PickT: A Decision-Making Tool for the Optimal Pickling Process Operation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Computer Tool Develoment Methodology
3. Results
3.1. Estimation of Inhibiton Efficency Based on Measurements
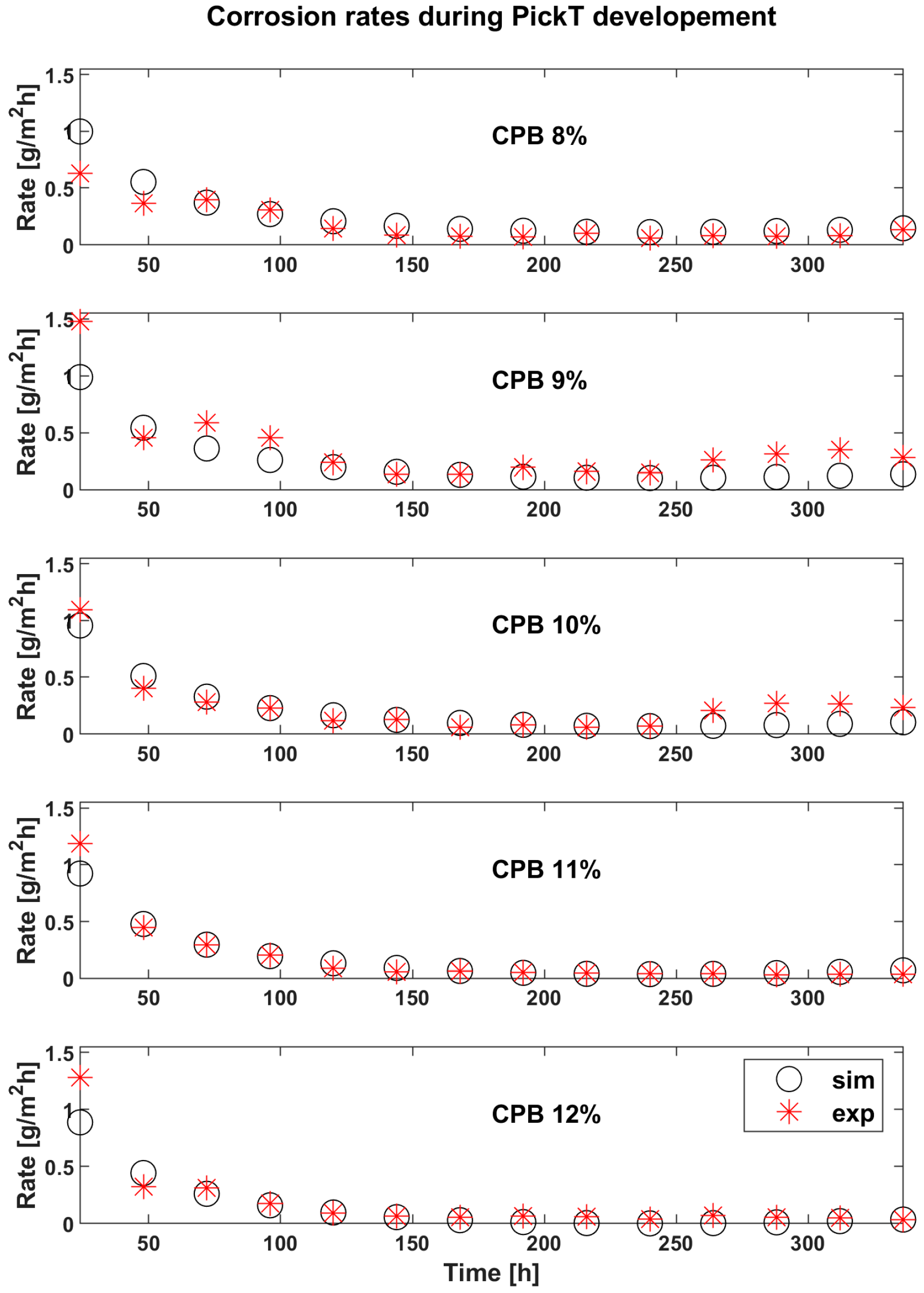
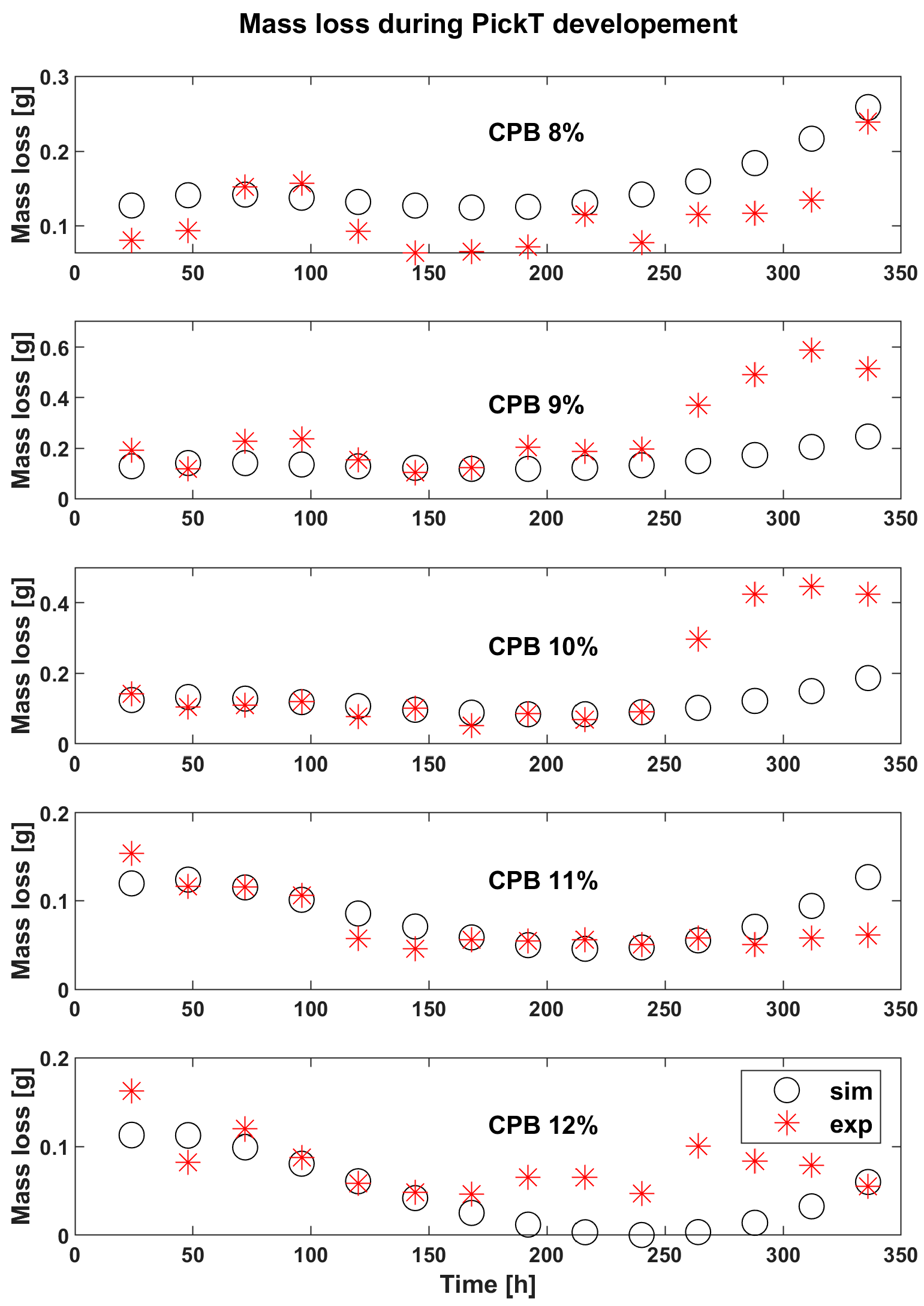
3.2. Results for CPB during the Developemnt and Verification of PickT
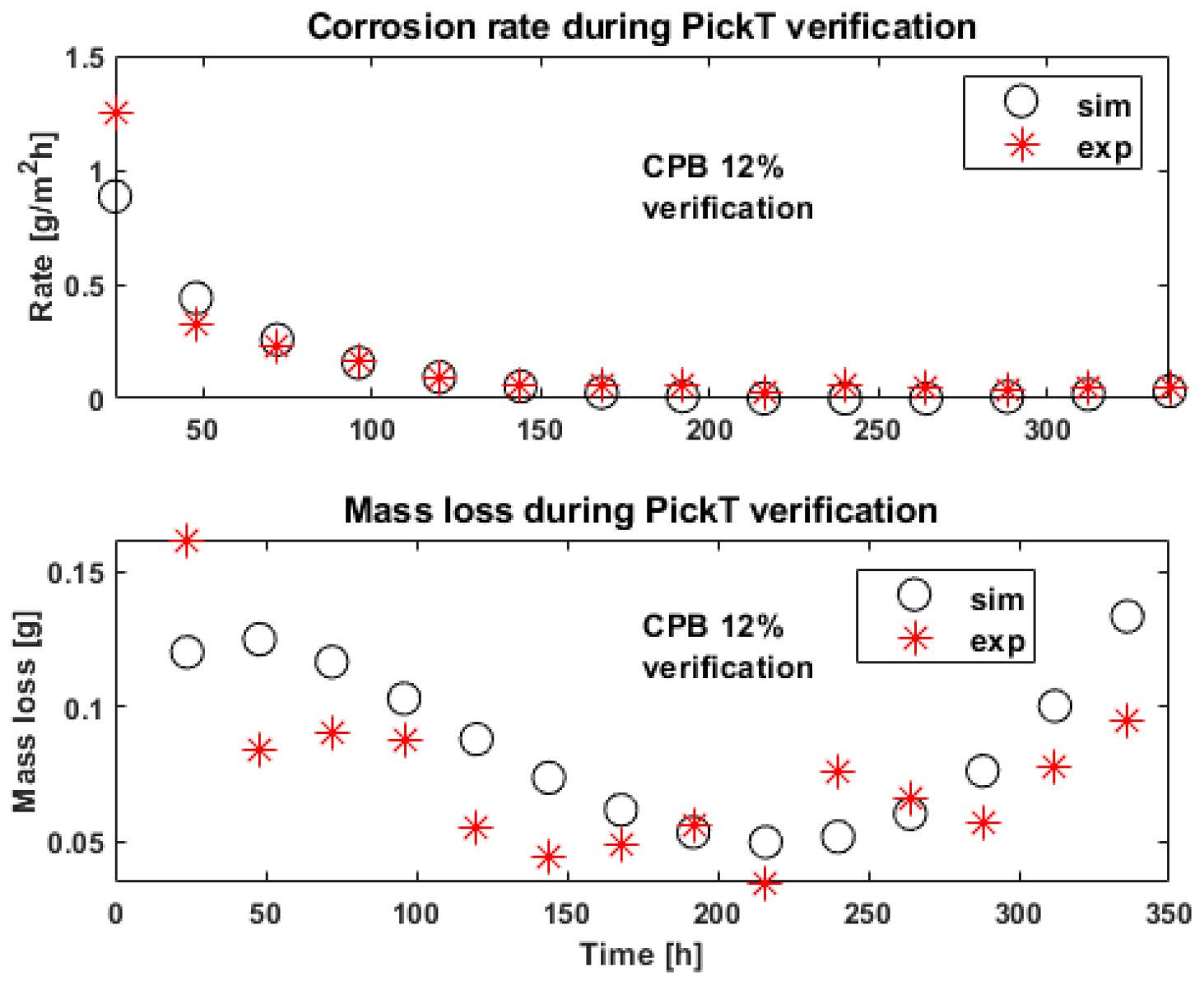
3.3. The Effectivness of PickT Transferability for MET
4. Discussion
4.1. Practical Assessment of the Bath Lifetime Using PickT Results
4.2. Discussion in the Context of Existing Literature
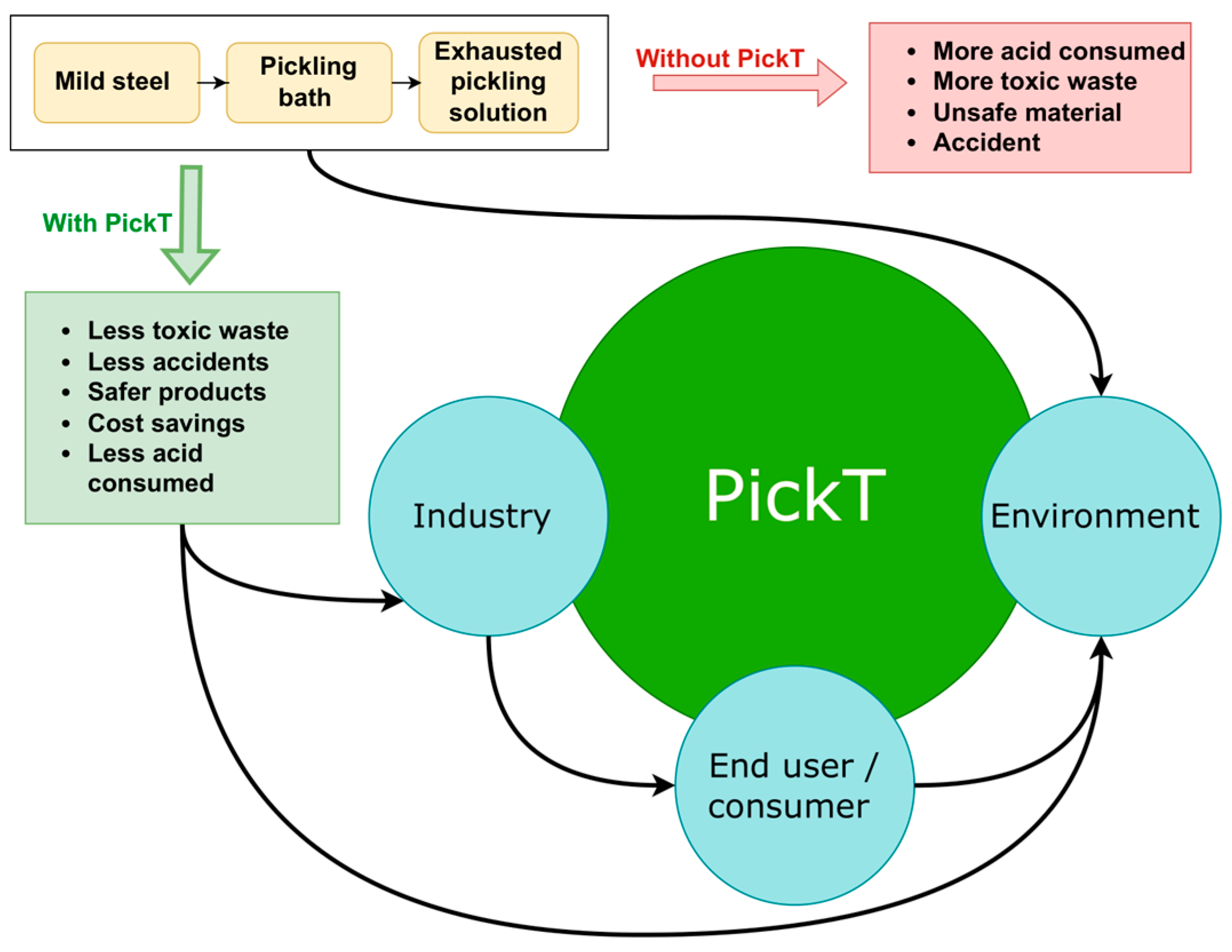
4.3. Industrial Use of PickT: Socio-Environmental and Economical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haque, J.; Srivastava, V.; Quraishi, M.A.; Singh Chauhan, D.; Lgaz, H.; Chung, I.-M. Polar Group Substituted Imidazolium Zwitterions as Eco-Friendly Corrosion Inhibitors for Mild Steel in Acid Solution. Corros. Sci. 2020, 172, 108665. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, X.; Wu, J.; Zhang, Y.; Lin, L.; Yang, G.; Deng, S.; Li, L.; Yu, X.; Qi, H.; et al. Sustainability Evaluation of a Steel Production System in China Based on Emergy. J. Clean. Prod. 2016, 112, 1498–1509. [Google Scholar] [CrossRef]
- Deyab, M.A. Understanding the Anti-Corrosion Mechanism and Performance of Ionic Liquids in Desalination, Petroleum, Pickling, de-Scaling, and Acid Cleaning Applications. J. Mol. Liq. 2020, 309, 113107. [Google Scholar] [CrossRef]
- Kuklík, V.; Kudláček, J. Chemical Pre-Treatment. In Hot-Dip Galvanizing of Steel Structures; Butterworth Heineman: Oxford, UK, 2016; pp. 17–27. [Google Scholar] [CrossRef]
- Hu, J.Y.; Zhang, S.S.; Chen, E.; Li, W.G. A Review on Corrosion Detection and Protection of Existing Reinforced Concrete (RC) Structures. Constr. Build. Mater. 2022, 325, 126718. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, Y.; Yuan, Y.; Zhou, X.; Liu, Y.; Meng, X. Recent Advances of Metal–Organic Frameworks in Corrosion Protection: From Synthesis to Applications. Chem. Eng. J. 2022, 430, 132823. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Nazdracheva, T. Anti-Corrosion Coatings for Protection of Steel Railway Structures Exposed to Atmospheric Environments: A Review. Constr. Build. Mater. 2021, 288, 123115. [Google Scholar] [CrossRef]
- Manoj, A.; Ramachandran, R.; Menezes, P.L. Self-Healing and Superhydrophobic Coatings for Corrosion Inhibition and Protection. Int. J. Adv. Manuf. Technol. 2020, 106, 2119–2131. [Google Scholar] [CrossRef]
- Chelaru, J.D.; Aylakov, D.; Mureşan, L.M. Corrosion Inhibition of Low Carbon Steel in Simulated Wastewater from Petroleum Industry. Stud. Univ. Babeș-Bolyai Chem. 2017, 62, 357–368. [Google Scholar] [CrossRef]
- Kamel, M.M.; Mohsen, Q.; Anwar, Z.M.; Sherif, M.A. An Expired Ceftazidime Antibiotic as an Inhibitor for Disintegration of Copper Metal in Pickling HCl Media. J. Mater. Res. Technol. 2021, 11, 875–886. [Google Scholar] [CrossRef]
- Fernandes, C.M.; da Ferreira Fagundes, T.S.; Escarpini dos Santos, N.; Rocha, T.S.d.M.; Garrett, R.; Borges, R.M.; Muricy, G.; Valverde, A.L.; Ponzio, E.A. Ircinia Strobilina Crude Extract as Corrosion Inhibitor for Mild Steel in Acid Medium. Electrochim. Acta 2019, 312, 137–148. [Google Scholar] [CrossRef]
- Guruprasad, A.M.; Sachin, H.P. Novel Cost-Effective Aqueous Amorphophallus Paeoniifolius Leaves Extract as a Green Corrosion Inhibitor for Mild Steel Corrosion in Hydrochloric Acid Medium: A Detailed Experimental and Surface Characterization Studies. Chem. Data Collect. 2021, 34, 100734. [Google Scholar] [CrossRef]
- Aribo, S.; Adeyeye, O.D.; Olusegun, S.J.; Ige, O.O.; Ogunbadejo, A.S.; Igbafen, E.O.; Olubambi, P.A. Inhibitive Performance of Sida Acuta and Monoethylene Glycol on the under Deposit Corrosion of UNS S30403 Stainless Steel. Int. J. Adv. Manuf. Technol. 2017, 93, 1853–1858. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Xie, B.; Gao, L.X.; Joo, H.G.; Lee, K.Y. Inhibition of Copper Corrosion in Acidic Chloride Solution by Methionine Combined with Cetrimonium Bromide/Cetylpyridinium Bromide. J. Appl. Electrochem. 2011, 41, 491–498. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J. yan The Performance of Complex Corrosion Inhibitor CFPS-Tb3+ for Aluminum Alloy. Chem. Pap. 2022, 76, 3507–3520. [Google Scholar] [CrossRef]
- Basheer, N.A.; Ali, A.A.; Allawi, R.H.; Mashaf, A.A. Preparation of New Nanocomposites and Evaluation of Their Efficiency as Corrosion Inhibitors in the Oil Industry. Chem. Pap. 2023, 77, 1655–1667. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, R.K.; Kaur, G.; Lata, S.; Singh, G. Synthesis, Electrochemical and Theoretical Characterization of Schiff Base as an Anticorrosive Molecule for Mild Steel in 0.5 M H2SO4 Medium. Chem. Pap. 2022, 76, 137–156. [Google Scholar] [CrossRef]
- Prabhu, P.R.; Hiremath, P.; Prabhu, D.; Gowrishankar, M.C.; Gurumurthy, B.M. Chemical, Electrochemical, Thermodynamic and Adsorption Study of EN8 Dual-Phase Steel with Ferrite-Martensite Structure in 0.5 M H2SO4 Using Pectin as Inhibitor. Chem. Pap. 2021, 75, 6083–6099. [Google Scholar] [CrossRef]
- Rahimi, A.; Abdouss, M.; Farhadian, A.; Guo, L.; Neshati, J. Development of a Novel Thermally Stable Inhibitor Based on Furfuryl Alcohol for Mild Steel Corrosion in a 15% HCl Medium for Acidizing Application. Ind. Eng. Chem. Res. 2021, 60, 11030–11044. [Google Scholar] [CrossRef]
- Cen, H.; Wu, C.; Chen, Z. Polydopamine Functionalized Graphene Oxide as an Effective Corrosion Inhibitor of Carbon Steel in HCl Solution. J. Mater. Sci. 2022, 57, 1810–1832. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Use of Asparagus Racemosus Extract as Green Corrosion Inhibitor for Mild Steel in 0.5 M H2SO4. J. Mater. Sci. 2018, 53, 8523–8535. [Google Scholar] [CrossRef]
- Tammam, R.H.; Saleh, M.M. Corrosion Inhibition of Copper-Iron Alloy in Acid Solution Using Cetylpyridinium Bromide as Cationic Surfactant. Prot. Met. Phys. Chem. Surf. 2019, 55, 761–769. [Google Scholar] [CrossRef]
- Haruna, K.; Saleh, T.A.; Quraishi, M.A. Expired Metformin Drug as Green Corrosion Inhibitor for Simulated Oil/Gas Well Acidizing Environment. J. Mol. Liq. 2020, 315, 113716. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Abdel-Azeim, S.; Chauhan, D.S.; Quraishi, M.A. Electrochemical and Computational Insights on the Application of Expired Metformin Drug as a Novel Inhibitor for the Sweet Corrosion of C1018 Steel. ACS Omega 2021, 6, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Jiang, X.; Zhou, L.; Liao, Y.; Duan, M.; Wang, H.; Pu, Q.; Zhou, J. Synergic Effect of Methyl Acrylate and N-Cetylpyridinium Bromide in N-Cetyl-3-(2-Methoxycarbonylvinyl)Pyridinium Bromide Molecule for X70 Steel Protection. Corros. Sci. 2015, 94, 224–236. [Google Scholar] [CrossRef]
- Fadl, A.M.; Sadeek, S.A.; Magdy, L.; Abdou, M.I.; El-Shiwiniy, W.H. Multi-Functional Epoxy Composite Coating Incorporating Mixed Cu(II) and Zr(IV) Complexes of Metformin and 2,2\-Bipyridine as Intensive Network Cross-Linkers Exhibiting Anti-Corrosion, Self-Healing and Chemical-Resistance Performances for Steel Petroleum Platforms. Arab. J. Chem. 2021, 14, 103367. [Google Scholar] [CrossRef]
- Kahyarian, A.; Nesic, S. On the Mechanism of Carbon Dioxide Corrosion of Mild Steel: Experimental Investigation and Mathematical Modeling at Elevated Pressures and Non-Ideal Solutions. Corros. Sci. 2020, 173, 108719. [Google Scholar] [CrossRef]
- Clarelli, F.; De Filippo, B.; Natalini, R. Mathematical Model of Copper Corrosion. Appl. Math. Model. 2014, 38, 4804–4816. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A Review on Methods of Regeneration of Spent Pickling Solutions from Steel Processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef]
- Hooshmand Zaferani, S.; Sharifi, M.; Zaarei, D.; Shishesaz, M.R. Application of Eco-Friendly Products as Corrosion Inhibitors for Metals in Acid Pickling Processes—A Review. J. Env. Chem. Eng. 2013, 1, 652–657. [Google Scholar] [CrossRef]
- Fu, J.J.; Li, S.N.; Wang, Y.; Liu, X.D.; Lu, L. De Computational and Electrochemical Studies on the Inhibition of Corrosion of Mild Steel by L-Cysteine and Its Derivatives. J. Mater. Sci. 2011, 46, 3550–3559. [Google Scholar] [CrossRef]
- Seddiqi, H.; Sadatshojaie, A.; Vaferi, B.; Yahyazadeh, E.; Salehi, A.; Wood, D.A. Mathematical Model for Iron Corrosion That Eliminates Chemical Potential Parameters. Chin. J. Chem. Eng. 2020, 28, 603–612. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Rodríguez, J.A.; Cruz-Borbolla, J.; Alvarado-Rodríguez, J.G.; Thangarasu, P. Development of a Predictive Model for Corrosion Inhibition of Carbon Steel by Imidazole and Benzimidazole Derivatives. Corros. Sci. 2016, 108, 23–35. [Google Scholar] [CrossRef]
- Tang, B.; Su, W.; Wang, J.; Fu, F.; Yu, G.; Zhang, J. Minimizing the Creation of Spent Pickling Liquors in a Pickling Process with High-Concentration Hydrochloric Acid Solutions: Mechanism and Evaluation Method. J. Environ. Manag. 2012, 98, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Lateef, H.M.; Sayed, A.R.; Shalabi, K. Synthesis and Theoretical Studies of Novel Conjugated Polyazomethines and Their Application as Efficient Inhibitors for C1018 Steel Pickling Corrosion Behavior. Surf. Interfaces 2021, 23, 101037. [Google Scholar] [CrossRef]
- Gebril, M.A.; Bedair, M.A.; Soliman, S.A.; Bakr, M.F.; Mohamed, M.B.I. Experimental and Computational Studies of the Influence of Non-Ionic Surfactants with Coumarin Moiety as Corrosion Inhibitors for Carbon Steel in 1.0 M HCl. J. Mol. Liq. 2022, 349, 118445. [Google Scholar] [CrossRef]
- Umoren, S.A.; Ogbobe, O.; Igwe, I.O.; Ebenso, E.E. Inhibition of Mild Steel Corrosion in Acidic Medium Using Synthetic and Naturally Occurring Polymers and Synergistic Halide Additives. Corros. Sci. 2008, 50, 1998–2006. [Google Scholar] [CrossRef]
- Guo, Y.; Jin, P.; Shao, M.; Dong, S.; Du, R.; Lin, C. Effect of an Environmentally Friendly Diisooctyl Sebacate-Based Mixed Corrosion Inhibitor on Reinforcing Steel. Acta Phys.-Chim. Sin. 2020, 38, 2003033. [Google Scholar] [CrossRef]
- Hossain, N.; Chowdhury, M.A.; Rana, M.; Hassan, M.; Islam, S. Terminalia Arjuna Leaves Extract as Green Corrosion Inhibitor for Mild Steel in HCl Solution. Results Eng. 2022, 14, 100438. [Google Scholar] [CrossRef]
- Peter, A.; Sharma, S.K.; Obot, I.B. Anticorrosive Efficacy and Adsorptive Study of Guar Gum with Mild Steel in Acidic Medium. J. Anal. Sci. Technol. 2016, 7, 26. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Fatyeyeva, K. Ionic Liquids as Green and Sustainable Steel Corrosion Inhibitors: Recent Developments. Chem. Eng. J. 2021, 425, 131480. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Saji, V.S. Heterocyclic Biomolecules as Green Corrosion Inhibitors. J. Mol. Liq. 2021, 341, 117265. [Google Scholar] [CrossRef]
- Krause, P.; Boyle, D.P.; Bäse, F. Comparison of Different Efficiency Criteria for Hydrological Model Assessment. Adv. Geosci. 2005, 5, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Koch, G.H.; Brongers, M.P.H.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Costs and Preventive Strategies in the United States; NACE International: Houston, TX, USA, 2001. [Google Scholar]
- Gueccia, R.; Winter, D.; Randazzo, S.; Cipollina, A.; Koschikowski, J.; Micale, G.D.M. An Integrated Approach for the HCl and Metals Recovery from Waste Pickling Solutions: Pilot Plant and Design Operations. Chem. Eng. Res. Des. 2021, 168, 383–396. [Google Scholar] [CrossRef]
- Gueccia, R.; Bogle, D.; Randazzo, S.; Tamburini, A.; Cipollina, A.; Winter, D.; Koschikowski, J.; Micale, G. Economic Benefits of Waste Pickling Solution Valorization. Membranes 2022, 12, 114. [Google Scholar] [CrossRef]
- Lorenz, M.; Seitfudem, G.; Randazzo, S.; Gueccia, R.; Gehring, F.; Prenzel, T.M. Combining Membrane and Zero Brine Technologies in Waste Acid Treatment for a Circular Economy in the Hot-Dip Galvanizing Industry: A Life Cycle Perspective. J. Sustain. Metall. 2023, 9, 537–549. [Google Scholar] [CrossRef]
- EUR-Lex–52011DC0571–EN–EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52011DC0571 (accessed on 4 August 2023).
- Foureaux, A.F.S.; Moreira, V.R.; Lebron, Y.A.R.; Santos, L.V.S.; Amaral, M.C.S. Direct Contact Membrane Distillation as an Alternative to the Conventional Methods for Value-Added Compounds Recovery from Acidic Effluents: A Review. Sep. Purif. Technol. 2020, 236, 116251. [Google Scholar] [CrossRef]
- Reig, M.; Bermejo, D. Innovative Process for Valorisation of Minerals and Added-Value Products from Metallurgical Wastewater; AEDyR: Madrid, Spain, 2021. [Google Scholar]
- Big Chemical Encyclopedia. Available online: https://chempedia.info/page/073223254216173198120098138174138231149080222169/ (accessed on 4 August 2023).
- Olsen, D.; Blumenschein, C. Regenerating Spent Pickling Liquor. US20030026746A1, 17 September 2002. [Google Scholar]
- Hazardous Wastes–Hazardous Wastes–Climate Policy Watcher. Available online: https://www.climate-policy-watcher.org/hazardous-wastes/hazardous-wastes-1.html (accessed on 4 August 2023).
- World Steel in Figures 2022. Available online: https://worldsteel.org/steel-topics/statistics/world-steel-in-figures-2022/ (accessed on 4 August 2023).
- Verma, C. Handbook of Science & Engineering of Green Corrosion Inhibitors: Modern Theory, Fundamentals & Practical Applications; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780323905893. [Google Scholar]




| Time [h] | Mass of the 7 Plates Immersed in 7 Different Solutions [g] | ||||||
|---|---|---|---|---|---|---|---|
| Blank | CI 8% | CI 9% | CI 10% | CI 11% | CI 12% | CI 12% Verification | |
| 0 | 15.1303 | 11.8791 | 13.8133 | 14.0309 | 14.0601 | 13.7025 | 13.9250 |
| 24 | 14.1971 | 11.7987 | 13.6220 | 13.8888 | 13.9061 | 13.5397 | 13.7630 |
| 48 | 13.5248 | 11.7052 | 13.5037 | 13.7848 | 13.7900 | 13.4572 | 13.6788 |
| 72 | 12.8135 | 11.5528 | 13.2757 | 13.6750 | 13.6746 | 13.3377 | 13.5887 |
| 96 | 12.1849 | 11.3956 | 13.0388 | 13.5559 | 13.5687 | 13.2501 | 13.5011 |
| 120 | 11.8349 | 11.3029 | 12.8846 | 13.4797 | 13.5118 | 13.1917 | 13.4458 |
| 144 | 11.5245 | 11.2397 | 12.7812 | 13.3791 | 13.4666 | 13.1433 | 13.4019 |
| 168 | 11.2320 | 11.1748 | 12.6584 | 13.3270 | 13.4106 | 13.0970 | 13.3532 |
| 192 | 10.9193 | 11.1031 | 12.4541 | 13.2417 | 13.3566 | 13.0318 | 13.2977 |
| 216 | 10.6091 | 10.9884 | 12.2669 | 13.1736 | 13.3009 | 12.9662 | 13.2630 |
| 240 | 10.2999 | 10.9117 | 12.0713 | 13.0824 | 13.2507 | 12.9191 | 13.1870 |
| 264 | 9.9774 | 10.7907 | 11.7012 | 12.7862 | 13.1933 | 12.8185 | 13.1210 |
| 288 | 9.6931 | 10.6805 | 11.2088 | 12.3617 | 13.1432 | 12.7345 | 13.0645 |
| 312 | 9.3959 | 10.5460 | 10.6209 | 11.9150 | 13.0853 | 12.6557 | 12.9870 |
| 336 | 9.0914 | 10.3067 | 10.1062 | 11.4896 | 13.0244 | 12.6006 | 12.8924 |
| Overall mass loss [g] | 6.0389 | 1.5724 | 3.7071 | 2.5413 | 1.0357 | 1.1019 | 1.0326 |
| IE% | - | 73.96 | 38.61 | 57.91 | 82.85 | 81.75 | 82.90 |
| Time [h] | Mass of the 4 Plates Immersed in 4 Different Solutions [g] | |||
|---|---|---|---|---|
| Blank | CI 3.3 [g/L] | CI 6.6 [g/L] | CI 10 [g/L] | |
| 0 | 4.8685 | 4.8739 | 5.0864 | 5.0260 |
| 2 | 4.8438 | 4.8555 | 5.0719 | 5.0172 |
| 4 | 4.8229 | 4.8377 | 5.0540 | 4.9995 |
| 6 | 4.8005 | 4.8184 | 5.0349 | 4.9809 |
| 8 | 4.7753 | 4.7979 | 5.0165 | 4.9627 |
| 10 | 4.7562 | 4.7826 | 5.0029 | 4.9512 |
| Overall mass loss [g] | 0.1133 | 0.0913 | 0.0835 | 0.0747 |
| IE% | - | 18.70 | 25.65 | 33.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crișan, C.A.; Timiș, E.C.; Vermeșan, H. PickT: A Decision-Making Tool for the Optimal Pickling Process Operation. Materials 2023, 16, 5567. https://doi.org/10.3390/ma16165567
Crișan CA, Timiș EC, Vermeșan H. PickT: A Decision-Making Tool for the Optimal Pickling Process Operation. Materials. 2023; 16(16):5567. https://doi.org/10.3390/ma16165567
Chicago/Turabian StyleCrișan, Claudia Alice, Elisabeta Cristina Timiș, and Horațiu Vermeșan. 2023. "PickT: A Decision-Making Tool for the Optimal Pickling Process Operation" Materials 16, no. 16: 5567. https://doi.org/10.3390/ma16165567
APA StyleCrișan, C. A., Timiș, E. C., & Vermeșan, H. (2023). PickT: A Decision-Making Tool for the Optimal Pickling Process Operation. Materials, 16(16), 5567. https://doi.org/10.3390/ma16165567








