Selective Oxidation of Cyclohexanone to Adipic Acid Using Molecular Oxygen in the Presence of Alkyl Nitrites and Transition Metals as Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedure for Catalytic Oxidation under Pressure in 100 mL Volume
2.3. General Procedure for Catalytic Oxidation under Pressure in 600 mL Volume
2.4. General Procedure for Catalytic Oxidation under Atmospheric Pressure
2.5. Analytical Methods
3. Results
3.1. Oxidation of C-ON with O2 to AA Using the Co2+/Mn2+/R-ONO System
3.2. Oxidation of Cyclohexanol, C-ON, or Their Mixtures with O2 Using the IPN/Co(acac)2/Mn(acac)2 System
3.3. Study of the Oxidation of Cyclic Ketones Using the IPN/Co(acac)2/Mn(acac)2 System
3.4. C-ON Oxidation with Air Using the IPN/Co(acac)2/Mn(acac)2 System
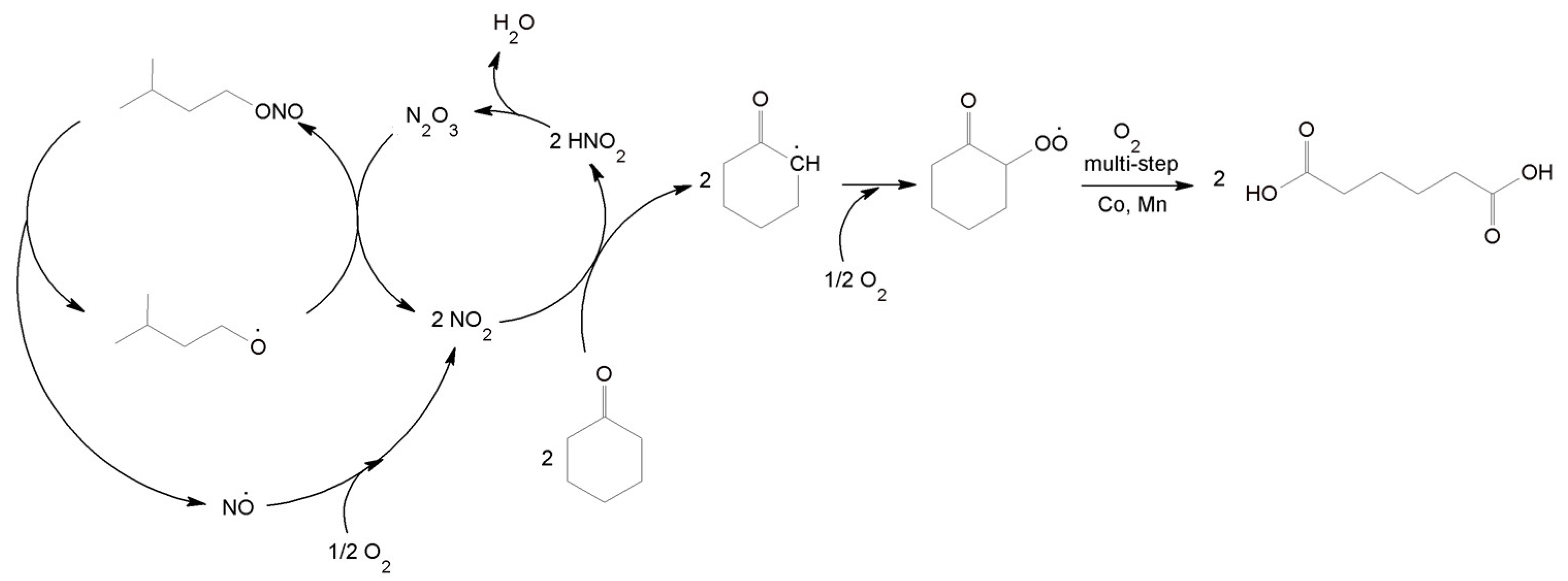
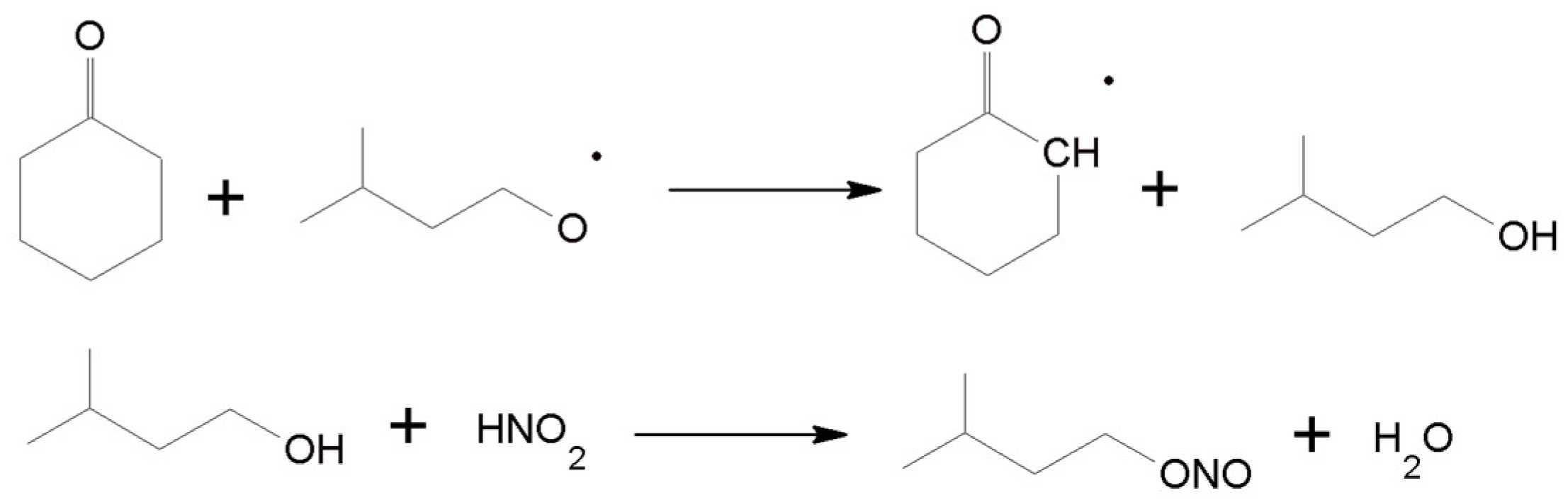
3.5. Proposed Mechanism for the Oxidation of C-ON with O2 Using the IPN/Co(acac)2/Mn(acac)2 System
4. Conclusions
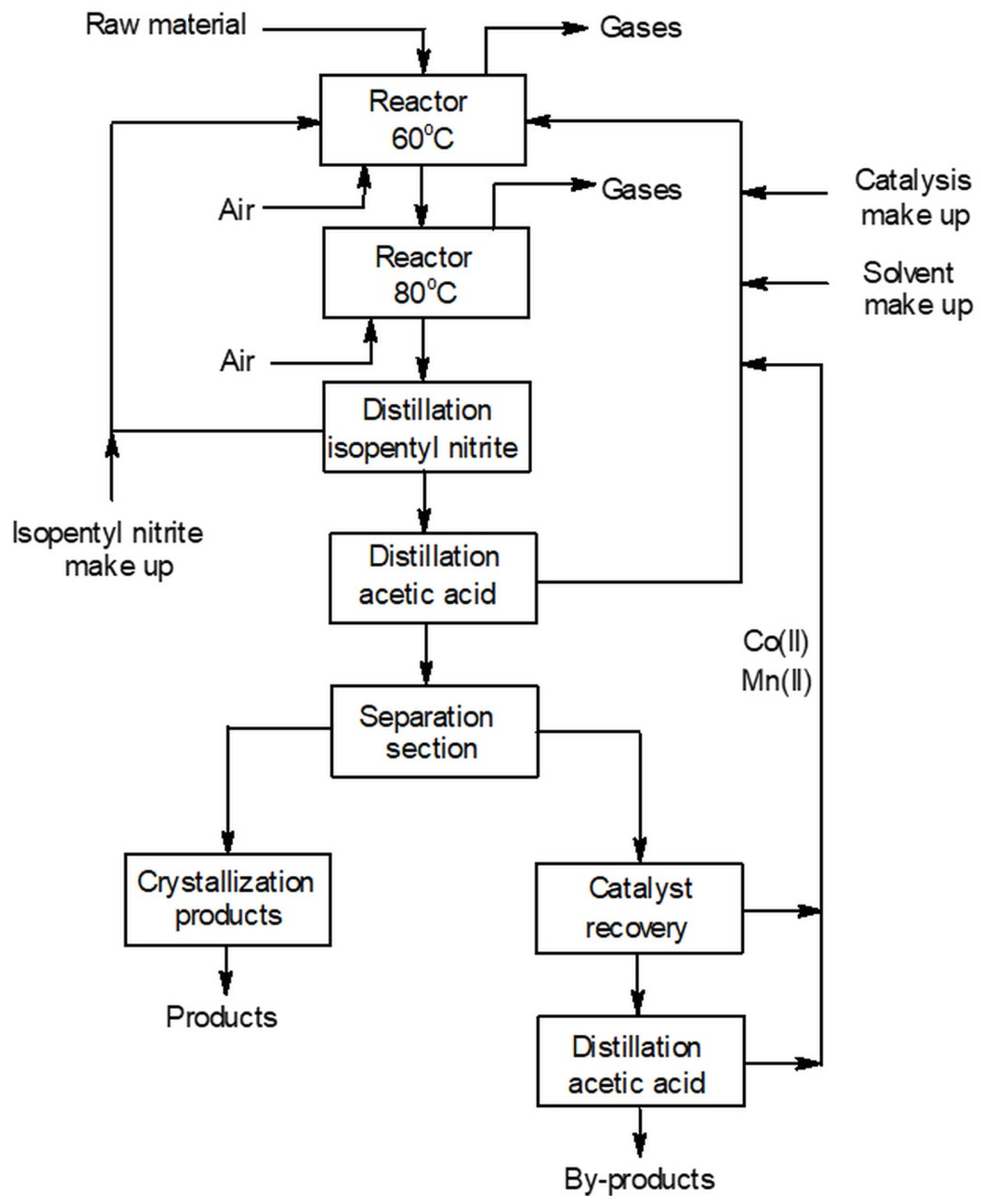
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rios, J.; Lebeau, J.; Yang, T.; Li, S.; Lynch, M.D. A critical review on the progress and challenges to a more sustainable, cost competitive synthesis of adipic acid. Green Chem. 2021, 23, 3172–3190. [Google Scholar]
- Lisicki, D.; Orlińska, B. Oxidation of cyclic ketones to dicarboxylic acids. Pol. J. Chem. Tech. 2018, 20, 102–107. [Google Scholar]
- Cornils, B.; Lappe, P. Dicarboxylic acids, aliphatic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Research and Markets. The World’s Largest Market Research Store. Available online: www.researchandmarkets.com/reports/3641969/global-markets-for-adipic-acid#relc2 (accessed on 30 June 2023).
- Lisicki, D.; Dobras, G.; Orlińska, B.; Zawadiak, J. Metody otrzymywania kwasu adypinowego o potencjalnym zastosowaniu przemysłowym. Przem. Chem. 2017, 96, 1485–1489. [Google Scholar]
- Abutaleb, A.; Ali, M.A. A comprehensive and updated review of studies on the oxidation of cyclohexane to produce ketone-alcohol (KA) oil. Rev. Chem. Eng. 2021, 38, 769–797. [Google Scholar]
- Li, G.; Liu, S.; Dou, X.; Wei, H.; Shang, M.; Luo, Z.H.; Su, Y. Synthesis of Adipic Acid through Oxidation of K/A oil and Its Kinetic Study in a Microreactor System. Reaction Engineering. AIChE J. 2020, 66, e16289. [Google Scholar]
- Musser, M.T. Adipic acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- UN Environment Programme. Drawing down N2O to Protect Climate and the Ozone Layer a UNEP Synthesis Report. Available online: https://wedocs.unep.org/handle/20.500.11822/8489 (accessed on 30 June 2023).
- Task Force on National Greenhouse Gas Inventories. Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories, Chapter 3 Industrial Processes. Available online: https://www.ipcc-nggip.iges.or.jp/public/gp/english/8489 (accessed on 30 June 2023).
- Artz, J.; Palkovits, S.; Palkovits, R.; Creusen, G.; Holzha, F.J. Producing Widespread Monomers from Biomass Using Economical Carbon and Ruthenium—Titanium Dioxide Electrocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 17108–17113. [Google Scholar]
- Hatakeyama, K.; Nakagawa, Y.; Tamura, M.; Tomishige, K. Efficient production of adipic acid from 2-methoxycyclohexanone by aerobic oxidation with a phosphotungstic acid catalyst. Green Chem. 2020, 22, 4962–4974. [Google Scholar]
- Corona, A.; Biddy, M.J.; Vardon, D.R.; Birkved, M.; Hauschild, M.Z.; Beckham, G.T. Life cycle assessment of adipic acid production from lignin. Green Chem. 2018, 20, 3857–3866. [Google Scholar]
- Pyo, S.H.; Park, J.H.; Srebny, B.; Hatti-Kaul, R. A sustainable synthetic route for biobased 6-hydroxyhexanoic acid, adipic acid and ε-caprolactone by integrating bio- and chemical catalysis. Green Chem. 2020, 22, 4450. [Google Scholar]
- Rauen, A.L.; Weineltb, F.; Waldvogel, S.R. Sustainable electroorganic synthesis of lignin-derived dicarboxylic acids. Green Chem. 2020, 22, 5956–5960. [Google Scholar]
- Pellis, A.; Byrne, F.P.; Sherwood, J.; Vastano, M.; Comerford, J.W.; Farmer, T.J. Safer bio-based solvents to replace toluene and tetrahydrofuran for the biocatalyzed synthesis of polyesters. Green Chem. 2019, 21, 1686–1694. [Google Scholar]
- Montazeri, M.; Zaimes, G.G.; Khanna, V.; Eckelman, M.J. Meta-Analysis of Life Cycle Energy and Greenhouse Gas Emissions for Priority Biobased Chemicals. ACS Sustain. Chem. Eng. 2016, 4, 6443–6454. [Google Scholar]
- Hwang, K.C.; Sagadevan, A. One-pot room-temperature conversion of cyclohexane to adipic acid by ozone and UV light. Science 2014, 346, 1495–1498. [Google Scholar]
- Matsumoto, Y.; Kuriyama, M.; Yamamoto, K.; Nashida, K.; Onomura, O. Metal-free synthesis of adipic acid via organocatalytic direct oxidation of cyclohexane under ambient temperature and pressure. Org. Process. Res. Dev. 2018, 22, 1312–1317. [Google Scholar]
- Rao, D.G.; Tirukkoyllur, R.S. Liquid-phase oxidation of cyclohexane to adipic acid in a single stage. Ind. Eng. Chem. Process Des. Dev. 1986, 25, 299–304. [Google Scholar]
- Lisicki, D.; Orlińska, B.; Marek, A.A.; Bińczak, J.; Dziuba, K.; Martyniuk, T. Oxidation of Cyclohexane/Cyclohexanone Mixture with Oxygen as Alternative Method of Adipic Acid Synthesis. Materials 2023, 16, 298. [Google Scholar]
- Kulsrestha, G.N.; Saxena, M.P.; Gupta, A.K.; Goyal, H.B.; Prasad, R.; Prasada, R.T.S.R.; Patel, P.D. Catalyst and a Process for Preparing Carboxylic Acids Using the Catalyst. U.S. Patent US5547905, 20 August 1996. [Google Scholar]
- Liang, F.; Zhong, W.; Xiang, L.; Mao, L.; Xu, Q.; Kirk, S.R.; Yin, D. Synergistic hydrogen atom transfer with the active role of solvent: Preferred one-step aerobic oxidation of cyclohexane to adipic acid by N-hydroxyphthalimide. J. Catal. 2019, 378, 256–269. [Google Scholar]
- Ishii, Y.; Iwahama, T.; Sakaguchi, S.; Nakayama, K.; Nishiyama, Y. Alkane oxidation with molecular oxygen using a new efficient catalytic system: N-hydroxyphthalimide (NHPI) Combined with Co(acac)n (n = 2 or 3). J. Org. Chem. 1996, 61, 4520–4526. [Google Scholar]
- Ishii, Y. A novel catalysis of N-hydroxyphthalimide (NHPI) combined with Co(acac)(n) (n=2 or 3) in the oxidation of organic substrates with molecular oxygen. J. Mol. Catal. A Chem. 1997, 117, 123–137. [Google Scholar]
- Iwahama, T.; Syojyo, K.; Sakaguchi, S.; Ishii, Y. Direct conversion of cyclohexane into adipic acid with molecular oxygen catalyzed by N-hydroxyphthalimide combined with Mn(acac)2 and Co(OAc)2. Org. Process Res. Dev. 1998, 2, 255–260. [Google Scholar]
- Ishii, Y.; Sakaguchi, S. A new strategy for alkane oxidation with O2 using N-hydroxyphthalimide (NHPI) as a radical catalyst. Catal. Surv. Jpn. 1999, 3, 27–35. [Google Scholar]
- Ishii, Y.; Sakaguchi, S.; Iwahama, T. Innovation of hydrocarbon oxidation with molecular oxygen and Related Reactions. Adv. Synth. Catal. 2001, 343, 393–427. [Google Scholar]
- Ishii, Y.; Sakaguchi, S. Recent progress in aerobic oxidation of hydrocarbons by N-hydroxyimides. Catal. Today 2006, 117, 105–113. [Google Scholar]
- Schulz, J.G.D.; Onopchenko, A. Process for Converting Cyclohexane to Adipic Acid. U.S. Patent US4263453, 21 April 1981. [Google Scholar]
- Thomas, J.M.; Raja, R. Catalytically active centres in porous oxides: Design and performance of highly selective new catalysts. Chem. Commun. 2001, 8, 675–687. [Google Scholar]
- Mikami, Y.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Superior performance of Fe(BTC) with respect to other metal-containing solids in the N-hydroxyphthalimide-promoted heterogeneous aerobic oxidation of cycloalkanes. ChemCatChem 2013, 5, 1964–1970. [Google Scholar]
- Yuan, Y.; Ji, H.; Chen, Y.; Han, Y.; Song, X.; She, Y.; Zhong, R. Oxidation of cyclohexane to adipic acid using Fe-porphyrin as a biomimetic catalyst. Org. Process Res. Dev. 2004, 8, 418–420. [Google Scholar]
- Sawatari, N.; Yokota, T.; Sakaguchi, S.; Ishii, Y. Alkane oxidation with air catalyzed by lipophilic N-hydroxyphthalimides without any solvent. J. Org. Chem. 2001, 66, 7889–7891. [Google Scholar]
- Chavan, S.A.; Srinivas, D.; Ratnasamy, P. Oxidation of cyclohexane, cyclohexanone, and cyclohexanol to adipic acid by a non-HNO3 Route over Co/Mn cluster complexes. J. Catal. 2002, 212, 39–45. [Google Scholar]
- Wang, T.; She, Y.; Fu, H.; Li, H. Selective cyclohexane oxidation catalyzed by manganese porphyrins and co-catalysts. Catal. Today 2016, 264, 3–8. [Google Scholar]
- Kerry Yu, K.M.; Abutaki, A.; Zhou, Y.; Yue, B.; He, H.Y.; Tsang, S.C. Selective oxidation of cyclohexane in supercritical carbon dioxide. Catal Lett. 2007, 113, 115–119. [Google Scholar]
- Kamath, S.S.; Chandalia, S.B. Liquid phase oxidation of cyclohexanone to adipic acid by air in acetic acid solution. J. Appl. Chem. Biotechnol. 1973, 23, 469–478. [Google Scholar]
- Flemming, W.; Speer, W. Catalytic Oxidation of Ketones. U.S. Patent US2005183, 18 June 1935. [Google Scholar]
- Flemming, W. Catalytic Oxidation of Ketones. U.S. Patent US2299013, 13 October 1942. [Google Scholar]
- Flemming, W. Production of Dibasic Acids. U.S. Patent US2452741, 2 November 1948. [Google Scholar]
- Dassel, M.W.; Decoster, D.C.; Rostami, A.M.; Aldrich, S.M.; Vassiliou, E. Methods for Preparing Dibasic Acids. U.S. Patent US5922908, 13 July 1999. [Google Scholar]
- Longley, K.D.; Sprowl, D. Process of Producing Adipic Acid. U.S. Patent US3869508, 4 March 1975. [Google Scholar]
- Zou, G.; Zhong, W.; Mao, L.; Xu, Q.; Xiao, J.; Yin, D.; Xiao, Z.; Kirk, S.R.; Shu, T. A non-nitric acid method of adipic acid synthesis: Organic solvent- and promoter-free oxidation of cyclohexanone with oxygen over hollow-structured Mn/TS-1 catalysts. Green Chem. 2015, 17, 1884–1892. [Google Scholar]
- Besson, M.; Gauthard, F.; Horvath, B.; Gallezot, P. Catalytic oxidation with air of cyclohexanone to dicarboxylic acids on synthetic carbons. Effect of supported metals and solvents. J. Phys. Chem. B 2005, 109, 2461–2467. [Google Scholar]
- Crezee, E.; Barendregt, A.; Kapteijn, F.; Moulijn, J. Carbon coated monolithic catalysts in the selective oxidation of cyclohexanone. Catal. Today 2001, 69, 283–290. [Google Scholar]
- Pigamo, A.; Besson, M.; Black, B.; Gallezot, P.; Blackburn, A.; Kozynchenko, O.; Tennison, S.; Crezee, E.; Kapteijn, F. Effect of oxygen functional groups on synthetic carbons on liquid phase oxidation of cyclohexanone. Carbon 2002, 40, 1267–1278. [Google Scholar]
- Bhanja, P.; Ghosh, K.; Islam, S.S.; Patra, A.K.; Islam, S.M.; Bhaumik, A. New Hybrid Iron Phosphonate Material as an Efficient Catalyst for the Synthesis of Adipic Acid in Air and Water. ACS Sustain. Chem. Eng. 2016, 4, 7147–7157. [Google Scholar]
- Shen, H.C.; Weng, H.S. Liquid-phase oxidation of cyclohexane to dibasic acids with immobilized cobalt catalyst. Ind. Eng. Chem. Res. 1988, 27, 2254–2260. [Google Scholar]
- Rae, D.G.; Raghunathan, T.S. Oxidation of cyclohexanone to adipic acid with a cobalt acetate/oxygen/acetic acid system. J. Chem. Technol. Biotechnol. Chem. Technol. 1984, 34, 381–386. [Google Scholar]
- Cavani, F.; Ferroni, L.; Frattini, A.; Lucarelli, C.; Mazzini, A.; Raabova, K.; Alini, S.; Accorinti, P.; Babini, P. Evidence for the presence of alternative mechanisms in the oxidation of cyclohexanone to adipic acid with oxygen, catalysed by Keggin polyoxometalates. Appl. Catal. A Gen. 2011, 391, 118–124. [Google Scholar]
- Atlamsani, A.; Brégeault, J.M.; Ziyad, M. Oxidation of 2-methylcyclohexanone and cyclohexanone by dioxygen catalyzed by vanadium-containing heteropolyanions. J. Org. Chem. 1993, 58, 5663–5665. [Google Scholar]
- Shimizu, A.; Tanaka, K.; Ogawa, H.; Matsuoka, Y.; Fujimori, M.; Nagamori, Y.; Hamachi, H.; Kimura, K. An industrial process for adipic acid production by the liquid-phase oxidation of cyclohexanone with molecular oxygen. Bull. Chem. Soc. Jpn. 2003, 76, 1991–2001. [Google Scholar]
- Jie, X.; Xiuquan, J.; Jiping, M.; Xiaofang, L.; Jin, G.; Yong-Ming, X.; Fei, X. Method for Preparation of Adipic Acid by Oxidizing Cyclohexanone. C.N. Patent CN108084012, 25 May 2018. [Google Scholar]
- She, M.; Gu, R.; Meng, D.; Yang, H.; Wen, Y.; Qian, X.; Guo, X.; Dang, W. Nanosheets of Ni-SAPO-34 Molecular Sieve for Selective Oxidation of Cyclohexanone to Adipic Acid. Chem. Eur. J. 2022, 28, e202200696. [Google Scholar]
- Suzuki, Y.; Harada, E.; Nakamaru, K.; Takeda, Y.; Sano, M.; Hashimoto, K.; Miyake, T. Direct oxidation of cycloalkanes with molecular oxygen to dicarboxylic acids using isoamyl nitrite. J. Mol. Catal. A Chem. 2007, 276, 1–7. [Google Scholar]
- Ning, X.; Wang, M.; Yao, C.; Chen, X.; Kang, Y. tert-Butyl Nitrite: Organic Redox Cocatalyst for Aerobic Aldehyde-Selective Wacker—Tsuji Oxidation. Org. Lett. 2016, 18, 2700–2703. [Google Scholar]
- Hu, K.; Ning, X.; Qu, J.; Kang, Y. Tuning Regioselectivity of Wacker Oxidation in One Catalytic System: Small Change Makes Big Step. J. Org. Chem. 2018, 83, 11327–11332. [Google Scholar]
- Links, D.A. Green Chemistry Tert-butyl nitrite: A metal-free radical initiator for aerobic cleavage of benzylic C=C bonds in compressed carbon dioxide†. Green Chem. 2011, 13, 541–544. [Google Scholar]
- Shen, Z.; Dai, J.; Xiong, J.; He, X.; Mo, W.; Hu, B. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)/tert-Butyl Nitrite/Oxygen: A Versatile Catalytic Oxidation System. Adv. Synth. Catal. 2011, 353, 3031–3038. [Google Scholar]
- Xie, Y.; Mo, W.; Xu, D.; Shen, Z.; Sun, N.; Hu, B.; Hu, X. Efficient NO Equivalent for Activation of Molecular Oxygen and Its Applications in Transition-Metal-Free Catalytic Aerobic Alcohol Oxidation alcohol oxidation. J. Org. Chem. 2007, 72, 4288–4291. [Google Scholar]
- He, X.; Shen, Z.; Mo, W.; Sun, N.; Hu, B. TEMPO-tert-Butyl Nitrite: An Efficient Catalytic System for Aerobic Oxidation of Alcohols. Adv. Synth. Catal. 2009, 351, 89–92. [Google Scholar]
- Dong, Y.; Zhao, X.; Liu, R. 4-OH-TEMPO/TCQ/TBN/HCl: A Metal-Free Catalytic System for Aerobic Oxidation of Alcohols under Mild Conditions. Chin. J. Chem. 2015, 33, 1019–1023. [Google Scholar]
- Karimi, B.; Farhangi, E. A Highly Recyclable Magnetic Core-Shell Nanoparticle-Supported TEMPO catalyst for Efficient Metal- and Halogen-Free Aerobic Oxidation of Alcohols in Water. Chem. Eur. J. 2011, 17, 6056–6060. [Google Scholar]
- Links, D.A. SBA-15-functionalized TEMPO confined ionic liquid: An efficient catalyst system for transition-metal-free aerobic oxidation of alcohols with improved selectivity. Org. Biomol. Chem. 2011, 9, 4194–4198. [Google Scholar]
- Walsh, K.; Sneddon, H.F.; Moody, C.J. Solar Photochemical Oxidations of Benzylic and Allylic Alcohols Using Catalytic Organo-oxidation with DDQ: Application to Lignin Models. Org. Lett. 2014, 16, 5224–5227. [Google Scholar]
- Ma, J.; Hong, C.; Wan, Y.; Li, M.; Hu, X.; Mo, W.; Hu, B.; Sun, N.; Jin, L.; Shen, Z. Aerobic oxidation of secondary alcohols in water with ABNO/tert-butyl nitrite/KPF 6 catalytic system. Tetrahedron Lett. 2017, 58, 652–657. [Google Scholar]
- Hu, Y.; Chen, L.; Li, B. NHPI/tert-butyl nitrite: A highly efficient metal-free catalytic system for aerobic oxidation of alcohols to carbonyl compounds using molecular oxygen as the terminal oxidant. Catal. Commun. 2016, 83, 82–87. [Google Scholar]
- Shibuya, M.; Furukawa, K.; Yamamoto, Y. Selective Aerobic Oxidation of Primary Alcohols to Aldehydes. Synlett 2017, 28, 1554–1557. [Google Scholar]
- Gunchenko, P.A.; Li, J.; Liu, B.; Chen, H.; Pashenko, A.E.; Bakhonsky, V.V.; Zhuk, T.S.; Fokin, A.A. Aerobic oxidations with N-hydroxyphthalimide in trifluoroacetic acid. Mol. Catal. 2018, 447, 72–79. [Google Scholar]
- Market Research Future. Available online: https://www.marketresearchfuture.com/reports/adipic-acid-market-5479 (accessed on 30 June 2023).

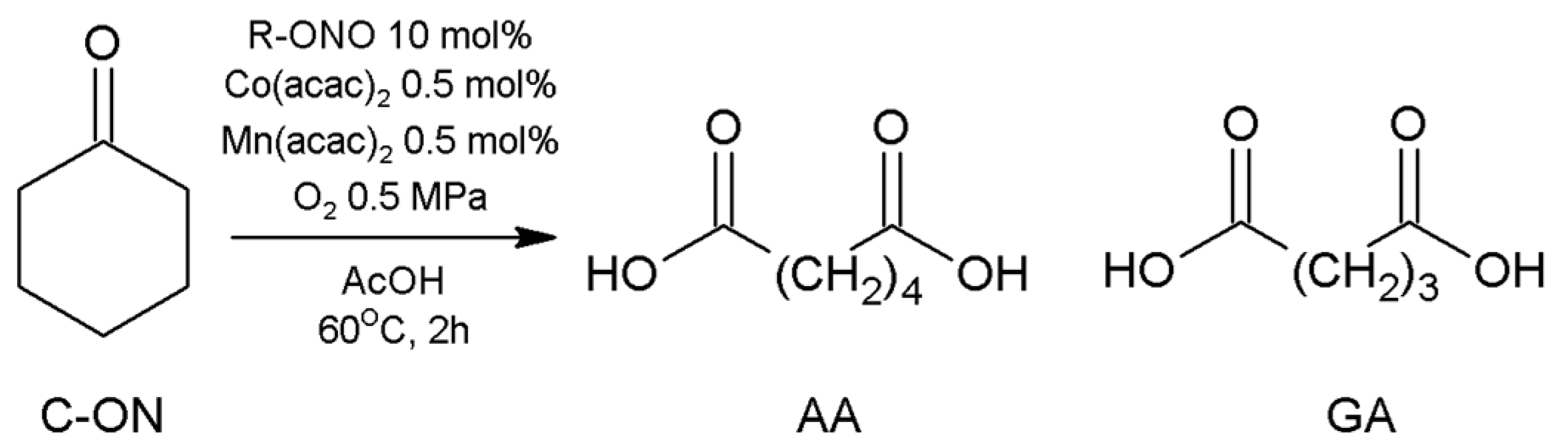
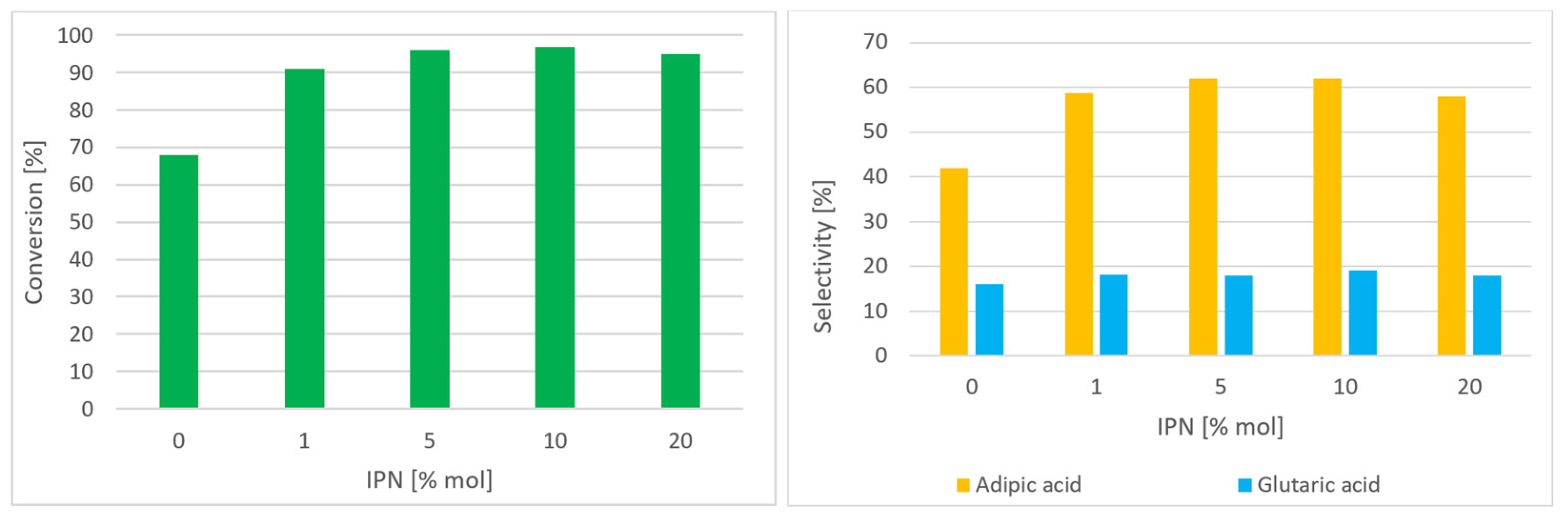
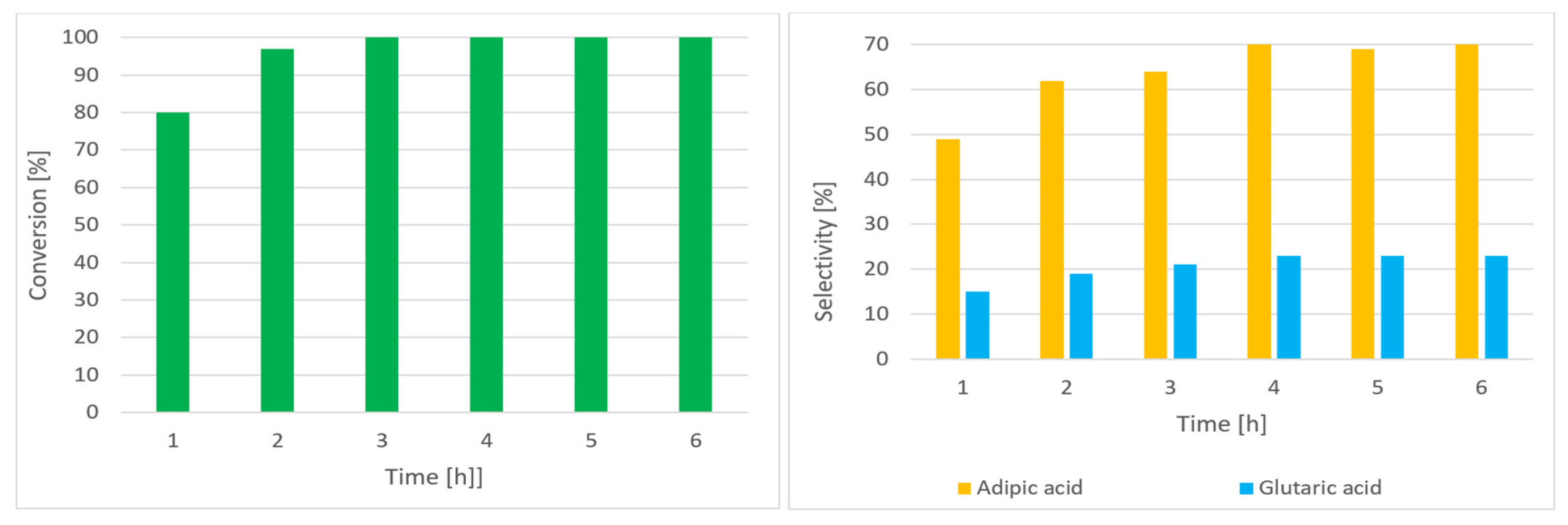
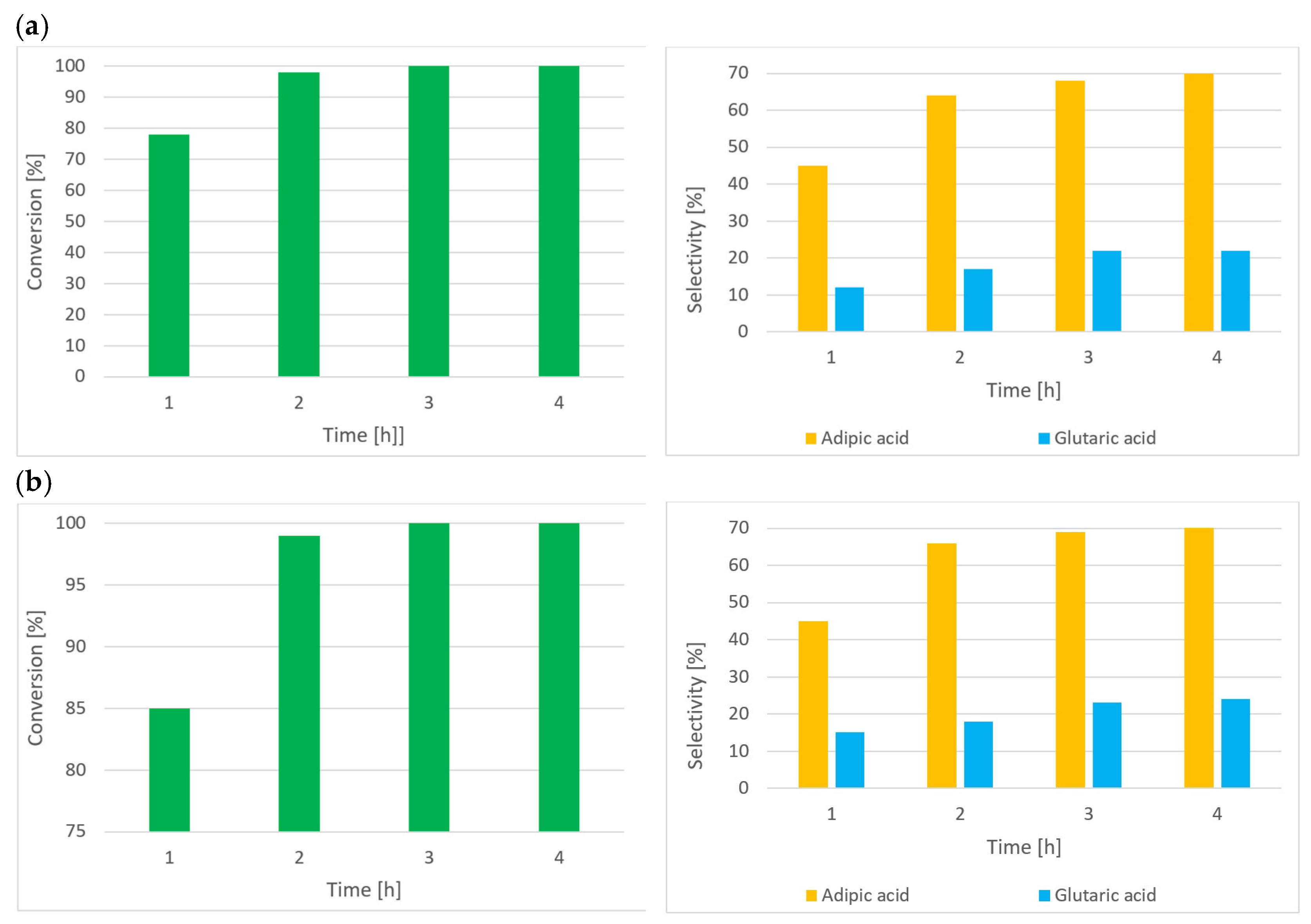

| Entry | Catalysis System | Solvent | Temp. (°C) | Pressure (MPa) | Time (h) | Conv. (%) a | Sel. (%) b | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Mn-HTS | - | 90 | 0.6 | 9 | 68 | 93 | [44] |
| 2 | Pt/carbon | H2O | 140 | 5.0 | - | 100 | 39 | [45] |
| 3 | Pt/carbon/monolith | H2O | 140 | 5.0 | 1 | 100 | 21 | [46] |
| 4 | Modified carbon material | H2O | 140 | 5.0 | 6 | 100 | 33 | [47] |
| 5 | Hybrid iron phosphonate material (FePO-1–2) | H2O | 75 | 0.1 | 10 | 96 | 72 | [48] |
| 6 | Mn-HTS | AcOH | 90 | 0.6 | 9 | 91 | 86 | [44] |
| 7 | NHPI/Mn(acac)2 | AcOH | 100 | 0.1 | 6 | 99 | 64 | [26] |
| 8 | Co(OAc)2/NaBr | AcOH | 80 | 0.1 | 1 | - | 36 | [49] |
| 9 | Co(OAc)2 | AcOH | 105 | 0.5 | - | 98 | 71 | [50] |
| 10 | POM Keggin | AcOH/H2O | 70 | 1.0 | 6 | 18 | 65 | [51] |
| 11 | Cluster Co/Mn/MEK [c] | AcOH/H2O | 100 | 3.8 | 8 | 98 | 87 | [35] |
| 12 | H5[PMo10V2O40]·30H2O | AcOH/H2O | 70 | 0.1 | 7 | 99 | 40 | [52] |
| 13 | H7[PMo8V4O40]·12H2O | AcOH/H2O | 70 | 0.1 | 7 | 99 | 51 | [52] |
| 14 | Mn(OAc)2/Co(OAc)2/p-TS [d] | AcOH/H2O | 70 | 0.1 | 5 | 97 | 78 | [53] |
| 15 | Mn(NO3)2/Co(NO3)2 | AcOH/H2O | 40 | 0.1 | - | 98 | 93 | [54] |
| 16 | Nanosheets of Ni-SAPO-34 molecular Sieve | acetone | 135 | 1.5 | 20 | 30 | 87 | [55] |
| Entry | Initiator | Mn2+ | Co2+ | Conv. (%) a | Sel. AA (%) | Sel. GA (%) |
|---|---|---|---|---|---|---|
| 1 | - | 0.5 | 0.5 | 68 | 42 | 16 |
| 2 | IPN | - | - | 38 | 45 | 10 |
| 3 | IPN | 0.5 | - | 94 | 56 | 13 |
| 4 | IPN | - | 0.5 | 66 | 43 | 12 |
| 5 | IPN | 0.5 | 0.5 | 97 | 62 | 19 |
| 6 | PN | 0.5 | 0.5 | 96 | 43 | 14 |
| 7 | TBN | 0.5 | 0.5 | 89 | 51 | 16 |
| 8 | HNO3 | 0.5 | 0.5 | 96 | 54 | 18 |
| 9 b | IPN | 0.5 | 0.5 | 95 | 59 | 19 |
| 10 c | IPN | 0.5 | 0.5 | 92 | 58 | 18 |
| Entry | Solvent | Temp. (°C) | Pressure (MPa) | Time (h) | Conv. (%) a | Sel. AA (%) | Sel. GA (%) |
|---|---|---|---|---|---|---|---|
| 1 | AcOH | 40 | 0.5 | 2 | 52 | 31 | 10 |
| 2 | AcOH | 50 | 0.5 | 2 | 91 | 41 | 11 |
| 3 | AcOH | 60 | 0.5 | 2 | 97 | 62 | 19 |
| 4 | AcOH | 80 | 0.5 | 2 | 100 | 68 | 18 |
| 5 | AcOH | 100 | 0.5 | 2 | 99 | 59 | 21 |
| 6 b | AcOH | 60–80 | 0.5 | 2 | 99 | 67 | 23 |
| 7 | AcOH | 60 | 0.1 | 2 | 83 | 61 | 17 |
| 8 | AcOH | 60 | 1.0 | 2 | 99 | 63 | 21 |
| 9 | AcOH | 60 | 1.5 | 2 | 97 | 64 | 21 |
| 10 c | - | 100 | 0.5 | 2 | 35 | 30 | 8 |
| 11 | MeCN | 100 | 0.5 | 2 | 99 | 31 | 16 |
| 12 | PhCN | 100 | 0.5 | 2 | 99 | 64 | 20 |
| 13 | AcOH | 40 | 0.5 | 2 | 52 | 31 | 10 |
| Entry | Raw Material | Time (h) | Conv. (%) a | Sel. AA (%) | Sel. C-ON (%) | Sel. GA (%) |
|---|---|---|---|---|---|---|
| 1 | C-ON | 2 | 97 | 62 | - | 19 |
| 2 | C-ON | 6 | 100 | 70 | - | 23 |
| 3 | C-OL | 2 | 95 | 11 | 47 | 5 |
| 4 | C-OL | 6 | 98 | 20 | 51 | 6 |
| 5 b | C-OL or C-ON | 2 | 100 | 58 | - | 17 |
| 6 b | C-OL or C-ON | 6 | 100 | 60 | - | 18 |
| Entry | Temp. (°C) | IPN (% mol) | Raw Material | Conv. (%) a | Sel. Main Product (%) | Sel. By-Product (%) |
|---|---|---|---|---|---|---|
| Cyclopentanone | Glutaric acid | Succinic acid | ||||
| 1 | 80 | - | 100 | 33 | 9 | |
| 2 | 80 | 10 | 100 | 47 | 11 | |
| 3 | 60 | 10 | 93 | 20 | 3 | |
| Cyclohexanone | Adipic acid | Glutaric acid | ||||
| 4 | 80 | - | 100 | 62 | 17 | |
| 5 | 80 | 10 | 100 | 70 | 18 | |
| 6 | 60 | 10 | 100 | 71 | 23 | |
| Cycloheptanone | Pimelic acid | Adipic acid | ||||
| 7 | 80 | - | 87 | 52 | 15 | |
| 8 | 80 | 10 | 99 | 54 | 18 | |
| 9 | 60 | 10 | 47 | 15 | 3 | |
| Cyclooktanone | Suberic acid | Pimelic acid | ||||
| 10 | 80 | - | 98 | 62 | 14 | |
| 11 | 80 | 10 | 100 | 70 | 12 | |
| 12 | 60 | 10 | 97 | 54 | 18 | |
| Cyclododecanone | Dodecane-1,12-dioic | Undecane-1,11-dioic acid | ||||
| 13 | 80 | - | 75 | 44 | 10 | |
| 14 | 80 | 10 | 100 | 59 | 9 | |
| 15 | 60 | 10 | 100 | 53 | 3 | |
| 2-Methyl cyclohexanone | 6-Oxyheptanoic acid | Glutaric acid | ||||
| 16 | 80 | - | 50 | 44 | 16 | |
| 17 | 80 | 10 | 100 | 68 | 14 | |
| 18 | 60 | 10 | 100 | 69 | 14 | |
| 3-Methyl cyclohexanone | 3-Methyladipic acid | 2-Methyladipic acid | ||||
| 19 | 80 | - | 69 | 45 | 18 | |
| 20 | 80 | 10 | 100 | 64 | 16 | |
| 21 | 60 | 10 | 100 | 59 | 16 | |
| 4-Methyl cyclohexanone | 3-Methyladipic acid | 2-Methylglutaric acid | ||||
| 22 | 80 | - | 68 | 61 | 9 | |
| 23 | 80 | 10 | 100 | 88 | 10 | |
| 24 | 60 | 10 | 99 | 75 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisicki, D.; Orlińska, B.; Martyniuk, T.; Dziuba, K.; Bińczak, J. Selective Oxidation of Cyclohexanone to Adipic Acid Using Molecular Oxygen in the Presence of Alkyl Nitrites and Transition Metals as Catalysts. Materials 2023, 16, 5722. https://doi.org/10.3390/ma16165722
Lisicki D, Orlińska B, Martyniuk T, Dziuba K, Bińczak J. Selective Oxidation of Cyclohexanone to Adipic Acid Using Molecular Oxygen in the Presence of Alkyl Nitrites and Transition Metals as Catalysts. Materials. 2023; 16(16):5722. https://doi.org/10.3390/ma16165722
Chicago/Turabian StyleLisicki, Dawid, Beata Orlińska, Tomasz Martyniuk, Krzysztof Dziuba, and Jakub Bińczak. 2023. "Selective Oxidation of Cyclohexanone to Adipic Acid Using Molecular Oxygen in the Presence of Alkyl Nitrites and Transition Metals as Catalysts" Materials 16, no. 16: 5722. https://doi.org/10.3390/ma16165722
APA StyleLisicki, D., Orlińska, B., Martyniuk, T., Dziuba, K., & Bińczak, J. (2023). Selective Oxidation of Cyclohexanone to Adipic Acid Using Molecular Oxygen in the Presence of Alkyl Nitrites and Transition Metals as Catalysts. Materials, 16(16), 5722. https://doi.org/10.3390/ma16165722





