation efficiency of the chloride ligand compared with the nitrate ligand, resulting in a reduction of the cationic form in solution and a decrease of its sorption capacity. Unfortunately, the values of the stability constants for Ac3+-Cl− and Ac3+-NO3− complexation could not be found in the available literature. However, there is a significant difference in the values of the dissociation constants of these acids; in the case of hydrochloric acid, the pKa has value −6, and in the case of nitric acid, the pKa has value −1.32. Thus, the concentration of the ligand in the dissociated state is an order of magnitude higher in the case of hydrochloric acid than in the case of nitric acid, which may contribute to a higher complexation efficiency of the cationic form of 225Ac3+ and consequently to a lower sorption capacity of α-ZrP in the hydrochloric acid environment compared with the corresponding values in the nitric acid environment [40].
- (d)
- In the range of concentrations below 0.001 M, i.e., at pH > 3 and especially at pH > 4, a gradual hydrolysis of the cationic forms and an increase in the concentration of hydroxo-complexes in solution and a decrease in the sorption of the cationic forms can be expected.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CR | chemical reaction in reaction zone |
| DM | mass transfer |
| FD | film diffusion |
| GD | gel diffusion |
| ID | diffusion in inert layer |
| ICP-MS | inductively coupled plasma mass spectrometry |
| RLD | diffusion in reacted layer |
| TAT | targeted alpha therapy |
| ZrP | zirconium(IV) phosphate |
References
- Clearfield, A.; Stynes, J.A. The preparation of crystalline zirconium phosphate and some observations on its ion exchange behavior. J. Inorg. Nucl. Chem. 1964, 26, 117–129. [Google Scholar] [CrossRef]
- Amphlett, C.B.; McDonald, L.A.; Redman, M.J. Synthetic inorganic ion-exchange materials—I zirconium phosphate. J. Inorg. Nucl. Chem. 1958, 6, 220–235. [Google Scholar] [CrossRef]
- Alberti, G.; Constantino, U. Recent progress in the field of synthetic inorganic exchangers having a layered or fibrous structure. J. Chromatogr. A 1974, 102, 5–29. [Google Scholar] [CrossRef]
- Clearfield, A.; Thakur, D.S. Zirconium and titanium phosphates as catalysts: A review. Appl. Catal. 1984, 26, 1–26. [Google Scholar] [CrossRef]
- Pica, M. Zirconium Phosphate Catalysts in the XXI Century: State of the Art from 2010 to Date. Catalysts 2017, 7, 190. [Google Scholar] [CrossRef]
- Colón, J.L.; Casañas, B. Drug carriers based on zirconium phosphate nanoparticles. In Tailored Organic-Inorganic Materials; Wiley: Hoboken, NJ, USA, 2015; pp. 395–437. [Google Scholar]
- Alongi, J.; Frache, A. Flame retardancy properties of α-zirconium phosphate based composites. Polym. Degrad. Stab. 2010, 95, 1928–1933. [Google Scholar] [CrossRef]
- Huang, T.C.; Lai, G.H.; Li, C.E.; Tsai, M.H.; Wan, P.Y.; Chung, Y.H.; Lin, M.H. Advanced anti-corrosion coatings prepared from α-zirconium phosphate/polyurethane nanocomposites. RSC Adv. 2017, 7, 9908–9913. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. Zirconium phosphate (ZrP)-based functional materials: Synthesis, properties and applications. Mater. Des. 2018, 155, 19–35. [Google Scholar] [CrossRef]
- Wiikinkoski, E.W.; Harjula, R.O.; Lehto, J.K.; Kemell, M.L.; Koivula, R.T. Effects of synthesis conditions on ion exchange properties of α-zirconium phosphate for Eu and Am. Radiochim. Acta 2017, 105, 1033–1042. [Google Scholar] [CrossRef][Green Version]
- Wiikinkoski, E.W.; Rautsola, I.; Xu, J.; Koivula, R. Column Separation of Am (III) and Eu (III) by α-Zirconium Phosphate Ion Exchanger in Nitric Acid. Chem. Eng. 2020, 4, 14. [Google Scholar] [CrossRef]
- Wiikinkoski, E.W.; Xu, J.; Zhang, W.; Hietala, S.; Koivula, R.T. Modification of α-Zirconium Phosphate Synthesis–Effects of Crystallinity and Acidity on Eu (III) and Am (III) Ion Exchange. ChemistrySelect 2018, 3, 9583–9588. [Google Scholar] [CrossRef]
- Lee, J.Y.; Vyas, C.K.; Kim, B.R.; Kim, H.J.; Hur, M.G.; Yang, S.D.; Park, J.H.; Kim, S.W. Acid resistant zirconium phosphate for the long term application of 68Ge/68Ga generator system. Appl. Radiat. Isot. 2016, 118, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, A. Group IV phosphates as catalysts and catalyst supports. J. Mol. Catal. 1984, 27, 251–262. [Google Scholar] [CrossRef]
- Troup, J.; Clearfield, A. Mechanism of ion exchange in zirconium phosphates. 20. Refinement of the crystal structure of α-zirconium phosphate. Inorg. Chem. 1977, 16, 3311–3314. [Google Scholar] [CrossRef]
- Clearfield, A. Inorganic ion exchangers with layered structures. Annu. Rev. Mater. Sci. 1984, 14, 205–229. [Google Scholar] [CrossRef]
- Albertsson, J.; Oskarsson, A.; Tellgren, R.; Thomas, J. Inorganic ion exchangers. 10. A neutron powder diffraction study of the hydrogen bond geometry in α-zirconium bis(monohydrogen orthophosphate) monohydrate. A model for the ion exchange. J. Phys. Chem. 1977, 81, 1574–1578. [Google Scholar] [CrossRef]
- Clearfield, A.; Smith, G.D. The crystallography and structure of α-zirconium bis(monohydrogenorthophosphate) monohydrate. Inorg. Chem. 1969, 8, 431. [Google Scholar] [CrossRef]
- Kullberg, L.; Clearfield, A. Mechanism of ion exchange in zirconium phosphates. 32. Thermodynamics of alkali metal ion exchange on crystalline α-ZrP. J. Phys. Chem. 1981, 85, 1585–1589. [Google Scholar] [CrossRef]
- Möller, T.; Bestaoui, N.; Wierzbicki, M.; Adams, T.; Clearfield, A. Separation of lanthanum, hafnium, barium and radiotracers yttrium-88 and barium-133 using crystalline zirconium phosphate and phosphonate compounds as prospective materials for a Ra-223 radioisotope generator. Appl. Radiat. Isot. 2011, 69, 947–954. [Google Scholar] [CrossRef]
- Mimura, H.; Akiba, K. Adsorption properties of europium on granulated α-zirconium phosphate. J. Nucl. Sci. Technol. 1996, 33, 592–596. [Google Scholar] [CrossRef]
- Jayswal, A.; Chudasama, U. Synthesis and Characterization of a New Phase of Zirconium Phosphate for the Separation of Metal Ions. J. Iran. Chem. Soc. 2007, 4, 510–515. [Google Scholar] [CrossRef]
- Xu, J.; Virolainen, S.; Zhang, W.; Kuva, J.; Sainio, T.; Koivula, R. Polyacrylonitrile-encapsulated amorphous zirconium phosphate composite adsorbent for Co, Nd and Dy separations. J. Chem. Eng. 2018, 351, 832–840. [Google Scholar] [CrossRef]
- Yan, M.; Wu, S.; Wang, Y.; Liang, M.; Wang, M.; Hu, W.; Yu, G.; Mao, Z.; Huang, F.; Zhou, J. Recent Progress of Supramolecular Chemotherapy Based on Host–Guest Interactions. Adv. Mater. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Larson, S.M.; Sgouros, G.; McDevitt, M.R.; Finn, R.D.; Divgi, C.R.; Ballangrud, A.M.; Hamacher, K.A.; Ma, D.; Humm, J.L.; et al. Targeted α particle immunotherapy for myeloid leukemia. Blood 2002, 100, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, M.E.; Seidl, C.; Bruchertseifer, F.; Horn, T.; Kurtz, F.; Feuerecker, B.; D’Alessandria, C.; Pfob, C.; Nekolla, S.; Apostolidis, C.; et al. Treatment of carcinoma in situ of the urinary bladder with an alpha-emitter immunoconjugate targeting the epidermal growth factor receptor: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1364–1371. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Allen, B.J.; Raja, C.; Rizvi, S.; Li, Y.; Tsui, W.; Graham, P.; Thompson, J.; Reisfeld, R.; Kearsley, J.; Morgenstern, A.; et al. Intralesional targeted alpha therapy for metastatic melanoma. Cancer Biol. Ther. 2005, 4, 1318–1324. [Google Scholar] [CrossRef]
- Kneifel, S.; Cordier, D.; Good, S.; Ionescu, M.C.; Ghaffari, A.; Hofer, S.; Kretzschmar, M.; Tolnay, M.; Apostolidis, C.; Waser, B.; et al. Local targeting of malignant gliomas by the diffusible peptidic vector 1, 4, 7, 10-tetraazacyclododecane-1-glutaric acid-4, 7, 10-triacetic acid-substance p. Clin. Cancer Res. 2006, 12, 3843–3850. [Google Scholar] [CrossRef]
- Kellerbauer, A.; Bruchertseifer, F.; Malmbeck, R.; Morgenstern, A. Targeted α therapy with 213Bi and 225Ac. J. Phys. Conf. Ser. 2020, 1643, 012205. [Google Scholar] [CrossRef]
- Ondrák, L.; Ondrák-Fialová, K.; Sakmár, M.; Vlk, M.; Štamberg, K.; Drtinová, B.; Šlouf, M.; Bruchertseifer, F.; Morgenstern, A.; Kozempel, J. Preparation and characterization of α-zirconium phosphate as a perspective material for separation of 225Ac and 213Bi. J. Radioanal. Nucl. Chem. 2023, 332, 1527–1532. [Google Scholar] [CrossRef]
- Yu, S.; Tang, H.; Zhang, D.; Wang, S.; Qiu, M.; Song, G.; Fu, D.; Hu, B.; Wang, X. MXenes as emerging nanomaterials in water purification and environmental remediation. Sci. Total Environ. 2022, 811, 152280. [Google Scholar] [CrossRef] [PubMed]
- Missana, T.; Alonso, U.; García-Gutiérrez, M. Evaluation of component additive modeling approach for europium adsorption on 2:1 clays: Experimental, thermodynamic databases, and models. Chemosphere 2021, 272, 129877. [Google Scholar] [CrossRef] [PubMed]
- Rabung, T.; Pierret, M.C.; Bauer, A.; Geckeis, H.; Bradbury, M.H.; Baeyens, B. Sorption of Eu (III)/Cm (III) on Ca-montmorillonite and Na-illite. Part 1: Batch sorption and time-resolved laser fluorescence spectroscopy experiments. Geochim. Cosmochim. 2005, 69, 5393–5402. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B.; Geckeis, H.; Rabung, T. Sorption of Eu (III)/Cm (III) on Ca-montmorillonite and Na-illite. Part 2: Surface complexation modeling. Geochim. Cosmochim. 2005, 69, 5403–5412. [Google Scholar] [CrossRef]
- Beneš, P.; Stamberg, K.; Štegman, R. Study of the Kinetics of Interaction of Cs-137 and Sr-85 with Soils using a Batch Method: Methodological Problems. Radiochim. Acta 1994, 66, 315–321. [Google Scholar] [CrossRef]
- Suchánková, P.; Kukleva, E.; Štamberg, K.; Nykl, P.; Sakmár, M.; Vlk, M.; Kozempel, J. Determination, modeling and evaluation of kinetics of 223Ra sorption on hydroxyapatite and titanium dioxide nanoparticle. Materials 2020, 13, 1915. [Google Scholar] [CrossRef]
- Manecke, G. Ionenaustauscher, Band I: Grundlagen. Struktur—Herstellung—Theorie, von F. Helfferich. Verlag Chemie GmbH, Weinheim/Bergstraße 1959. 1. Aufl., VIII, 520 S., 153 Abb., 14 Tab., geb. DM 48.–. Angew. Chem. 1962, 74, 596. [Google Scholar] [CrossRef]
- Starý, J.; Kyrš, M.; Marhol, M. Separační metody v radiochemii (Separation methods in radiochemistry); Academia: Praha, Czech Republic, 1975; 253p. [Google Scholar]
- Wu, C.; Brechbiel, M.W.; Gansow, O.A. An improved generator for the production of 213Bi from 225Ac. Radiochim. Acta 1997, 79, 141–144. [Google Scholar] [CrossRef]
- McAlister, D.R.; Horwitz, E.P. Automated two column generator systems for medical radionuclides. Appl. Radiat. Isot. 2009, 67, 1985–1991. [Google Scholar] [CrossRef]
- Bray, L.A.; Tingey, J.M.; DesChane, J.R.; Egorov, O.B.; Tenforde, T.S.; Wilbur, D.S.; Hamlin, D.K.; Pathare, P.M. Development of a unique bismuth (Bi-213) automated generator for use in cancer therapy. Ind. Eng. Chem. Res. 2000, 39, 3189–3194. [Google Scholar] [CrossRef]


| Control Process | Model | Differential Equation | |
|---|---|---|---|
| Mass transfer | DM | (1) | |
| Film diffusion | FD | (2) | |
| (3) | |||
| Diffusion in an inert layer | ID | (4) | |
| (5) | |||
| Diffusion in a reacted layer | RLD | (6) | |
| (7) | |||
| Chemical reaction in reaction zone | CR | (8) | |
| (9) | |||
| (10) | |||
| Gel diffusion | GD | (11) | |
| (12) |
| t [min] | F [-] |
|---|---|
| 0 | 1.000 |
| 0.5 | 0.179 |
| 1 | 0.129 |
| 5 | 0.071 |
| 10 | 0.071 |
| 15 | 0.070 |
| 20 | 0.058 |
| 25 | 0.054 |
| Model | WSOS/DF [-] | Kmodel [min−1] |
|---|---|---|
| DM | 1.38 × 102 | (2.48 ± 0.01) × 10−4 |
| FD | 7.79 × 100 | (3.17 ± 0.01) × 102 |
| ID | 4.14 × 102 | (2.21 ± 3.28) × 10−1 |
| RLD | 1.42 × 101 | (9.68 ± 0.03) × 10−4 |
| CR | 1.20 × 102 | (6.96 ± 20.7) × 102 |
| GD | 2.42 × 103 | (2.56 ± 7.40) × 10−5 |
| c [M] | Dw [mL/g] | σDw [mL/g] |
|---|---|---|
| 0.0001 | 267 | 11 |
| 0.0010 | 537 | 15 |
| 0.0025 | 5181 | 56 |
| 0.0050 | 11,206 | 126 |
| 0.0075 | 14,303 | 153 |
| 0.0100 | 4240 | 51 |
| 0.0125 | 2293 | 56 |
| 0.1000 | 59 | 4 |
| 1.0000 | 45 | 4 |
| c [M] | Dw [mL/g] | σDw [mL/g] |
|---|---|---|
| 0.0001 | 2662 | 28 |
| 0.0010 | 4482 | 42 |
| 0.0050 | 65,272 | 612 |
| 0.0100 | 19,486 | 187 |
| 0.1000 | 41 | 1 |
| 1.0000 | 11 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ondrák, L.; Ondrák Fialová, K.; Vlk, M.; Štamberg, K.; Bruchertseifer, F.; Morgenstern, A.; Kozempel, J. α-Zirconium(IV) Phosphate: Static Study of 225Ac Sorption in an Acidic Environment and Its Kinetic Sorption Study Using natEu as a Model System for 225Ac. Materials 2023, 16, 5732. https://doi.org/10.3390/ma16175732
Ondrák L, Ondrák Fialová K, Vlk M, Štamberg K, Bruchertseifer F, Morgenstern A, Kozempel J. α-Zirconium(IV) Phosphate: Static Study of 225Ac Sorption in an Acidic Environment and Its Kinetic Sorption Study Using natEu as a Model System for 225Ac. Materials. 2023; 16(17):5732. https://doi.org/10.3390/ma16175732
Chicago/Turabian StyleOndrák, Lukáš, Kateřina Ondrák Fialová, Martin Vlk, Karel Štamberg, Frank Bruchertseifer, Alfred Morgenstern, and Ján Kozempel. 2023. "α-Zirconium(IV) Phosphate: Static Study of 225Ac Sorption in an Acidic Environment and Its Kinetic Sorption Study Using natEu as a Model System for 225Ac" Materials 16, no. 17: 5732. https://doi.org/10.3390/ma16175732
APA StyleOndrák, L., Ondrák Fialová, K., Vlk, M., Štamberg, K., Bruchertseifer, F., Morgenstern, A., & Kozempel, J. (2023). α-Zirconium(IV) Phosphate: Static Study of 225Ac Sorption in an Acidic Environment and Its Kinetic Sorption Study Using natEu as a Model System for 225Ac. Materials, 16(17), 5732. https://doi.org/10.3390/ma16175732







