Effects of Oxygen Flow during Fabrication by Magnetron Sputtering on Structure and Performance of Zr-Doped HfO2 Thin Films
Abstract
:1. Introduction
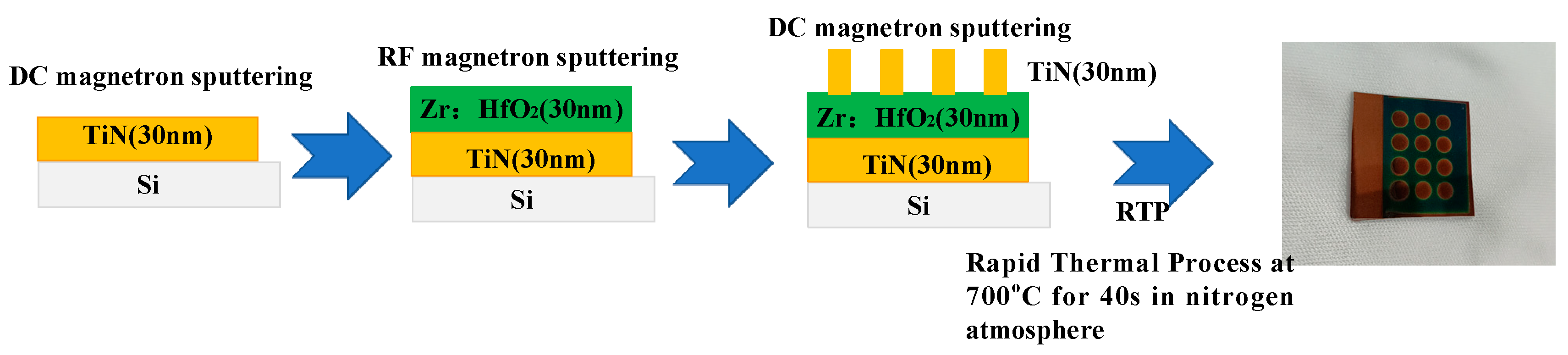
2. Film Preparation and Characterization
3. Results and Discussion
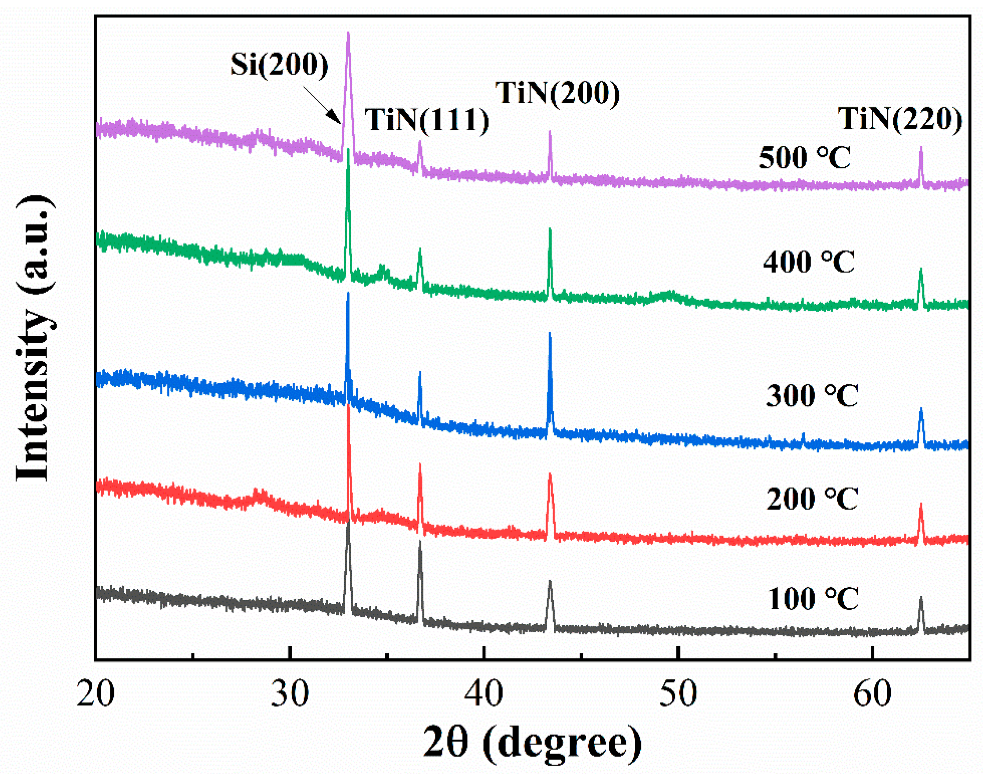
3.1. Variations in Deposition Rate and Components of Zr-Doped HfO2 Thin Films at Various Oxygen Flow Rates
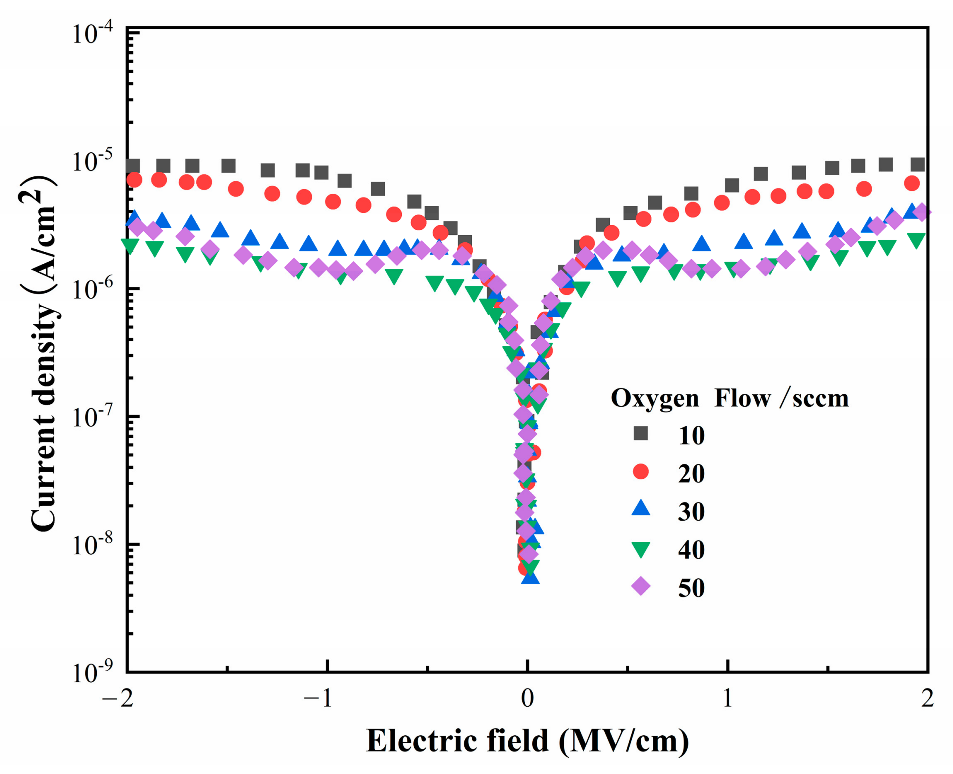
3.2. HZO-Based Heterojunction I–V Test and Polarization Curve Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, Z.; Cheng, X.; Shahid, T.; Anwar, M.; Zhenlun, S. Effects of O2 Flux on Structure, Optical Properties and Hydrophobicity of Highly Emissive Antireflective HfO2 Thin Films by Magnetron Sputtering. Vacuum 2022, 197, 110824. [Google Scholar]
- Khan, S.B.; Wu, H.; Ma, L.; Hou, M.; Zhang, Z. HfO2 Nanorod Array as High-Performance and High-Temperature Antireflective Coating. Adv. Mater. Interfaces 2017, 4, 1600892. [Google Scholar] [CrossRef]
- Lin, S. Optical properties of HfO2 nanoceramic films as a function of N–Bi co-doping. Ceram. Int. 2014, 40, 5707–5713. [Google Scholar] [CrossRef]
- Buckley, J.; De Salvo, B.; Ghibaudo, G.; Gely, M.; Damlencourt, J.F.; Martin, F.; Nicotra, G.; Deleonibus, S. Investigation of SiO2/HfO2 gate stacks for application to non-volatile memory devices. Solid State Electron. 2005, 49, 1833–1840. [Google Scholar] [CrossRef]
- Fan, Y.; Ovchinnikov, I.V.; Jiang, W.; Schwartz, R.N.; Wang, K.L. Fluctuations in nanoscale magnetoelectronics devices. J. Appl. Phys. 2012, 112, 114–174. [Google Scholar] [CrossRef] [Green Version]
- Boscke, T.S.; Muller, J.; Brauhaus, D.; Schroder, U.; Bottger, U. Ferroelectricity in hafnium oxide thin films. Appl. Phys. Lett. 2011, 99, 112903. [Google Scholar] [CrossRef]
- Jena, S.; Tokas, R.B.; Misal, J.S.; Rao, K.D.; Udupa, D.V.; Thakur, S.; Sahoo, N.K. Effect of O2/Ar gas flow ratio on the optical properties and mechanical stress of sputtered HfO2 thin films. Thin Solid Film. 2015, 592, 135–142. [Google Scholar] [CrossRef]
- Min, H.P.; Han, J.K.; Yu, J.K.; Moon, T.; Kim, K.D.; Hwang, C.S. Thin HfxZr1-xO2 films: A new lead-free system for electrostatic supercapacitors with large energy storage density and robust thermal stability. Adv. Energy Mater. 2014, 16, 1400610. [Google Scholar]
- Aarik, J.; Mändar, H.; Kirm, M.; Pung, L. Optical characterization of HfO2 thin films grown by atomic layer deposition. Thin Solid Film. 2004, 466, 41–47. [Google Scholar] [CrossRef]
- Dave, V.; Dubey, P.; Gupta, H.O.; Chandra, R. Influence of sputtering pressure on the structural, optical and hydrophobic properties of sputtered deposited HfO2 coatings. Thin Solid Film. 2013, 549, 2–7. [Google Scholar] [CrossRef]
- Tan, R.; Azuma, Y.; Kojima, I. Suppression of interfacial diffusion by a predeposited Hf metal layer on SiO2/Si. Appl. Surf. Sci. 2004, 22, 346–350. [Google Scholar] [CrossRef]
- Aygun, G.; Yildiz, I. Interfacial and structural properties of sputtered HfO2 layers. J. Appl. Phys. 2009, 106, 014312. [Google Scholar] [CrossRef] [Green Version]
- Lehan, J.; Mao, Y.; Bovard, B.G.; Macleod, H.A. Optical and microstructural properties of hafnium dioxide thin films. Thin Solid Film. 1991, 203, 227–250. [Google Scholar] [CrossRef]
- Smirnova, T.P.; Yakovkina, L.V.; Kitchai, V.N.; Kaichev, V.; Shubin, Y.; Morozova, N.; Zherikova, K. Chemical vapor deposition and characterization of hafnium oxide films. J. Phys. Chem. Solids 2008, 69, 685–687. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.G.; Feng, Y. Thermal stability and electrical properties of pulsed laser-deposited Hf-silicate thin films for high-k gate dielectric applications. J. Phys. D Appl. Phys. 2003, 36, 3051–3056. [Google Scholar] [CrossRef]
- Mittmann, T.; Materano, M.; Lomenzo, P.D.; Park, M.H.; Stolichnov, I.; Cavalieri, M.; Zhou, C.; Chung, C.-C.; Jones, J.L.; Szyjka, T.; et al. Origin of Ferroelectric Phase in Undoped HfO2 Films Deposited by Sputtering. Adv. Mater. Interfaces 2019, 6, 1900042. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhou, D.; Sun, N.; Wang, J.; Li, S. Effect of Bias Voltage on Substrate for the Structure and Electrical Properties of Y:HfO2 Thin Films Deposited by Reactive Magnetron Co-Sputtering. Adv. Electron. Mater. 2021, 7, 2100488. [Google Scholar] [CrossRef]
- Musil, J.; Baroch, P.; Vlček, J.; Nam, K.H.; Han, J.G. Reactive magnetron sputtering of thin films: Present status and trends. Thin Solid Film. 2005, 475, 208–218. [Google Scholar] [CrossRef]
- Mimura, T.; Shimizu, T.; Funakubo, H. Ferroelectricity in YO1.5-HfO2 films around 1 μm in thickness. Appl. Phys. Lett. 2019, 115, 032901. [Google Scholar] [CrossRef]
- Böescke, T.S.; Teichert, S.; Bräuhaus, D.; Müller, J.; Schröder, U.; Böttger, U.; Mikolajick, T. Phase transitions in ferroelectric Silicon doped Hafnium oxide. Appl. Phys. Lett. 2011, 99, 112904. [Google Scholar] [CrossRef]
- Mueller, S.; Mueller, J.; Singh, A.; Riedel, S.; Sundqvist, J.; Schroeder, U. Mikolajick.Incipient ferroelectricity in Al-doped HfO2 thin films. Adv. Funct. Mater. 2014, 22, 2412–2417. [Google Scholar] [CrossRef]
- Schroeder, U.; Richter, C.; Park, M.H.; Schenk, T.; Pešić, M.; Hoffmann, M.; Fengler, F.; Pohl, D.; Rellinghaus, B.; Zhou, C.; et al. Lanthanum-Doped Hafnium Oxide: A Robust Ferroelectric Material. Appl. Phys. Lett. 2018, 57, 2752–2765. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.; Boescke, T.S.; Schroeder, U.; Mueller, S.; Braeuhaus, D.; Boettger, U.; Frey, L.; Mikolajick, T. Ferroelectricity in simple binary ZrO2 and HfO2. Nano Lett. 2012, 12, 4318–4323. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, H.J.; Kim, Y.J.J.; Moon, T.; Kim, K.D.; Hwang, C.S. Toward a multifunctional monolithic device based on pyroelectricity and the electrocaloric effect of thin antiferroelectric HfxZr1-xO2 films. Nano Energy 2015, 12, 131–140. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, H.J.; Kim, Y.J.; Lee, W.; Moon, T.; Hwang, C.S. Evolution of phases and ferroelectric properties of thin Hf0.5Zr0.5O2 films according to the thickness and annealing temperature. Appl. Phys. Lett. 2013, 102, 242905. [Google Scholar] [CrossRef]
- Mittmann, T.; Materano, M.; Chang, S.-C.; Karpov, I.; Mikolajick, T.; Schroeder, U. Impact of oxygen vacancy content in ferroelectric HZO films on the device performance. In Proceedings of the 2020 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 12–18 December 2020; pp. 18.4.1–18.4.4. [Google Scholar]
- Islamov, D.R.; Zalyalov, T.M.; Orlov, O.M.; Gritsenko, V.A.; Krasnikov, G.Y. Impact of oxygen vacancy on the ferroelectric properties of lanthanum-doped hafnium oxide. Appl. Phys. Lett. 2020, 117, 162901. [Google Scholar] [CrossRef]
- Baumgarten, L.; Szyjka, T.; Mittmann, T.; Materano, M.; Schlueter, C.; Schroeder, U.; Müller, M. Impact of vacancies and impurities on ferroelectricity in PVD- and ALD- grown HfO2 films. Appl. Phys. Lett. 2020, 118, 032903. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.K.; Yang, Q.; Jiang, J.; Fan, P.; Liao, M.; Zhou, Y.C. The effects of oxygen vacancies on ferroelectric phase transition of HfO2-based thin film from first-principle. Comput. Mater. Sci. 2019, 167, 143–150. [Google Scholar] [CrossRef]
- Akkaya, A.; Kahveci, O.; Şahin, B.; Ayyıldız, E. Simultaneously-doping of HfO2 thin films by Ni with sputtering technique and effect of post annealing on structural and electrical properties. Phys. B Condens. Matter 2023, 665, 415034. [Google Scholar] [CrossRef]
- Olsen, T.; Schröder, U.; Müller, S.; Krause, A.; Martin, D.; Singh, A.; Müller, J.; Geidel, M.; Mikolajick, T. Co-sputtering yttrium into hafnium oxide thin films to produce ferroelectric properties. Appl. Phys. Lett. 2012, 101, 082905. [Google Scholar] [CrossRef]
- Xu, L.; Shibayama, S.; Izukashi, K.; Nishimura, T.; Yajima, T.; Migita, S.; Toriumi, A. General relationship for cation and anion doping effects on ferroelectric HfO2 formation. In Proceedings of the 2016 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 3–7 December 2016; pp. 25.2.1–25.2.4. [Google Scholar]
- Xu, L.; Nishimura, T.; Shibayama, S.; Yajima, T.; Migita, S.; Toriumi, A. Kinetic pathway of the ferroelectric phase formation in doped HfO2 films. J. Appl. Phys. 2017, 122, 124104. [Google Scholar] [CrossRef]
- Sun, N.; Xu, J.; Zhou, D.; Zhao, P.; Li, S.; Wang, J.; Chu, S.; Ali, F. DC reactively sputtered TiNx thin films for capacitor electrodes. J. Mater. Sci. Mater. Electron. 2018, 29, 10170–10176. [Google Scholar] [CrossRef]
- Stutzin, G.C.; Rózsa, K.; Gallagher, A. Deposition rates in direct current diode sputtering. J. Vaccuum Sci. Technol. A 1993, 11, 647–656. [Google Scholar] [CrossRef]
- Heo, S.; Tahir, D.; Chung, J.G.; Lee, J.C.; Kim, K.; Lee, J.; Lee, H.; Park, G.S.; Oh, S.K.; Kang, H.J.; et al. Band alignment of atomic layer deposited (HfZrO4)1-x(SiO2)x gate dielectrics on Si(100). Appl. Phys. Lett. 2015, 107, 182101. [Google Scholar] [CrossRef]
- Ganapathi, K.L.; Bhat, N.; Mohan, S. Influence of O2 flow rate on HfO2 gate dielectrics for back-gated graphene transistors. Semicond. Sci. Technol. 2014, 29, 055007. [Google Scholar] [CrossRef]
- Foster, A.S.; Gejo, F.L.; Shluger, A.L.; Nieminen, R.M. Vacancy and interstitial defects in hafnia. Phys. Rev. B 2006, 65, 174117. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.; Oh, S.-J.; Chang, Y.J.; Noh, T.W.; Jung, R.; Lee, J.-C. Role of oxygen vacancy in HfO2/SiO2/Si(100) interfaces. Appl. Phys. Lett. 2006, 88, 193502. [Google Scholar] [CrossRef]
- Rushchanskii, K.Z.; Blügel, S.; Ležaić, M. Ordering of oxygen vacancies and related ferroelectric Properties in HfO2-δ. Phys. Rev. Lett. 2021, 127, 087602. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Lee, Y.H.; Kim, H.J.; Moon, T.; Kim, K.D.; Müller, J.; Kersch, A.; Schroeder, U.; Mikolajick, T.; Hwang, C.S. Ferroelectricity and antiferroelectricity of doped thin HfO2-based films. Adv. Mater. 2015, 27, 1811–1831. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Y.; Liu, L.; Zhao, J.; Qin, X.; Zhang, J.; Zhang, C.; Liu, W. Effects of Oxygen Flow during Fabrication by Magnetron Sputtering on Structure and Performance of Zr-Doped HfO2 Thin Films. Materials 2023, 16, 5559. https://doi.org/10.3390/ma16165559
Xi Y, Liu L, Zhao J, Qin X, Zhang J, Zhang C, Liu W. Effects of Oxygen Flow during Fabrication by Magnetron Sputtering on Structure and Performance of Zr-Doped HfO2 Thin Films. Materials. 2023; 16(16):5559. https://doi.org/10.3390/ma16165559
Chicago/Turabian StyleXi, Yingxue, Lei Liu, Jiwu Zhao, Xinhui Qin, Jin Zhang, Changming Zhang, and Weiguo Liu. 2023. "Effects of Oxygen Flow during Fabrication by Magnetron Sputtering on Structure and Performance of Zr-Doped HfO2 Thin Films" Materials 16, no. 16: 5559. https://doi.org/10.3390/ma16165559
APA StyleXi, Y., Liu, L., Zhao, J., Qin, X., Zhang, J., Zhang, C., & Liu, W. (2023). Effects of Oxygen Flow during Fabrication by Magnetron Sputtering on Structure and Performance of Zr-Doped HfO2 Thin Films. Materials, 16(16), 5559. https://doi.org/10.3390/ma16165559






