Comparative Study on UV Degradation of Black Chinese Lacquers with Different Additives
Abstract
:1. Introduction
Fe(OH) (CH3COO)2 + H2O → Fe (OH)2↓ + CH3COOH
Fe (OH)2 + O2 + H2O → Fe(OH)3↓
2. Materials and Methods
2.1. Materials
2.2. Preparation of Black Chinese Lacquer
2.3. Sample Preparation
2.4. Accelerated Aging through Ultraviolet Irradiation
2.5. Physicochemical Measurements
2.6. Statistical Analysis
3. Results and Discussion
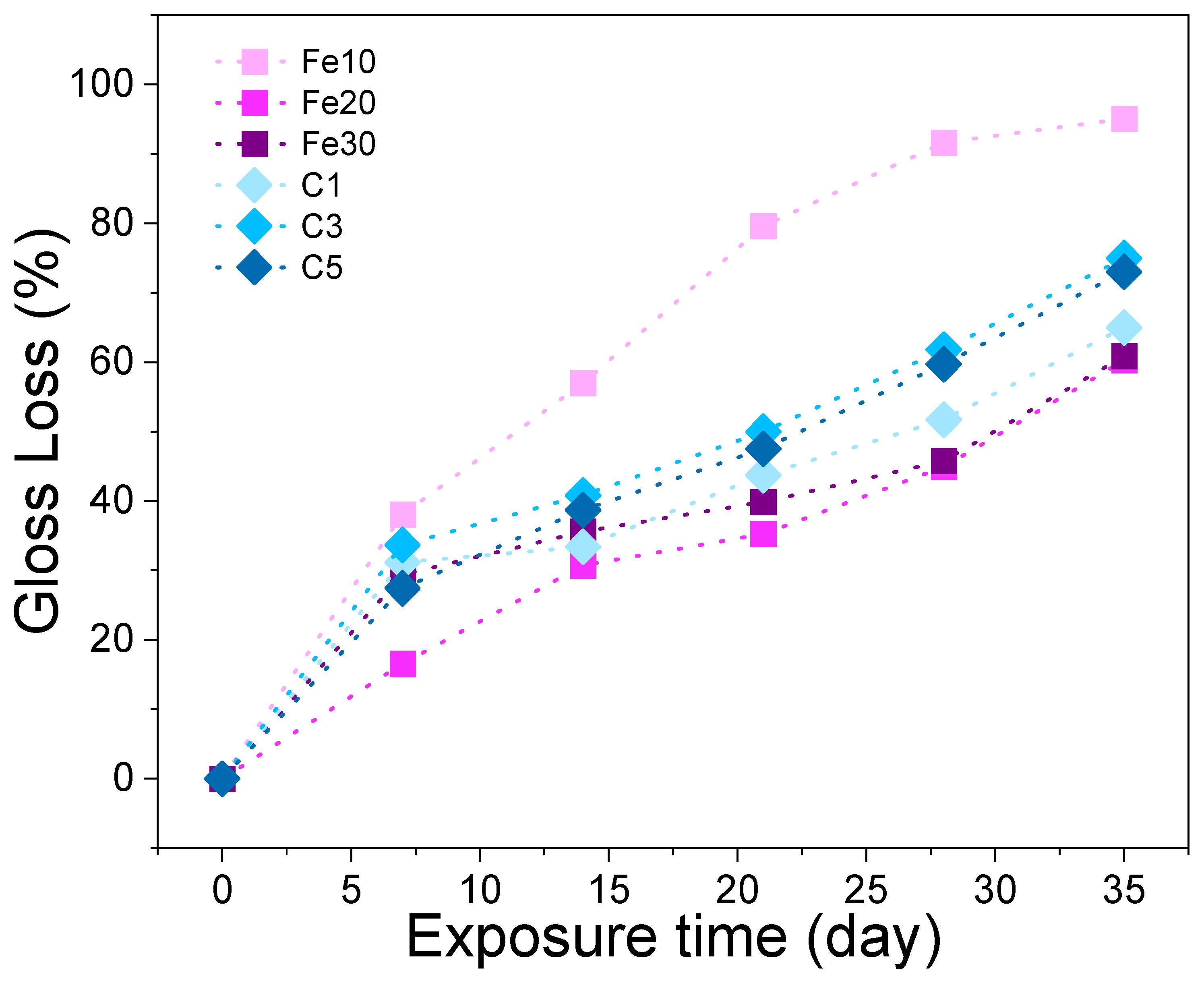
3.1. Gloss Loss
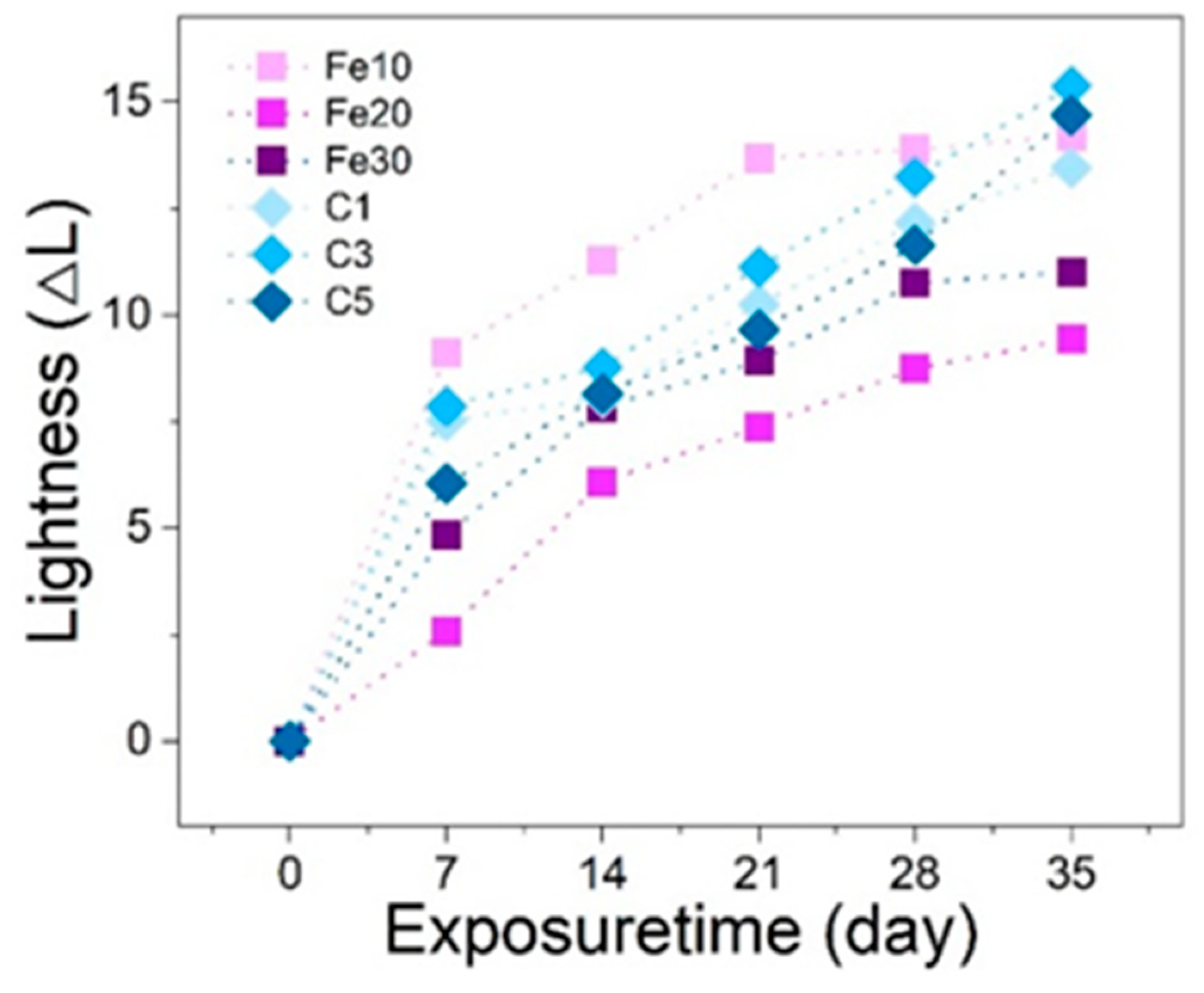
3.2. Color Change
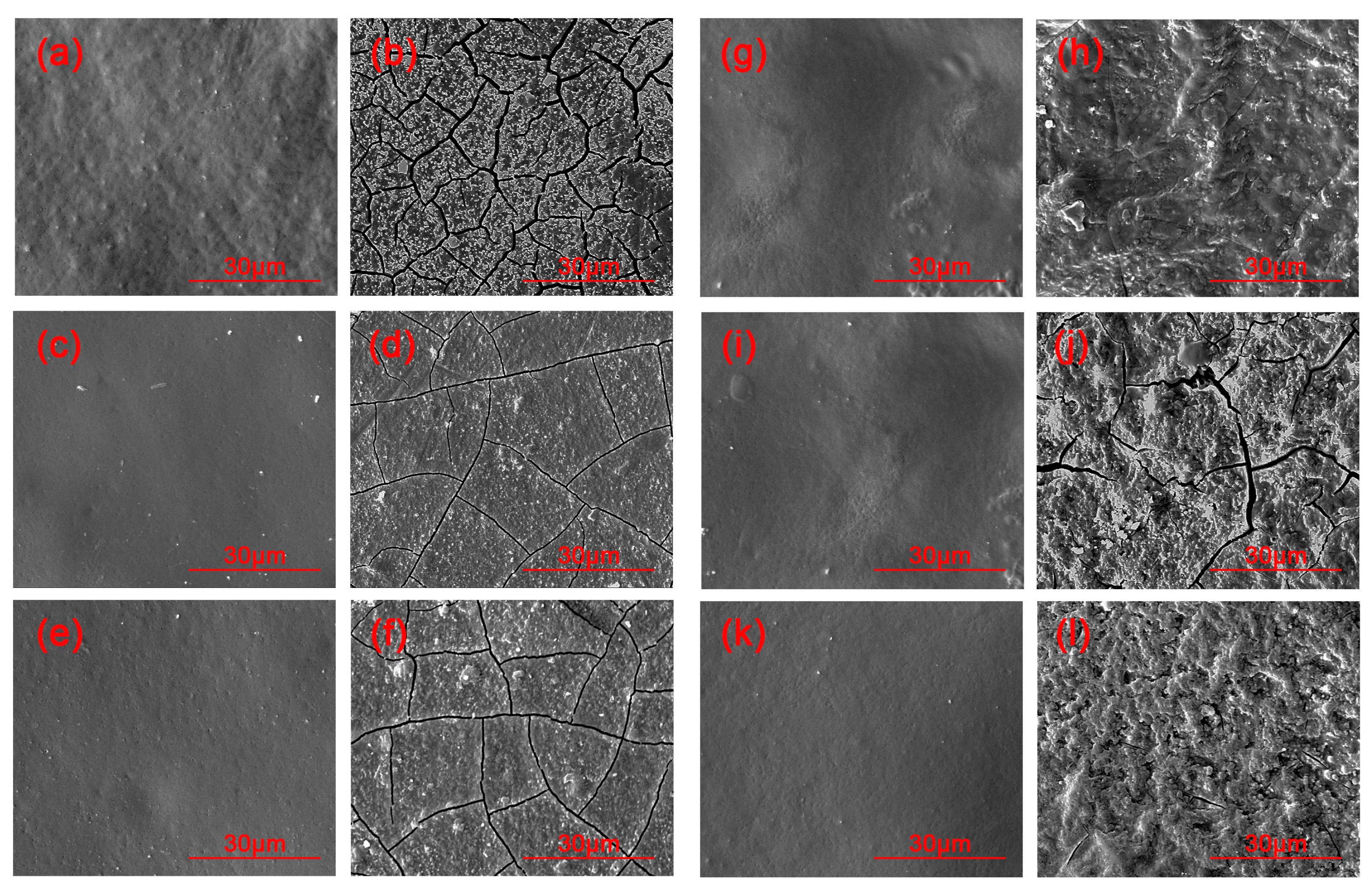
3.3. SEM
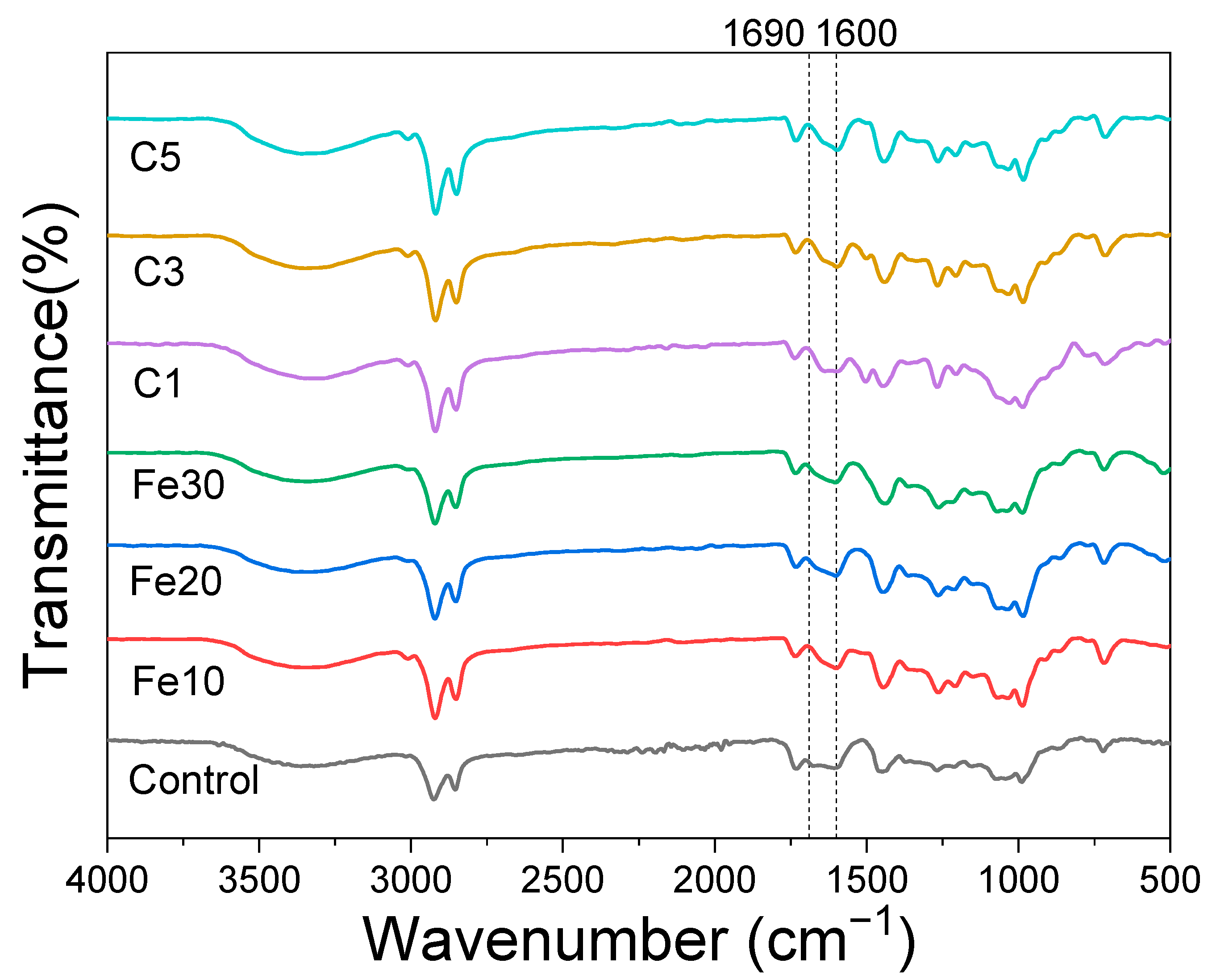
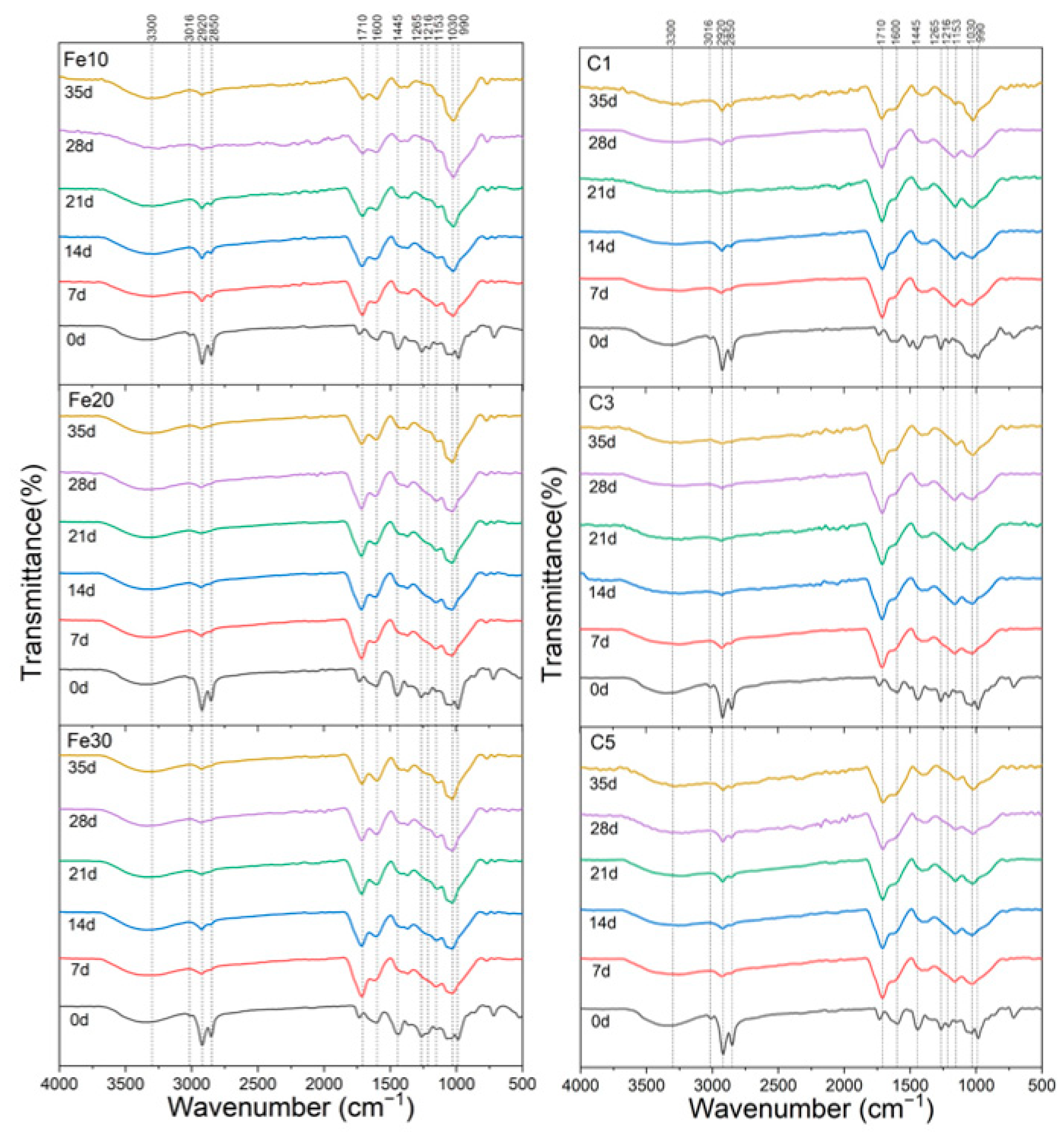
3.4. Infrared Spectroscopy Analysis
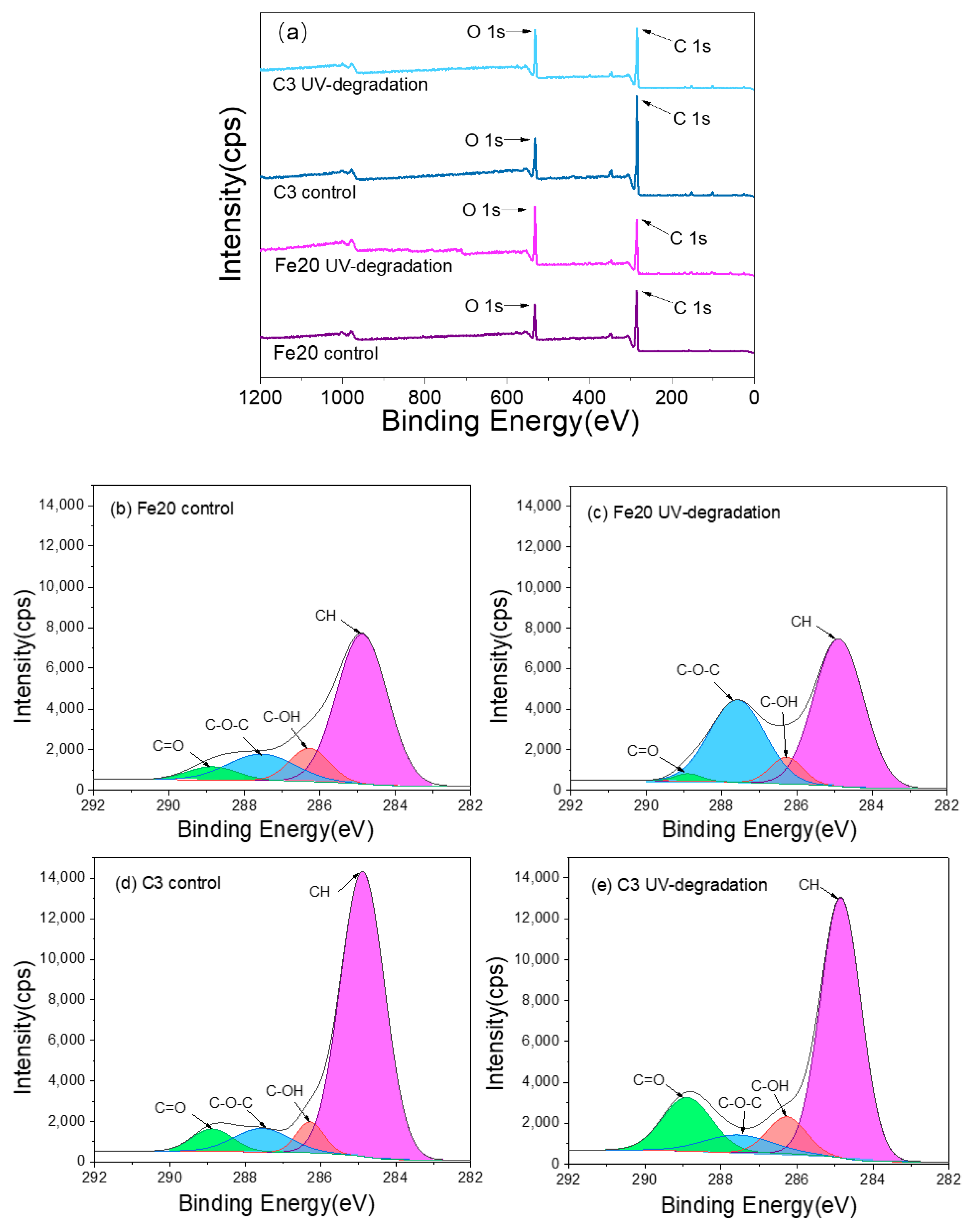
3.5. XPS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Y.; Bai, W.; Zhang, X.; Chen, J.; Wang, S.; Lin, J.; Xu, Y. Effect of Silane on the Active Aging Resistance and Anticorrosive Behaviors of Natural Lacquer. ACS Omega 2018, 3, 4129–4140. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Fujimoto, A.; Takahara, A. Characterization of catechol-containing natural thermosetting polymer “urushiol” thin film. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3688–3692. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, B.; Jiang, L.; Wu, J.; Sun, G. Natural lacquer was used as a coating and an adhesive 8000 years ago, by early humans at Kuahuqiao, determined by ELISA. J. Archaeol. Sci. 2018, 100, 80–87. [Google Scholar] [CrossRef]
- Zhai, K.; Sun, G.; Zheng, Y.; Wu, M.; Zhang, B.; Zhu, L.; Hu, Q. The earliest lacquerwares of China were discovered at Jingtoushan site in the Yangtze River Delta. Archaeometry 2021, 64, 218–226. [Google Scholar] [CrossRef]
- Li, T.; Xie, Y.-F.; Yang, Y.-M.; Wang, C.-S.; Fang, X.-Y.; Shi, J.-L.; He, Q.-J. Pigment identification and decoration analysis of a 5th century Chinese lacquer painting screen: A micro-Raman and FTIR study. J. Raman Spectrosc. 2009, 40, 1911–1918. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Zhao, Y.; Wen, Q.; Xie, Z.; Yuan, Y.; Tong, T.; Shen, X.; Tong, H. Composition/structure and lacquering craft analysis of Wenzhou Song dynasty lacquerware. Anal. Methods 2016, 8, 6529–6536. [Google Scholar] [CrossRef]
- Hao, X.; Wu, H.; Zhao, Y.; Tong, T.; Li, X.; Yang, C.; Tang, Y.; Shen, X.; Tong, H. Analysis on the Composition/structure and Lacquering Techniques of the Coffin of Emperor Qianlong Excavated from the Eastern Imperial Tombs. Sci. Rep. 2017, 7, 8446. [Google Scholar] [CrossRef] [Green Version]
- Kim, S. Observation by the Microscopic Analysis of Lacquer Layer for Identification of Lacquer-ware Function. J. Korean Wood Sci. Technol. 2008, 36, 96–104. [Google Scholar]
- Zongyi, T. Nan Cun Chuo Geng Lu南村辍耕录; Zhonghua Book Company: Beijing, China, 2004. [Google Scholar]
- Zheng, L.P.; Wang, L.Q.; Zhao, X.; Yang, J.L.; Zhang, M.X.; Wang, Y.F. Characterization of the materials and techniques of a birthday inscribed lacquer plaque of the qing dynasty. Herit. Sci. 2020, 8, 10. [Google Scholar] [CrossRef]
- Chang, B. Collection of Chinese Traditional Crafts. Natural Lacquer Painting; China Science and Technology Press: Beijing, China, 2018. [Google Scholar]
- Wang, S.X. The Explanation of the Book of Xiu Shi Lu髹饰录; Life—Reading—Xinzhi Sanlian Bookstore: Beijing, China, 2013. [Google Scholar]
- Coueignoux, C.; Rivers, S. Conservation of Photodegraded Asian Lacquer Surfaces: Four Case Studies. J. Am. Inst. Conserv. 2015, 54, 14–28. [Google Scholar] [CrossRef]
- Le Ho, A.S.; Duhamel, C.; Daher, C.; Bellot-Gurlet, L.; Paris, C.; Regert, M.; Sablier, M.; Andre, G.; Desroches, J.P.; Dumas, P. Alteration of Asian lacquer: In-depth insight using a physico-chemical multiscale approach. Analyst 2013, 138, 5685–5696. [Google Scholar] [CrossRef]
- Han, J.; Khanjian, H.; Schilling, M.R.; Webb, M. Study of water-soluble light-aging products on Asian lacquer surfaces. In Proceedings of the ICOM-CC 19th Triennial Conference 2021, Beijing, China, 17–21 May 2021. [Google Scholar]
- Webb, M.; Schilling, M.R.; Chang, J. The reproduction of realistic samples of Chinese export lacquer for research. Stud. Conserv. 2016, 61, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Nakagoshi, K.; Yoshizumi, K. Degradation of Japanese Lacquer under Wavelength Sensitivity of Light Radiation. Mater. Sci. Appl. 2011, 2, 1507–1515. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Jinnai, H.; Osawa, S. Wavelength Dependence on Light Deterioration of Oriental Lacquer Film. Kobunshi Ronbunshu 2001, 58, 442–447. [Google Scholar] [CrossRef]
- Toyoshima. Study of Deterioration of Urushi Film with Ultraviolet Radiation. Mater. Life 1996, 8, 28–35. [Google Scholar]
- Lu, R.; Kamiya, Y.; Kumamoto, T.; Honda, T.; Miyakoshi, T. Deterioration of surface structure of lacquer films due to ultraviolet irradiation. Surf. Interface Anal. 2006, 38, 1311–1315. [Google Scholar] [CrossRef]
- Yutaka, O. Conservation of cultural properties (Part 2) Urushi (Japanese lacquer) science for the restoration and the replicas production of cultural properties. Wood Prot. 2021, 47, 262–268. [Google Scholar]
- Ogawa, T.; Jinnai, H. Deterioration of Oriental Lacquer. Mater. Raifu Gakkaishi 2005, 17, 94–98. [Google Scholar]
- Shimadzu, Y.; Kawanobe, W. Deterioration Phenomena of Urushi Films:Combined Effects of Ultraviolet Radiation and Water. J. Jpn. Soc. Colour Mater. 2003, 76, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Elmahdy, A.E.; Wildman, R.D.; Ashcroft, I.A.; Ruiz, P.D. Experimental investigation and material modelling of fresh and UV aged Japanese lacquer (Urushi). Prog. Org. Coat. 2011, 70, 160–169. [Google Scholar] [CrossRef]
- Shimode, Y.; Endo, A.; Narita, C.; Higashi, S.; Hamada, H. Study on the Degradation Mechanism of the Urushi Products. In Proceedings of the ASME 2012 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 9–15 November 2012. [Google Scholar]
- McSharry, C.; Faulkner, R.; Rivers, S.; Shaffer, M.S.P.; Welton, T. The chemistry of East Asian lacquer: A review of the scientific literature. Stud. Conserv. 2013, 52, 29–40. [Google Scholar] [CrossRef]
- Zhang, F.L. Chinese Lacquer Craft and Lacquerware Conservation; Science Press: Beijing, China, 2010. [Google Scholar]
- Honda, T.; Lu, R.; Miyakoshi, T. Chrome-free corrosion protective coating based on lacquer hybridized with silicate. Prog. Org. Coat. 2006, 56, 279–284. [Google Scholar] [CrossRef]
- Feng, X.H.; Sheng, Y.T.; Ge, X.W.; Wu, Z.H.; Huang, Q.T. Evaluation of the properties of hybrid yellow poplar (Liriodendron sino-americanum): A comparison study with yellow poplar (Liriodendron tulipifera). Maderas-Cienc. Tecnol. 2021, 23, 15. [Google Scholar] [CrossRef]
- Haoliang, H. Methods of Refining Chinese Lacquer and Categories of Refined Chinese Lacquer. Chin. Raw Lacq. 2003, 2, 19–30. [Google Scholar] [CrossRef]
- Miaoqing, X.; Guoqing, L.; Jinhuo, L. The coordination reaction between urushiol and ferrous sulfate. Chin. Raw Lacq. 2003, 1, 9–13. [Google Scholar] [CrossRef]
- Kesel, W.D.; Dhondt, G. Coromandel Lacquer Screens; Art Media Resources Ltd.: Chicago, IL, USA, 2002. [Google Scholar]
- Yang, L.; Zheng, J.T.; Huang, N. The Characteristics of Moisture and Shrinkage of Eucalyptus urophylla x E. Grandis Wood during Conventional Drying. Materials 2022, 15, 3386. [Google Scholar] [CrossRef]
- Miklin-Kniefacz, S.; Pitthard, V.; Parson, W.; Berger, C.; Stanek, S.; Griesser, M.; Kučková, Š.H. Searching for blood in Chinese lacquerware: Zhū xiě huī豬血灰. Stud. Conserv. 2016, 61, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.S.; Park, J.H.; Kim, S.-C. A Study on the Optical Characteristics According to the Lacquer Drying Conditions for the Conservation of Lacquerwares. J. Korean Wood Sci. Technol. 2018, 46, 610–621. [Google Scholar] [CrossRef]
- Zheng, B.; Bai, W.; Chen, J.; Jian, R.; Yang, K.; Lin, Q.; Xu, Y. Inorganic salts as effective additive for adjusting the curing of natural oriental lacquer. Prog. Org. Coat. 2021, 161, 106494. [Google Scholar] [CrossRef]
- ASTM G154-16; Standard Practice for Operating Fluorescent Ultraviolet (UV) Lamp Apparatus for Exposure of Nonmetallic Materials. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D523-2014; Standard Test Method for Specular Gloss. ASTM International: West Conshohocken, PA, USA, 2014.
- GB/T 4893.6-2013; Physical and Chemical Properties Test of Furniture Surface Paint Film. China National Standards: Shenzhen, China, 2013.
- ASTM D2244-2009b; Standard Practice for Calculation of Color Tolerances and Color Differences from Instrumentally Measured Color Coordinates. ASTM International: West Conshohocken, PA, USA, 2009.
- Peng, W.; Yan, X. Preparation of Tung Oil Microcapsule and Its Effect on Wood Surface Coating. Polymers 2022, 14, 1536. [Google Scholar] [CrossRef]
- Narita, C.; Shimode, Y.; Yamada, K. Influence of the finishing methods of Urushi products on degradation. Prog. Org. Coat. 2016, 101, 379–384. [Google Scholar] [CrossRef]
- Kamiya, Y.; Nishimura, S. Basic Study on Issue of Change of Color of the Black Lacquer Film—Surface Change Due to the Ultraviolet Irradiation of the Reacted Black Lacquer with Iron. Bull. TIRI 2013, 8, 88–91. [Google Scholar]
- Thei, J.; Rivers, S.; Taylor, A.C. A preliminary examination of urushi-based conservation options for the treatment of photodegraded Japanese lacquer using scanning electron microscopy and profilometry. Stud. Conserv. 2016, 61, 131–148. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.J.; Wang, L.W.; Lin, Q. Effect of hexamethylenetetramine on the property of Chinese lacquer film. Prog. Org. Coat. 2019, 133, 169–173. [Google Scholar] [CrossRef]
- Wu, Y.; Gan, J.; Yang, F.; Zhang, H.Q.; Wang, W. Preparation and antibacterial properties of waterborne UV cured coating modified by quaternary ammonium compounds. J. Appl. Polym. Sci. 2021, 138, 11. [Google Scholar] [CrossRef]
- Park, J.; Park, C.; Kim, S. A Study of Different Drying Condition for Conservation of Lacquer Wares and Durability of Urushi Lacquer Coats with Enzyme Addition. J. Korea Furnit. Soc. 2021, 32, 313–322. [Google Scholar]
- Kenjo, T.; Mihar, K. Studies on Films of Japanese Lacquer (VI) Influence of Soybean, Linseed and Japanese Tung Oils Added. J. Jpn. Soc. Colour. Mater. 1976, 49, 600–604. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Tibirna, C.-M.; Vasile, C. XPS characterization of naturally aged wood. Appl. Surf. Sci. 2009, 256, 1355–1360. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Zhao, J.; Li, W.; Wang, B.; Yang, Y.; Sun, J.; Fang, X.; Xia, R.; Liu, Y.; et al. A route of alkylated carbon black with hydrophobicity, high dispersibility and efficient thermal conductivity. Appl. Surf. Sci. 2021, 538, 147858. [Google Scholar] [CrossRef]

| Code | Additives | Number of Specimens |
|---|---|---|
| L-Fe10 | 10% Fe(OH)2 | 8 |
| L-Fe20 | 20% Fe(OH)2 | 8 |
| L-Fe30 | 30% Fe(OH)2 | 8 |
| L-C1 | 1% carbon black | 8 |
| L-C3 | 3% carbon black | 8 |
| L-C5 | 5% carbon black | 8 |
| Code | H0 (%) | H7 (%) | H14 (%) | H21 (%) | H28 (%) | H35 (%) |
|---|---|---|---|---|---|---|
| Fe10 | 21.4 c | 13.3 b | 9.3 c | 4.4 c | 1.8 c | 1.8 c |
| Fe20 | 32.9 b | 27.4 a | 22.7 b | 21.3 b | 18.1 b | 13.0 b |
| Fe30 | 47.2 a | 33.4 a | 30.4 a | 28.4 a | 25.6 a | 18.5 a |
| p values | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C1 | 34.7 a | 23.7 a | 23.0 a | 19.5 a | 16.7 a | 12.1 a |
| C3 | 29.6 b | 19.7 b | 17.6 b | 14.9 b | 11.4 b | 7.4 b |
| C5 | 28.7 b | 20.8 b | 17.5 b | 14.9 b | 11.4 b | 7.7 b |
| p values | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Code | L0 | L7 | L14 | L21 | L28 | L35 |
|---|---|---|---|---|---|---|
| Fe10 | 9.15 a | 18.24 a | 20.42 a | 22.83 a | 23.02 a | 23.34 a |
| Fe20 | 9.14 a | 11.71 c | 15.21 b | 16.51 b | 17.88 c | 18.56 b |
| Fe30 | 9.13 a | 13.98 b | 16.98 b | 17.03 b | 19.89 b | 20.16 b |
| p values | 0.910 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C1 | 9.02 a | 14.95 a | 16.54 a | 18.50 a | 21.18 a | 22.48 a |
| C3 | 8.88 a | 16.74 a | 16.90 a | 20.01 a | 22.13 a | 24.25 a |
| C5 | 9.24 a | 15.29 a | 17.41 a | 18.01 a | 20.88 a | 23.94 a |
| p values | 0.755 | 0.776 | 0.406 | 0.527 | 0.734 | 0.522 |
| Code | %C | %O | C/O |
|---|---|---|---|
| Fe20 control | 77.77 | 22.23 | 3.50 |
| Fe20 UV degradation | 62.58 | 31.07 | 2.01 |
| C3 control | 80.83 | 19.17 | 4.22 |
| C3 UV degradation | 72.88 | 25.58 | 2.85 |
| Code | CH | C-OH | C-O-C | C=O |
|---|---|---|---|---|
| Fe20 control | 66.86 | 11.68 | 15.73 | 5.73 |
| Fe20 UV degradation | 56.70 | 6.75 | 35.11 | 1.45 |
| C3 control | 80.47 | 5.37 | 8.38 | 5.79 |
| C3 UV degradation | 66.77 | 8.97 | 7.53 | 16.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Liu, X.; Lv, J. Comparative Study on UV Degradation of Black Chinese Lacquers with Different Additives. Materials 2023, 16, 5607. https://doi.org/10.3390/ma16165607
Liu W, Liu X, Lv J. Comparative Study on UV Degradation of Black Chinese Lacquers with Different Additives. Materials. 2023; 16(16):5607. https://doi.org/10.3390/ma16165607
Chicago/Turabian StyleLiu, Wenjia, Xinyou Liu, and Jiufang Lv. 2023. "Comparative Study on UV Degradation of Black Chinese Lacquers with Different Additives" Materials 16, no. 16: 5607. https://doi.org/10.3390/ma16165607
APA StyleLiu, W., Liu, X., & Lv, J. (2023). Comparative Study on UV Degradation of Black Chinese Lacquers with Different Additives. Materials, 16(16), 5607. https://doi.org/10.3390/ma16165607





