Synthesis, Properties and Adsorption Kinetic Study of New Cross-Linked Composite Materials Based on Polyethylene Glycol Polyrotaxane and Polyisoprene/Semi-Rotaxane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis
2.3.1. Synthesis of Diamine Polyethylene Glycol
2.3.2. Synthesis of PEG/HPβCD
2.3.3. Synthesis of PI/HPβCD SR
2.3.4. Synthesis of the Pure Cross-Linked Material 1
2.3.5. Synthesis of the Cross-Linked Materials 2 and 3
3. Results and Discussion
3.1. Chemical and Physical Properties of the Composite Materials
3.1.1. Chemical Analysis
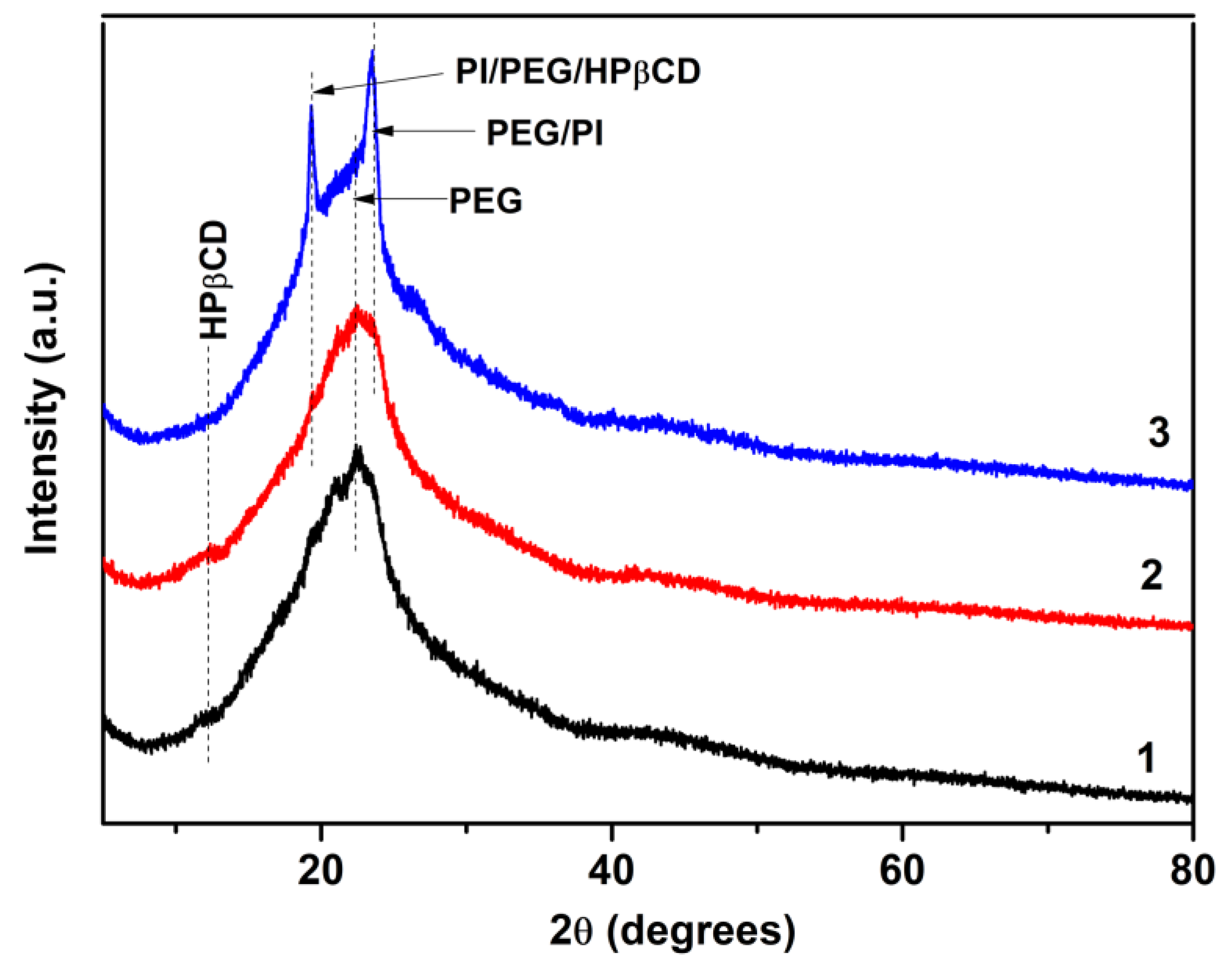
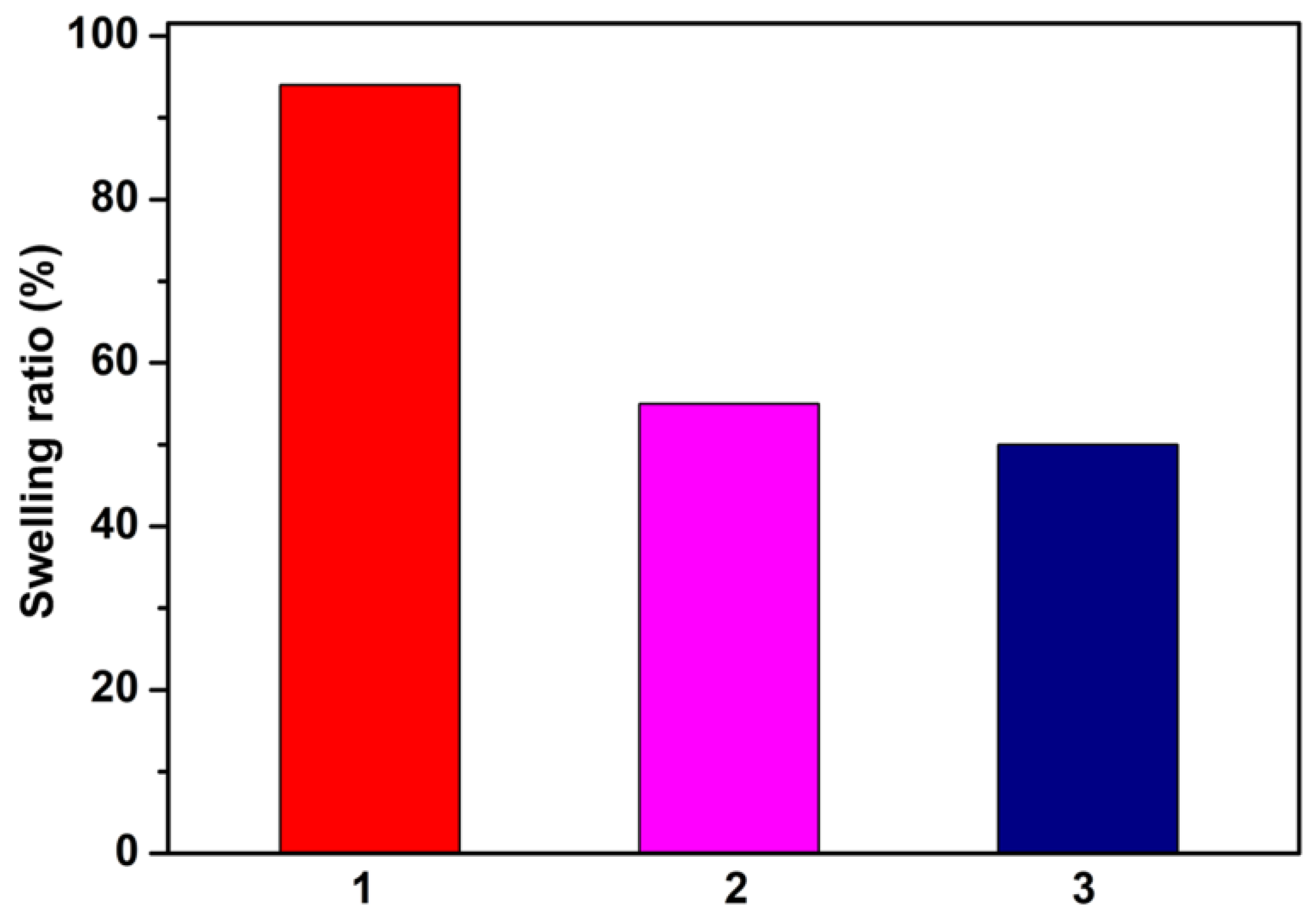
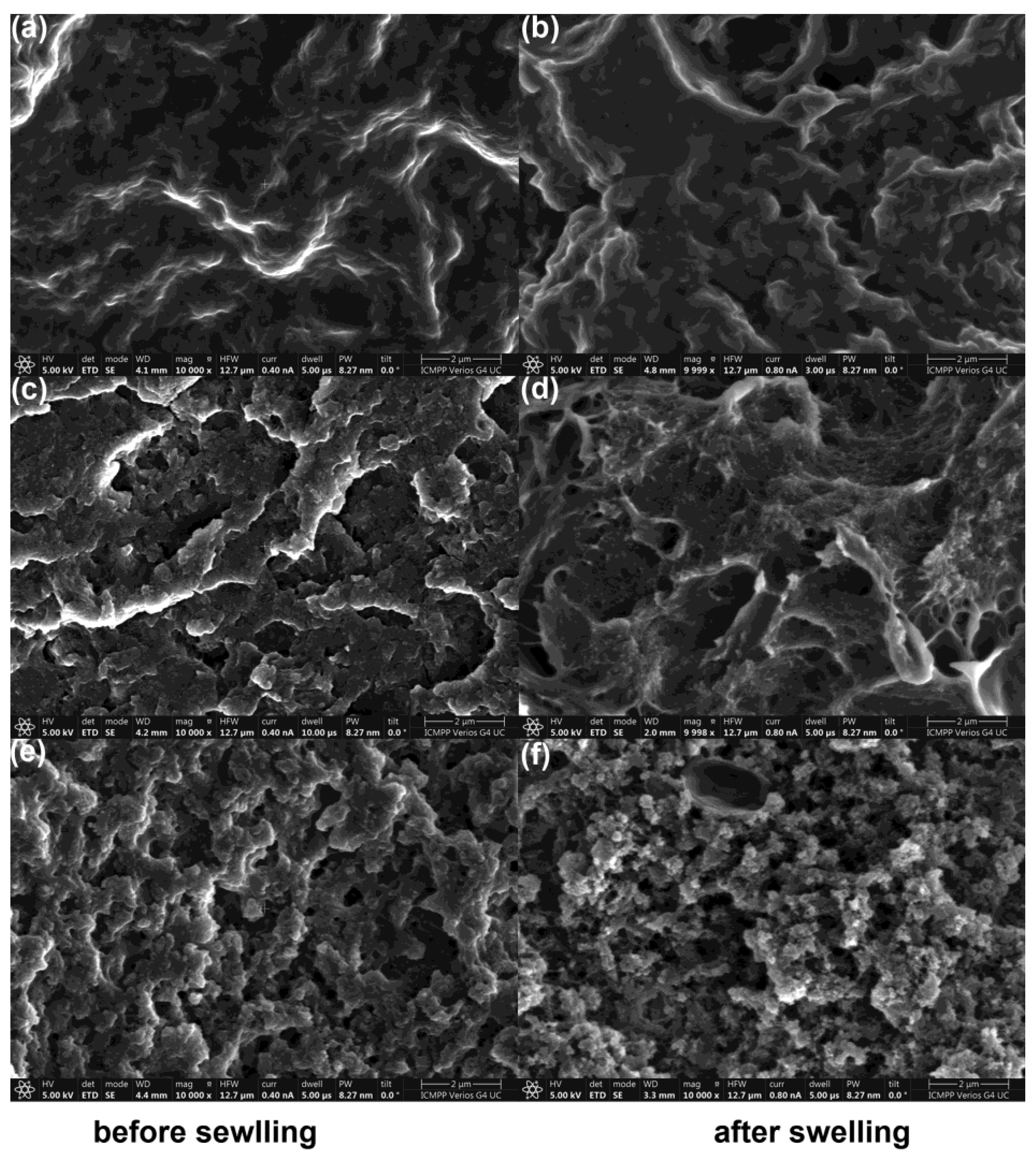
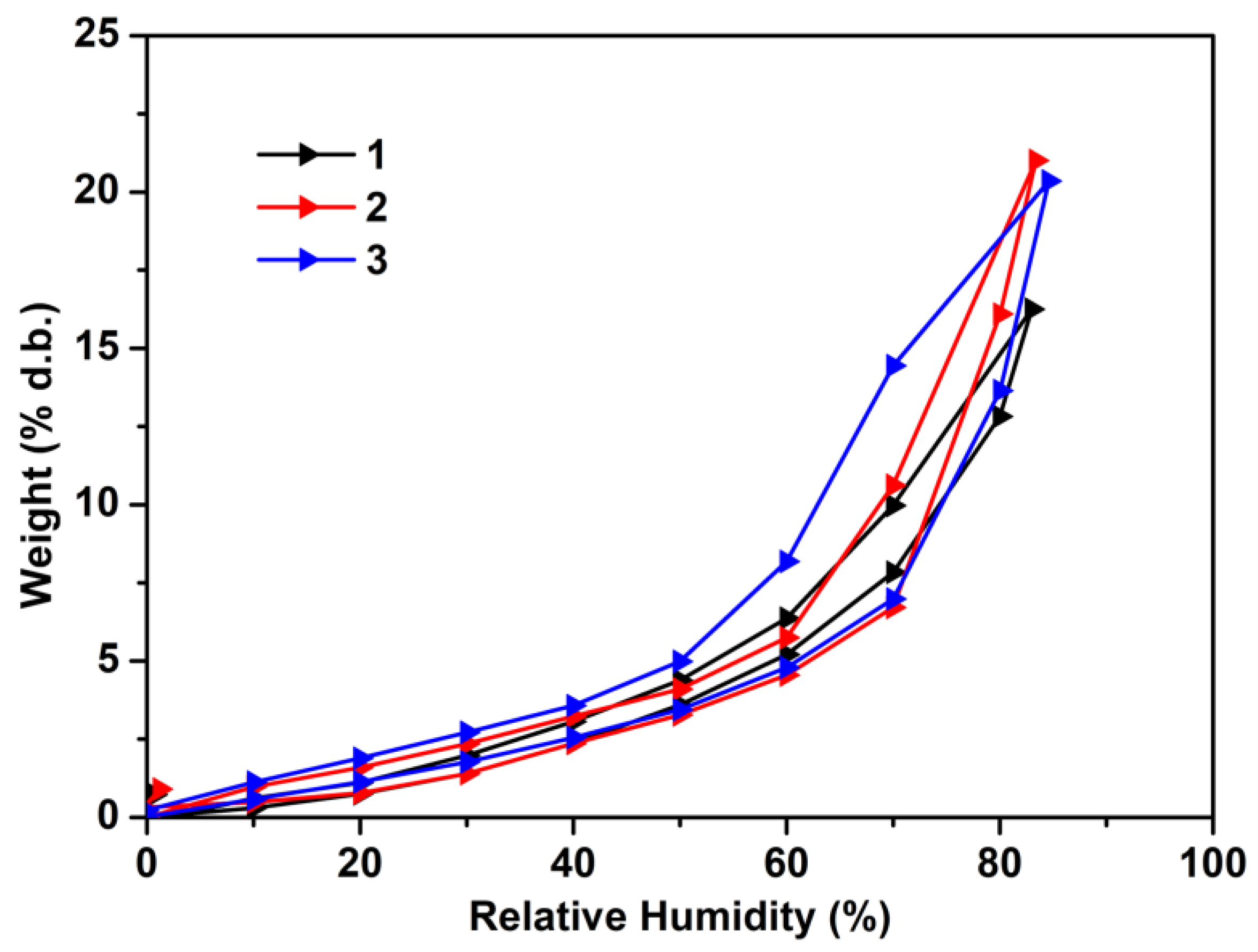
3.1.2. Physical and Morphological Properties of the Materials
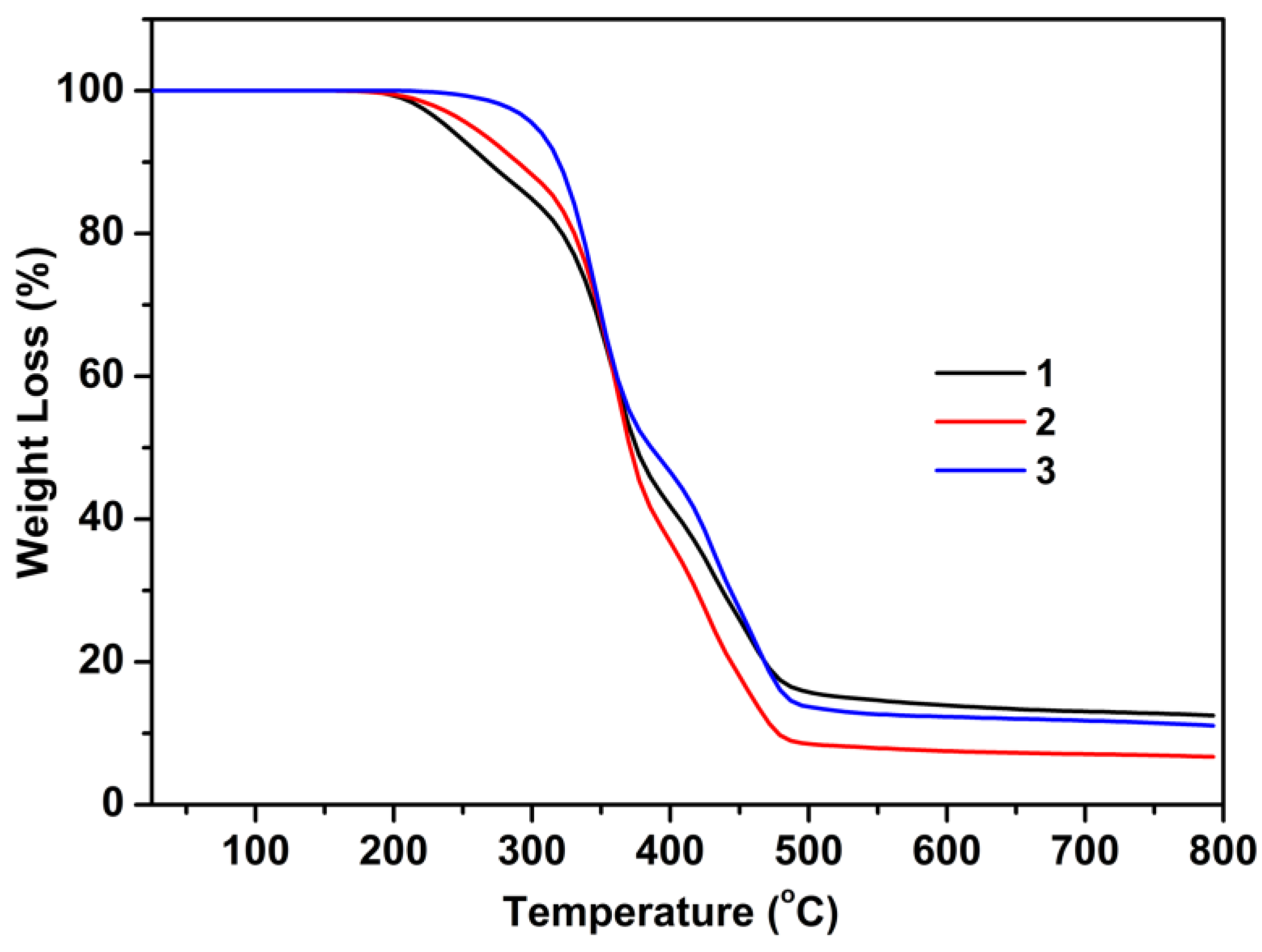
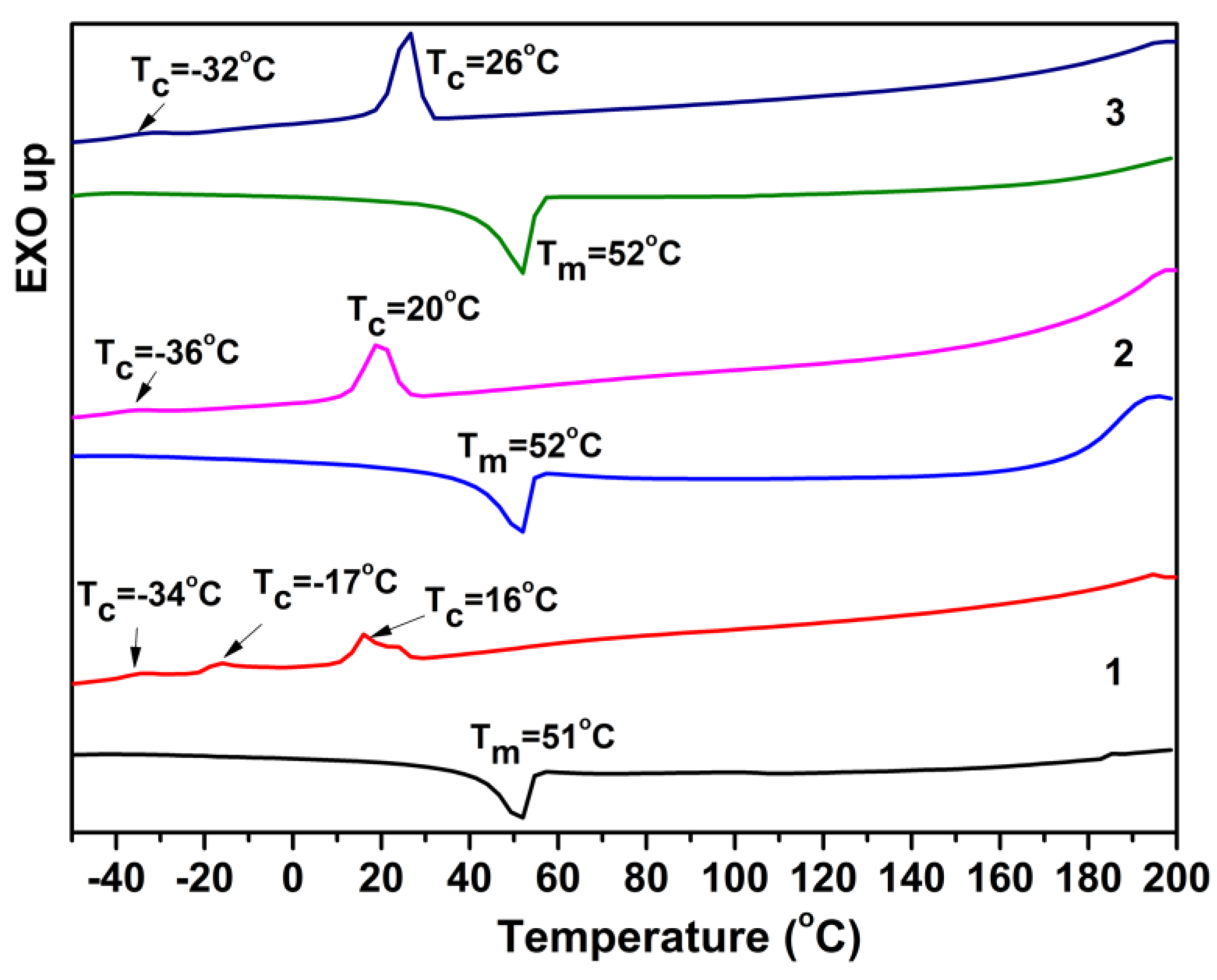
3.1.3. Thermal Analysis
3.2. Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araki, J.; Ito, K. Recent advances in the preparation of cyclodextrin-based polyrotaxanes and their applications to soft materials. Soft Matter 2007, 3, 1456–1473. [Google Scholar] [CrossRef] [PubMed]
- Inutsuka, M.; Inoue, K.; Hayashi, Y.; Inomata, A.; Sakai, Y.; Yokoyama, H.; Ito, K. Highly dielectric and flexible polyrotaxane elastomer by introduction of cyano groups. Polymer 2015, 59, 10–15. [Google Scholar] [CrossRef]
- Kato, K.; Yasuda, T.; Ito, K. Viscoelastic properties of slide-ring gels reflecting sliding dynamics of partial chains and entropy of ring components. Macromolecules 2013, 46, 310–316. [Google Scholar] [CrossRef]
- Dikshit, K.; Bruns, C.J. Post-synthesis modification of slide-ring gels for thermal and mechanical reconfiguration. Soft Matter 2021, 17, 5248–5257. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Wang, Y.-X.; Li, D.; Coen, C.-T.; Hwaun, E.; Chen, G.; Wu, H.-C.; Zhong, D.; Niu, S.; et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 2022, 375, 1411–1417. [Google Scholar] [CrossRef]
- Ogawa, M.; Kawasaki, A.; Koyama, Y.; Takata, T. Synthesis and properties of a polyrotaxane network prepared from a Pd-templated bis-macrocycle as a topological cross-linker. Polym. J. 2011, 43, 909–915. [Google Scholar] [CrossRef][Green Version]
- Zheng, J.; Yang, Z.; Sang, Y.; Zhang, F.; Zhang, Y.; Zhang, P. A simple preparation method of rotaxane cross-linker based on γ-cyclodextrin and its application in hydrogel materials. Mater. Today Commun. 2022, 33, 104760. [Google Scholar] [CrossRef]
- Ito, K. Slide-ring materials using topological supramolecular architecture. Curr. Opin. Solid State Mater. Sci. 2010, 14, 28–34. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Sun, Y.; Qin, Y.; Zhang, H.; Yu, X.; Liu, Y. Stretchable slide-ring supramolecular hydrogel for flexible electronic devices. Commun. Mater. 2022, 3, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, D.; Yang, J.; Nishi, T.; Ito, K.; Zhao, X.; Zhang, L. Novel slide-ring material/natural rubber composites with high damping property. Scientific Report 2016, 6, 22810. [Google Scholar] [CrossRef]
- Resmerita, A.-M.; Asandulesa, M.; Farcas, A. Evaluation of the chemical, morphological and dielectric properties of supramolecular networks consisting of polyethylene glycol polyrotaxanes and polystyrene/semi-rotaxane with hydroxypropyl-β-cyclodextrins. Macromol. Chem. Phys. 2022, 223, 2100383. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, Y.; Wang, Z.; Liu, H.; Le, M.; Lin, C.; Wu, G.; Wang, L.; Shi, X.; Jia, Y.-G.; et al. Polymerizable rotaxane hydrogels for three-dimensional printing fabrication of wearable sensors. Nat. Commun. 2023, 14, 1331. [Google Scholar] [CrossRef]
- Noda, Y.; Hayashi, Y.; Ito, K. From topological gels to slide-ring materials. J. Appl. Polym. Sci. 2014, 131, 40509. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular polymeric materials via cyclodextrins-guest interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Nakamitsu, T.; Kamachi, M. Preparation and characterization of polyrotaxanes containing many threaded a-cyclodextrins. J. Org. Chem. 1993, 58, 7524–7528. [Google Scholar] [CrossRef]
- Resmerita, A.-M.; Asandulesa, M.; Bulai, G.; Farcas, A. Novel supramolecular networks based on PEG and PEDOT cross-linked polyrotaxanes as electrical conductive materials. Eur. Polym. J. 2019, 119, 39–46. [Google Scholar] [CrossRef]
- Resmerita, A.-M.; Silion, M.; Cojocaru, C.; Farcas, A. Structural and morphological characterization of a new semi-polyrotaxane architecture based on 2-hydroxypropyl-β-cyclodextrins and polyisoprene. React. Funct. Polym. 2022, 181, 105459. [Google Scholar] [CrossRef]
- Kato, K.; Naito, M.; Ito, K.; Takahara, A.; Kojio, K. Freestanding tough glassy membranes produced by simple solvent casting of polyrotaxane derivatives. ACS Appl. Polym. Mater. 2021, 3, 4177–4183. [Google Scholar] [CrossRef]
- Munir, M.; Nazar, M.F.; Zafar, M.N.; Zubair, M.; Ashfaq, M.; Hosseini-Bandegharaei, A.; Ud-Din Khan, S.; Ahmad, A. Effective adsorptive removal of methylene blue from water by didodecyldimethylammonium bromide-modified brown clay. ACS Omega 2020, 5, 16711–16721. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons-a comparative study. Dyes Pigments 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Güleç, F.; Williams, O.; Kostas, E.T.; Samson, A.; Stevens, L.A.; Lester, E. A comprehensive comparative study on methylene blue removal from aqueous solution using biochars produced from rapeseed, whitewood, and seaweed via different thermal conversion technologies. Fuel 2022, 330, 125428. [Google Scholar] [CrossRef]
- Ivanets, A.; Prozorovich, V.; Roshchina, M.; Sychova, O.; Srivastava, V.; Sillanpää, M. Methylene blue adsorption on magnesium ferrite: Optimization study, kinetics and reusability. Mater. Today Commun. 2022, 31, 10359. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Kumar, V.B.; Luong, J.H.T.; Gedanken, A. Kinetics, isotherm, and thermodynamic studies of methylene blue adsorption on polyaniline and polypyrrole macro-nanoparticles synthesized by c-dot-initiated polymerization. ASC Omega 2018, 3, 7196–7203. [Google Scholar] [CrossRef]
- Sultana, S.; Mohammad, R.; Khan, Z.; Umar, K. Synthesis and characterization of copper ferrite nanoparticles doped polyaniline. J. Alloys Compd. 2012, 535, 44–49. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Versatility of polyethylene glycol (PEG) in designing solid-solid phase change materials (PCMs) for thermal management and their application to innovative technologies. J. Mater. Chem. A 2017, 5, 18379–18396. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Homocianu, M.; Samoila, P.; Grecu, I.; Bele, A. New composite membranes based on PVDF fibers loaded with TiO2: Sm nanostructures and reinforced with graphene/graphene oxide for photocatalytic applications. Surf. Interfaces 2022, 34, 102382. [Google Scholar] [CrossRef]
- Bushra, R.; Shahadat, M.; Ahmad, A.; Nabi, S.A.; Umar, K.; Oves, M.; Raeissi, A.S.; Muneer, M. Synthesis, characterization, antimicrobial activity and applications of polyanilineTi(IV)arsenophosphate adsorbent for the analysis of organic and inorganic pollutants. J. Hazard. Mater. 2014, 264, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Resmerita, A.-M.; Assaf, K.I.; Lazar, A.I.; Nau, W.M.; Farcas, A. Polyrotaxanes based on PEG-amine with cucurbit[7]uril, α-cyclodextrin and its tris-O-methylated derivative. Eur. Polym. J. 2017, 93, 323–333. [Google Scholar] [CrossRef]
- Inomata, A.; Sakai, Y.; Zhao, C.; Ruslim, C.; Shinohara, Y.; Yokoyama, H.; Amemiya, Y.; Ito, K. Crystallinity and cooperative motions of cyclic molecules in partially threaded solid-state polyrotaxanes. Macromolecules 2010, 43, 4660. [Google Scholar] [CrossRef]
- Mayumi, K.; Ito, K.; Kato, K. Polyrotaxanes and Slide-Ring Materials (Monographs in Supramolecular Chemistry No. 15); Gale, P., Steed, J., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Meng, J.; Liu, X.; Xia, L. Thermal-triggered Trans-1, 4 polyisoprene/polyethylene wax shape memory and self-healing composites. Polym. Test. 2022, 111, 107601. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, C.; Yang, B.; Zhang, X.; Dong, X.; Wang, D.; Liu, G. Achieving high elasticity of trans-1,4-polyisoprene with a combination of radiation crosslinking and thiol-ene grafting. Polym. Chem. 2023, 14, 81–91. [Google Scholar] [CrossRef]
- Watanabe, H.; Chen, Q.; Kawasaki, Y.; Matsumiya, Y.; Inoue, T.; Urakawa, O. Entanglement dynamics in miscible polyisoprene/poly(p-tert-butylstyrene) blends. Macromolecules 2011, 44, 1570–1584. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1983, 60, 309. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Bakar, N.F.A.; Him, N.R.N.; Lau, W.J. Adsorption kinetics of methylene blue dyes onto magnetic graphene oxide. J. Environ. Chem. Eng. 2018, 6, 2803–2811. [Google Scholar] [CrossRef]
- Harrou, A.; Gharibi, E.; Nasri, H.; Ouahabi, E.M. Thermodynamics and kinetics of the removal of methylene blue from aqueous solution by raw kaolin. SN Appl. Sci. 2020, 2, 277. [Google Scholar] [CrossRef]
- Gürses, A.; Doğar, Ç.; Yalçına, M.; Açıkyıldız, M.; Bayrak, R.; Karaca, S. The adsorption kinetics of the cationic dye, methylene blue, onto clay. J. Hazard. Mater. 2006, 131, 217–228. [Google Scholar] [CrossRef]
- Enache, A.-C.; Samoila, P.; Cojocaru, C.; Apolzan, R.; Predeanu, G.; Harabagiu, V. An eco-friendly modification of a walnut shell biosorbent for increased efficiency in wastewater treatment. Sustainability 2023, 15, 2704. [Google Scholar] [CrossRef]

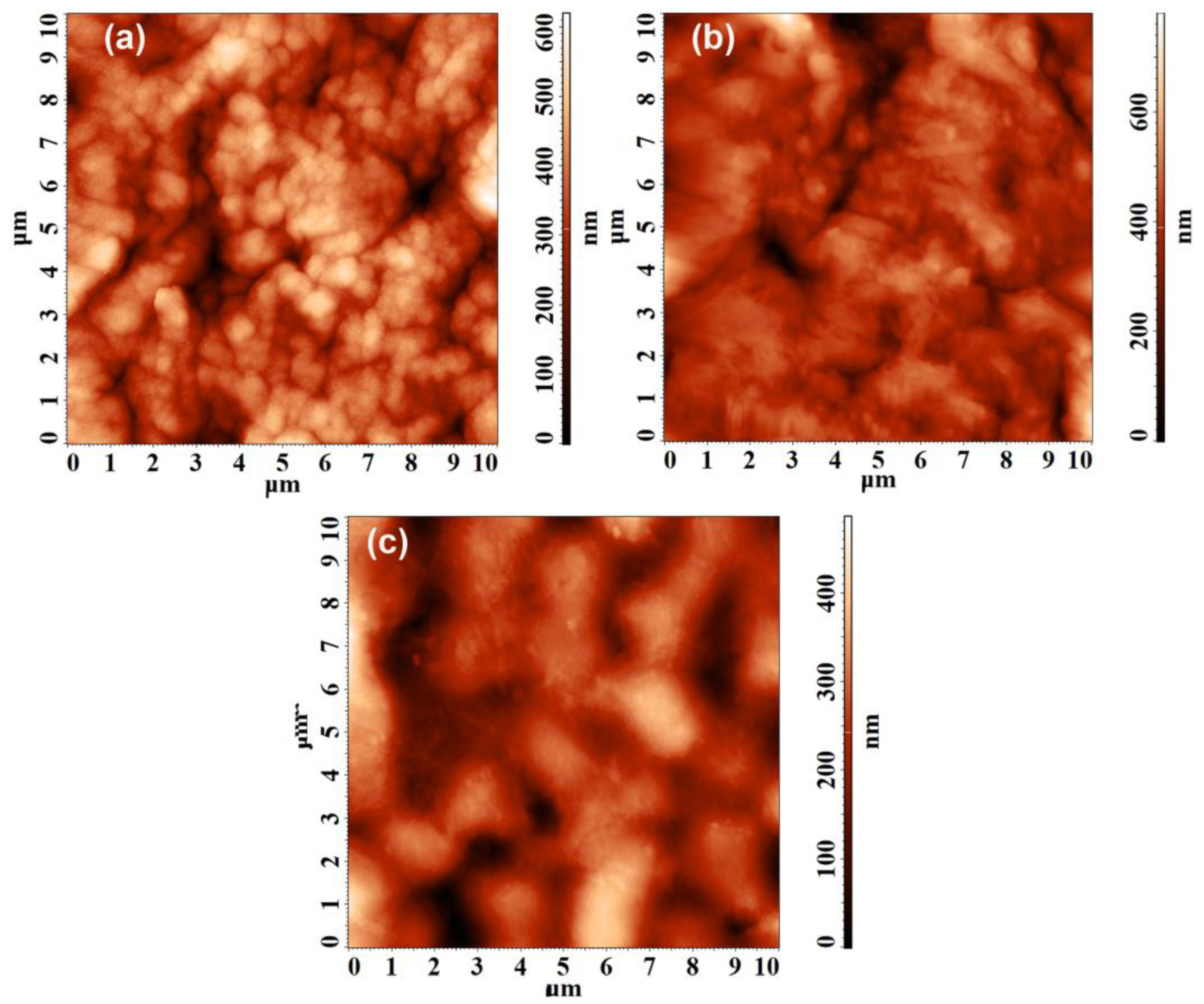

| Sample | Sa (nm) | Sq (nm) | Ssk | Sku | Sz (nm) |
|---|---|---|---|---|---|
| 1 | 70.69 | 89.02 | −0.382 | 0.157 | 308.94 |
| 2 | 64.73 | 86.08 | −0.429 | 1.599 | 358.29 |
| 3 | 58.16 | 73.09 | −0.125 | 0.040 | 239.54 |
| Sample | W a [%] | rpm b [nm] | BET c | |
|---|---|---|---|---|
| A d [m2 g−1] | Wm e [g g−1] | |||
| 1 | 20.2272 | 3.97 | 101.944 | 0.0290 |
| 2 | 20.9997 | 4.70 | 89.250 | 0.0254 |
| 3 | 20.3485 | 4.04 | 100.842 | 0.0287 |
| Sample | Step | Tonset a [°C] | Tpeak b [°C] | Tendset c [°C] | W d [%] | Rezidue e [%] |
|---|---|---|---|---|---|---|
| 1 | I | 205 | 241 | 263 | 15.06 | |
| II | 325 | 358 | 377 | 46.30 | 11.60 | |
| III | 419 | 445 | 477 | 27.04 | ||
| 2 | I | 220 | 263 | 303 | 13.09 | 6.32 |
| II | 329 | 360 | 376 | 49.53 | ||
| III | 410 | 426 | 471 | 31.06 | ||
| 3 | I | 309 | 346 | 368 | 53.31 | 9.51 |
| II | 413 | 455 | 483 | 37.18 |
| Model | Kinetic Model (Rate Equation) | Kinetic Model (Non-Linear Equation) | Kinetic Parameters |
|---|---|---|---|
| PFO a | qe = 2.5588 (mg/g) K1 = 2.6781 × 10−2 ε2 = 0.2429 | ||
| PSO b | qe = 2.9841 (mg/g) K2 = 1.112 × 10−2 ε2 = 0.1553 | ||
| MOE c | qe = 2.9835 (mg/g) K1 = 1.4296 × 10−5 K2 = 1.112 × 10−2 ε2 = 0.1553 | ||
| ID d | kd = 0.1757 J = 0.3124 ε2 = 0.4125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resmerita, A.-M.; Bargan, A.; Cojocaru, C.; Farcas, A. Synthesis, Properties and Adsorption Kinetic Study of New Cross-Linked Composite Materials Based on Polyethylene Glycol Polyrotaxane and Polyisoprene/Semi-Rotaxane. Materials 2023, 16, 5594. https://doi.org/10.3390/ma16165594
Resmerita A-M, Bargan A, Cojocaru C, Farcas A. Synthesis, Properties and Adsorption Kinetic Study of New Cross-Linked Composite Materials Based on Polyethylene Glycol Polyrotaxane and Polyisoprene/Semi-Rotaxane. Materials. 2023; 16(16):5594. https://doi.org/10.3390/ma16165594
Chicago/Turabian StyleResmerita, Ana-Maria, Alexandra Bargan, Corneliu Cojocaru, and Aurica Farcas. 2023. "Synthesis, Properties and Adsorption Kinetic Study of New Cross-Linked Composite Materials Based on Polyethylene Glycol Polyrotaxane and Polyisoprene/Semi-Rotaxane" Materials 16, no. 16: 5594. https://doi.org/10.3390/ma16165594
APA StyleResmerita, A.-M., Bargan, A., Cojocaru, C., & Farcas, A. (2023). Synthesis, Properties and Adsorption Kinetic Study of New Cross-Linked Composite Materials Based on Polyethylene Glycol Polyrotaxane and Polyisoprene/Semi-Rotaxane. Materials, 16(16), 5594. https://doi.org/10.3390/ma16165594








