Three-Dimensional-Printed Photocatalytic Sponges Decorated with Mn-Doped ZnO Nanoparticles
Abstract
:1. Introduction
2. Experimental Part
2.1. Fabrication of 3D-Printed Sponges
2.2. Synthesis of Photocatalytic Nanostructures—Decoration of HDPE Sponges
2.3. Characterization and Photocatalytic Experiments
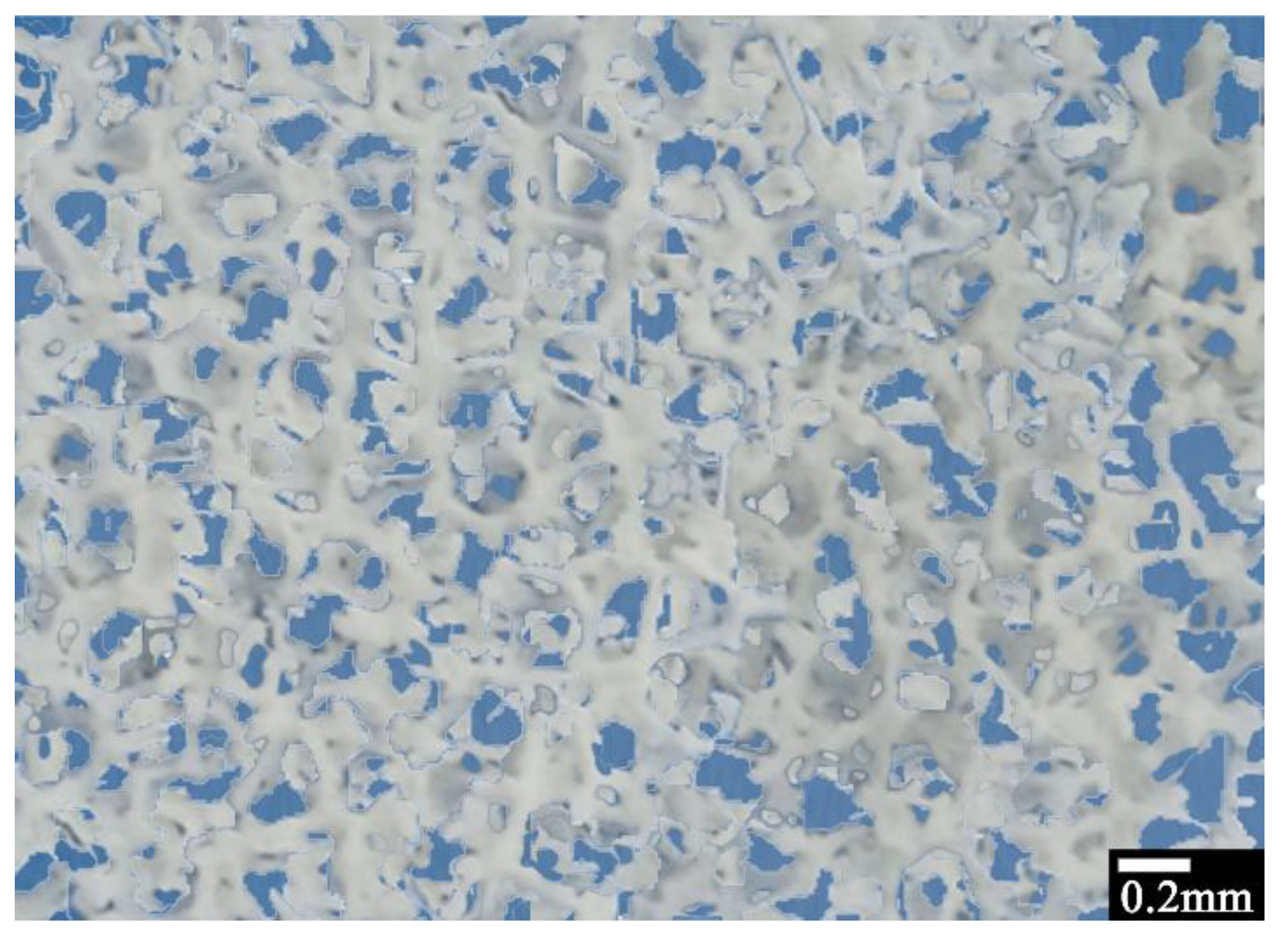
2.3.1. Optical and Scanning Electron Microscopy
2.3.2. X-ray Diffraction X-ray
2.3.3. Raman Spectroscopy Studies
2.3.4. Photocatalytic Efficiency Measurements
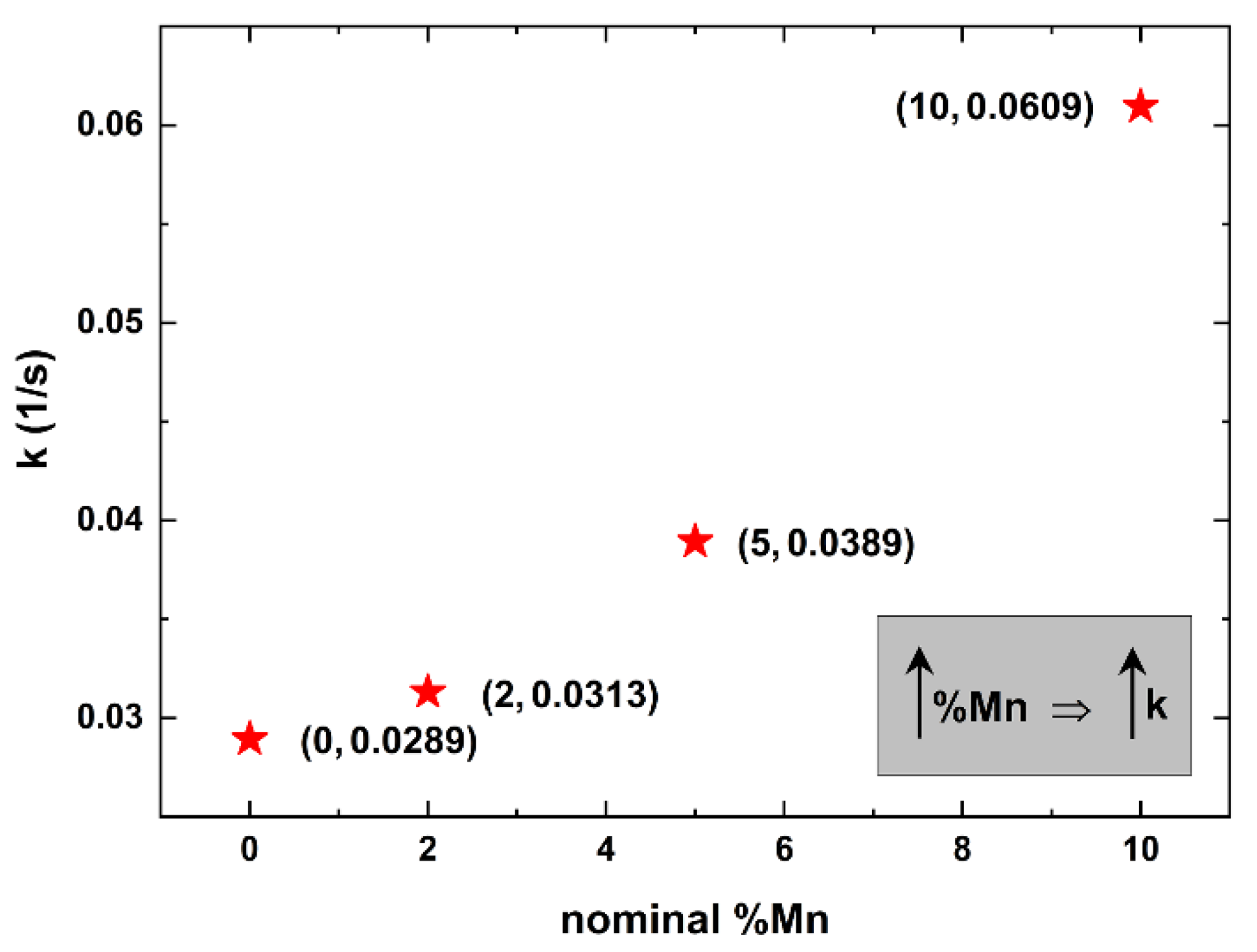
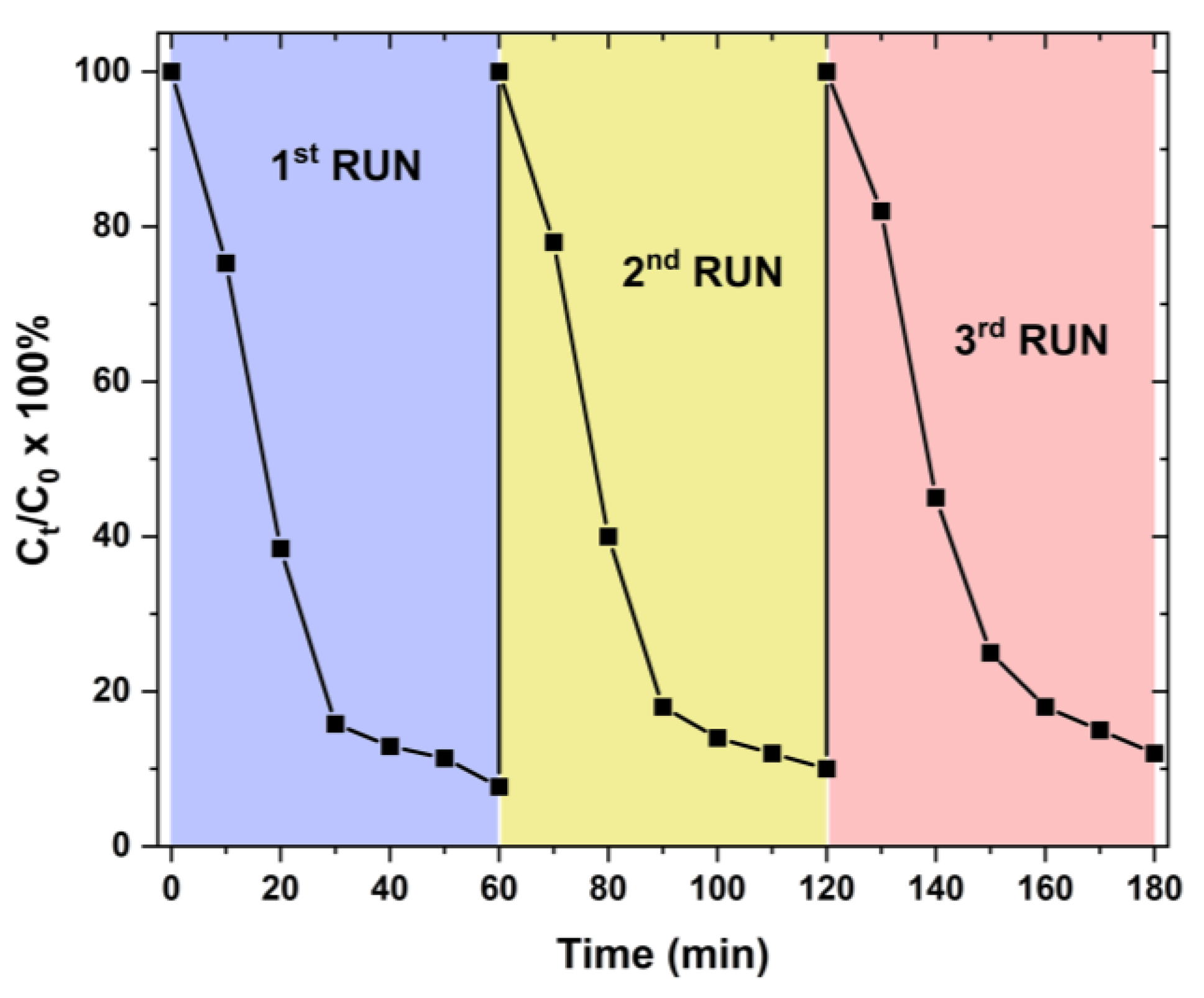
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, S.K.; Tyagi, R.; Nandi, B.K. Removal of Residual Surfactants from Laundry Wastewater: A Review. J. Dispers. Sci. Technol. 2013, 34, 1526–1534. [Google Scholar] [CrossRef]
- Singh, J.; Yadav, P.; Pal, A.K.; Mishra, V. Water Pollutants: Origin and Status. In Sensors in Water Pollutants Monitoring: Role of Material; Pooja, D., Kumar, P., Singh, P., Patil, S., Eds.; Advanced Functional Materials and Sensors; Springer: Singapore, 2020; pp. 5–20. [Google Scholar] [CrossRef]
- Sevastaki, M.; Suchea, M.P.; Kenanakis, G. 3D Printed Fully Recycled TiO2-Polystyrene Nanocomposite Photocatalysts for Use against Drug Residues. Nanomaterials 2020, 10, 2144. [Google Scholar] [CrossRef] [PubMed]
- Detergent Chemicals Market Size, Growth, Report 2021–2030. Available online: https://www.precedenceresearch.com/detergent-chemicals-market (accessed on 24 January 2023).
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, properties, and applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- Ge, J.; Qu, J.; Lei, P.; Liu, H. New bipolar electrocoagulation–electroflotation process for the treatment of laundry wastewater. Sep. Purif. Technol. 2004, 36, 33–39. [Google Scholar] [CrossRef]
- Kowalska, I. Surfactant removal from water solutions by means of ultrafiltration and ion-exchange. Desalination 2008, 221, 351–357. [Google Scholar] [CrossRef]
- Khan, M.N.; Zareen, U. Sand sorption process for the removal of sodium dodecyl sulfate (anionic surfactant) from water. J. Hazard. Mater. 2006, 133, 269–275. [Google Scholar] [CrossRef]
- Ariapad, A.; Zanjanchi, M.; Arvand, M. Efficient removal of anionic surfactant using partial template-containing MCM-41. Desalination 2012, 284, 142–149. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; Van Langenhove, H. Heterogeneous Photocatalysis as an Advanced Oxidation Process for the Abatement of Chlorinated, Monocyclic Aromatic and Sulfurous Volatile Organic Compounds in Air: State of the Art. Crit. Rev. Environ. Sci. Technol. 2007, 37, 489–538. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Luque, R. Heterogeneous Photocatalysis. Chemengineering 2021, 5, 26. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269. [Google Scholar] [CrossRef]
- Kenanakis, G.; Katsarakis, N. Light-induced photocatalytic degradation of stearic acid by c-axis oriented ZnO nanowires. Appl. Catal. A Gen. 2010, 378, 227–233. [Google Scholar] [CrossRef]
- Mills, A.; Lee, S.-K. A web-based overview of semiconductor photochemistry-based current commercial applications. J. Photochem. Photobiol. A Chem. 2002, 152, 233–247. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: Intermediates and degradation pathways. Appl. Catal. B Environ. 2003, 42, 319–335. [Google Scholar] [CrossRef]
- Tanaka, K.; Padermpole, K.; Hisanaga, T. Photocatalytic degradation of commercial azo dyes. Water Res. 2000, 34, 327–333. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Liu, J.; Ma, M.; Chen, W.; Li, L. Activated carbon supported TiO2-photocatalysis doped with Fe ions fo continuous treatment of dye wastewater in a dynamic reactor. J. Environ. Sci. 2010, 22, 1290–1296. [Google Scholar] [CrossRef]
- van Grieken, R.; Marugán, J.; Sordo, C.; Martínez, P.; Pablos, C. Photocatalytic inactivation of bacteria in water using suspended and immobilized silver-TiO2. Appl. Catal. B Environ. 2009, 93, 112–118. [Google Scholar] [CrossRef]
- Mills, A.; Elliott, N.; Hill, G.; Fallis, D.; Durrant, J.R.; Willis, R.L. Preparation and characterisation of novel thick sol-gel titania film photocatalysts. Photochem. Photobiol. Sci. 2003, 2, 591–596. [Google Scholar] [CrossRef]
- Liao, G.; Li, Z.; Cheng, Y.; Xu, D.; Zhu, D.; Jiang, S.; Guo, J.; Chen, X.; Xu, G.; Zhu, Y. Properties of oriented carbon fiber/polyamide 12 composite parts fabricated by fused deposition modeling. Mater. Des. 2018, 139, 283–292. [Google Scholar] [CrossRef]
- Prabhakar, M.M.; Saravanan, A.; Lenin, A.H.; Leno, I.J.; Mayandi, K.; Ramalingam, P.S. A short review on 3D printing methods, process parameters and materials. Mater. Today Proc. 2020, 45, 6108–6114. [Google Scholar] [CrossRef]
- Jin, M.; Giesa, R.; Neuber, C.; Schmidt, H. Filament Materials Screening for FDM 3D Printing by Means of Injection-Molded Short Rods. Macromol. Mater. Eng. 2018, 303, 1800507. [Google Scholar] [CrossRef]
- Arceo, F. Is PLA Recyclable or even Biodegradable?—3D Solved. Available online: https://3dsolved.com/is-pla-recyclable/ (accessed on 3 April 2023).
- Majgaonkar, P.; Hanich, R.; Malz, F.; Brüll, R. Chemical Recycling of Post-Consumer PLA Waste for Sustainable Production of Ethyl Lactate. Chem. Eng. J. 2021, 423, 129952. [Google Scholar] [CrossRef]
- Rodríguez-Panes, A.; Claver, J.; Camacho, A.M. The Influence of Manufacturing Parameters on the Mechanical Behaviour of PLA and ABS Pieces Manufactured by FDM: A Comparative Analysis. Materials 2018, 11, 1333. [Google Scholar] [CrossRef]
- McDaniel, M.P.; DesLauriers, P.J. Ethylene Polymers, HDPE. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 1–40. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Schmidt, T.C.; Nabipour, I.; Spitz, J. Worldwide bottled water occurrence of emerging contaminants: A review of the recent scientific literature. J. Hazard. Mater. 2020, 392, 122271. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Lee, S.C.; Son, B.; Park, S.Y.; Lee, J.W.; Oh, Y.-K.; Kim, Y.; Choi, S.; Lee, Y.-S.; Lee, J. Innovative three-dimensional (3D) eco-TiO2 photocatalysts for practical environmental and bio-medical applications. Sci. Rep. 2014, 4, 6740. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.C.; Tarancón, A.; Canales-Vázquez, J.; Méndez-Ramos, J.; Hernández-Afonso, L.; Acosta-Mora, P.; Rueda, J.R.M.; Fernández-González, R. Three dimensional printing of components and functional devices for energy and environmental applications. Energy Environ. Sci. 2017, 10, 846–859. [Google Scholar] [CrossRef]
- Giakoumaki, A.N.; Kenanakis, G.; Klini, A.; Androulidaki, M.; Viskadourakis, Z.; Farsari, M.; Selimis, A. 3D micro-structured arrays of ZnO nanorods. Sci. Rep. 2017, 7, 2100. [Google Scholar] [CrossRef] [PubMed]
- Skorski, M.R.; Esenther, J.M.; Ahmed, Z.; Miller, A.E.; Hartings, M.R. The chemical, mechanical, and physical properties of 3D printed materials composed of TiO2-ABS nanocomposites. Sci. Technol. Adv. Mater. 2016, 17, 89–97. [Google Scholar] [CrossRef]
- Viskadourakis, Z.; Sevastaki, M.; Kenanakis, G. 3D structured nanocomposites by FDM process: A novel approach for large-scale photocatalytic applications. Appl. Phys. A 2018, 124, 585. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.; Mantke, S.; Mills, A.; Robinson, A.; Sheel, D. A comparative study of three techniques for determining photocatalytic activity. J. Photochem. Photobiol. A Chem. 2007, 188, 387–391. [Google Scholar] [CrossRef]
- Uddin, M.; Hasnat, M.; Samed, A.; Majumdar, R. Influence of TiO2 and ZnO photocatalysts on adsorption and degradation behaviour of Erythrosine. Dye. Pigment. 2007, 75, 207–212. [Google Scholar] [CrossRef]
- Evgenidou, E.; Fytianos, K.; Poulios, I. Semiconductor-sensitized photodegradation of dichlorvos in water using TiO2 and ZnO as catalysts. Appl. Catal. B Environ. 2005, 59, 81–89. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, Z.Y.; Wang, B.; Wang, C.X.; Chen, D.H.; Yang, G.W. Self-assembled ZnO agave-like nanowires and anomalous superhydrophobicity. J. Physics Condens. Matter 2005, 17, 5441–5446. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Rajendran, K.; Banerjee, S.; Chini, T.; Sengodan, V. Influence of Mn doping on the microstructure and optical property of ZnO. Mater. Sci. Semicond. Process. 2008, 11, 6–12. [Google Scholar] [CrossRef]
- Kenanakis, G.; Katsarakis, N. ZnO nanowires on glass via chemical routes: A prospective photocatalyst for indoors applications. J. Environ. Chem. Eng. 2014, 2, 1416–1422. [Google Scholar] [CrossRef]
- Frysali, M.A.; Papoutsakis, L.; Kenanakis, G.; Anastasiadis, S.H. Anastasiadis. Functional Surfaces with Photocatalytic Behavior and Reversible Wettability: ZnO Coating on Silicon Spikes. J. Phys. Chem. C 2015, 119, 25401–25407. [Google Scholar] [CrossRef]
- Černohorský, O.; Grym, J.; Faitová, H.; Bašinová, N.; Kučerová, S.; Yatskiv, R.; Veselý, J. Modeling of Solution Growth of ZnO Hexagonal Nanorod Arrays in Batch Reactors. Cryst. Growth Des. 2020, 20, 3347–3357. [Google Scholar] [CrossRef]
- Kenanakis, G.; Vernardou, D.; Koudoumas, E.; Kiriakidis, G.; Katsarakis, N. Ozone sensing properties of ZnO nanostructures grown by the aqueous chemical growth technique. Sensors Actuators B Chem. 2007, 124, 187–191. [Google Scholar] [CrossRef]
- Tanaka, K.; Fukui, K.; Murai, S.; Fujita, K. Mechanical milling-induced room-temperature ferromagnetic phase in MnO2–ZnO system. Appl. Phys. Lett. 2006, 89, 052501. [Google Scholar] [CrossRef]
- Bhatti, K.P.; Chaudhary, S.; Pandya, D.K.; Kashyap, S.C. On the room-temperature ferromagnetism in (ZnO)0.98(MnO2)0.02. Solid State Commun. 2005, 136, 384–388. [Google Scholar] [CrossRef]
- Putri, N.A.; Fauzia, V.; Iwan, S.; Roza, L.; Umar, A.A.; Budi, S. Mn-doping-induced photocatalytic activity enhancement of ZnO nanorods prepared on glass substrates. Appl. Surf. Sci. 2018, 439, 285–297. [Google Scholar] [CrossRef]
- Yan, X.; Hu, D.; Li, H.; Li, L.; Chong, X.; Wang, Y. Nanostructure and optical properties of M doped ZnO (M = Ni, Mn) thin films prepared by sol–gel process. Phys. B Condens. Matter 2011, 406, 3956–3962. [Google Scholar] [CrossRef]
- Verma, A.K.; Singh, D.; Singh, S.; Yadav, R.R. Surfactant-free synthesis and experimental analysis of Mn-doped ZnO–glycerol nanofluids: An ultrasonic and thermal study. Appl. Phys. A 2019, 125, 253. [Google Scholar] [CrossRef]
- Renitta, A.; Vijayalakshmi, K. High performance hydrogen sensor based on Mn implanted ZnO nanowires array fabricated on ITO substrate. Mater. Sci. Eng. C 2017, 77, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.; Ghica, D.; Nistor, S.V.; Maraloiu, A.V.; Plugaru, R. Mn2+ ions distribution in doped sol–gel deposited ZnO films. Appl. Surf. Sci. 2017, 396, 1880–1889. [Google Scholar] [CrossRef]
- Wang, D.; Shang, W.; Zhang, B.; Jiang, C.; Qu, F.; Yang, M. Manganese-doped zinc oxide hollow balls for chemiresistive sensing of acetone vapors. Microchim. Acta 2019, 186, 44. [Google Scholar] [CrossRef]
- Ozgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Baranowska-Korczyc, A.; Kościński, M.; Coy, E.L.; Grześkowiak, B.F.; Jasiurkowska-Delaporte, M.; Peplińska, B.; Jurga, S. ZnS coating for enhanced environmental stability and improved properties of ZnO thin films. RSC Adv. 2018, 8, 24411–24421. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, M.; Shishodia, P.K. Ferromagnetism In Sol-gel Derived ZnO: Mn Nanocrystalline Thin Films. Adv. Mater. Lett. 2016, 7, 116–122. [Google Scholar] [CrossRef]
- Akyol, M.; Çetin, S.K.; Ekicibil, A. Microstructural and magnetic properties of Ax:Zn1-xO (A = Mn, Gd and Mn/Gd) nanocrystals. Philos. Mag. 2015, 96, 31–44. [Google Scholar] [CrossRef]
- Vernardou, D.; Kenanakis, G.; Couris, S.; Koudoumas, E.; Kymakis, E.; Katsarakis, N. pH effect on the morphology of ZnO nanostructures grown with aqueous chemical growth. Thin Solid Films 2007, 515, 8764–8767. [Google Scholar] [CrossRef]
- Phan, T.L.; Vincent, R.; Cherns, D.; Nghia, N.X.; Ursaki, V.V. Raman scattering in Me-doped ZnO nanorods (Me = Mn, Co, Cu and Ni) prepared by thermal diffusion. Nanotechnology 2008, 19, 475702. [Google Scholar] [CrossRef]
- Strelchuk, V.; Kolomys, O.; Rarata, S.; Lytvyn, P.; Khyzhun, O.; Chey, C.O.; Nur, O.; Willander, M. Raman Submicron Spatial Mapping of Individual Mn-doped ZnO Nanorods. Nanoscale Res. Lett. 2017, 12, 351. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Kalathil, S.; Nisar, A.; Lee, J.; Cho, M.H. Oxygen vacancy induced band gap narrowing of ZnO nanostructures by an electrochemically active biofilm. Nanoscale 2013, 5, 9238–9246. [Google Scholar] [CrossRef] [PubMed]
- Raskar, N.D.; Dake, D.V.; Mane, V.A.; Stathatos, E.; Deshpande, U.; Dole, B. One step synthesis of vertically grown Mn-doped ZnO nanorods for photocatalytic application. J. Mater. Sci. Mater. Electron. 2019, 30, 10886–10899. [Google Scholar] [CrossRef]
- Shah, W.H.; Alam, A.; Javed, H.; Rashid, K.; Ali, A.; Ali, L.; Safeen, A.; Ali, M.R.; Imran, N.; Sohail, M.; et al. Tuning of the band gap and dielectric loss factor by Mn doping of Zn1-xMnxO nanoparticles. Sci. Rep. 2023, 13, 8646. [Google Scholar] [CrossRef] [PubMed]
- Ab Wahab, N.; Sairi, N.A.; Alias, Y. Photocatalytic activities enhancement of manganese doped ZnO by decoration on CNT for degradation of organic pollutants under solar irradiation. Appl. Phys. A 2021, 128, 59. [Google Scholar] [CrossRef]
- Biswas, B.D.; Purkayastha, M.D.; Tiwari, E.; Denrah, S.; Sarkar, M.; Darbha, G.K.; Majumder, T.P. Study of the photocatalytic activity of Mn-doped ZnO nanocomposites depending on their morphology and structure with the variation of manganese concentration. Surf. Interfaces 2021, 23, 100902. [Google Scholar] [CrossRef]
- Aadnan, I.; Zegaoui, O.; El Mragui, A.; Daou, I.; Moussout, H.; da Silva, J.C.G.E. Structural, Optical and Photocatalytic Properties of Mn Doped ZnO Nanoparticles Used as Photocatalysts for Azo-Dye Degradation under Visible Light. Catalysts 2022, 12, 1382. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrithias, N.R.; Katsara, K.; Papoutsakis, L.; Papadakis, V.M.; Viskadourakis, Z.; Remediakis, I.N.; Kenanakis, G. Three-Dimensional-Printed Photocatalytic Sponges Decorated with Mn-Doped ZnO Nanoparticles. Materials 2023, 16, 5672. https://doi.org/10.3390/ma16165672
Vrithias NR, Katsara K, Papoutsakis L, Papadakis VM, Viskadourakis Z, Remediakis IN, Kenanakis G. Three-Dimensional-Printed Photocatalytic Sponges Decorated with Mn-Doped ZnO Nanoparticles. Materials. 2023; 16(16):5672. https://doi.org/10.3390/ma16165672
Chicago/Turabian StyleVrithias, Nikolaos Rafael, Klytaimnistra Katsara, Lampros Papoutsakis, Vassilis M. Papadakis, Zacharias Viskadourakis, Ioannis N. Remediakis, and George Kenanakis. 2023. "Three-Dimensional-Printed Photocatalytic Sponges Decorated with Mn-Doped ZnO Nanoparticles" Materials 16, no. 16: 5672. https://doi.org/10.3390/ma16165672
APA StyleVrithias, N. R., Katsara, K., Papoutsakis, L., Papadakis, V. M., Viskadourakis, Z., Remediakis, I. N., & Kenanakis, G. (2023). Three-Dimensional-Printed Photocatalytic Sponges Decorated with Mn-Doped ZnO Nanoparticles. Materials, 16(16), 5672. https://doi.org/10.3390/ma16165672










