Development of Liposomal and Liquid Crystalline Lipidic Nanoparticles with Non-Ionic Surfactants for Quercetin Incorporation
Abstract
:1. Introduction
2. Materials and Methods
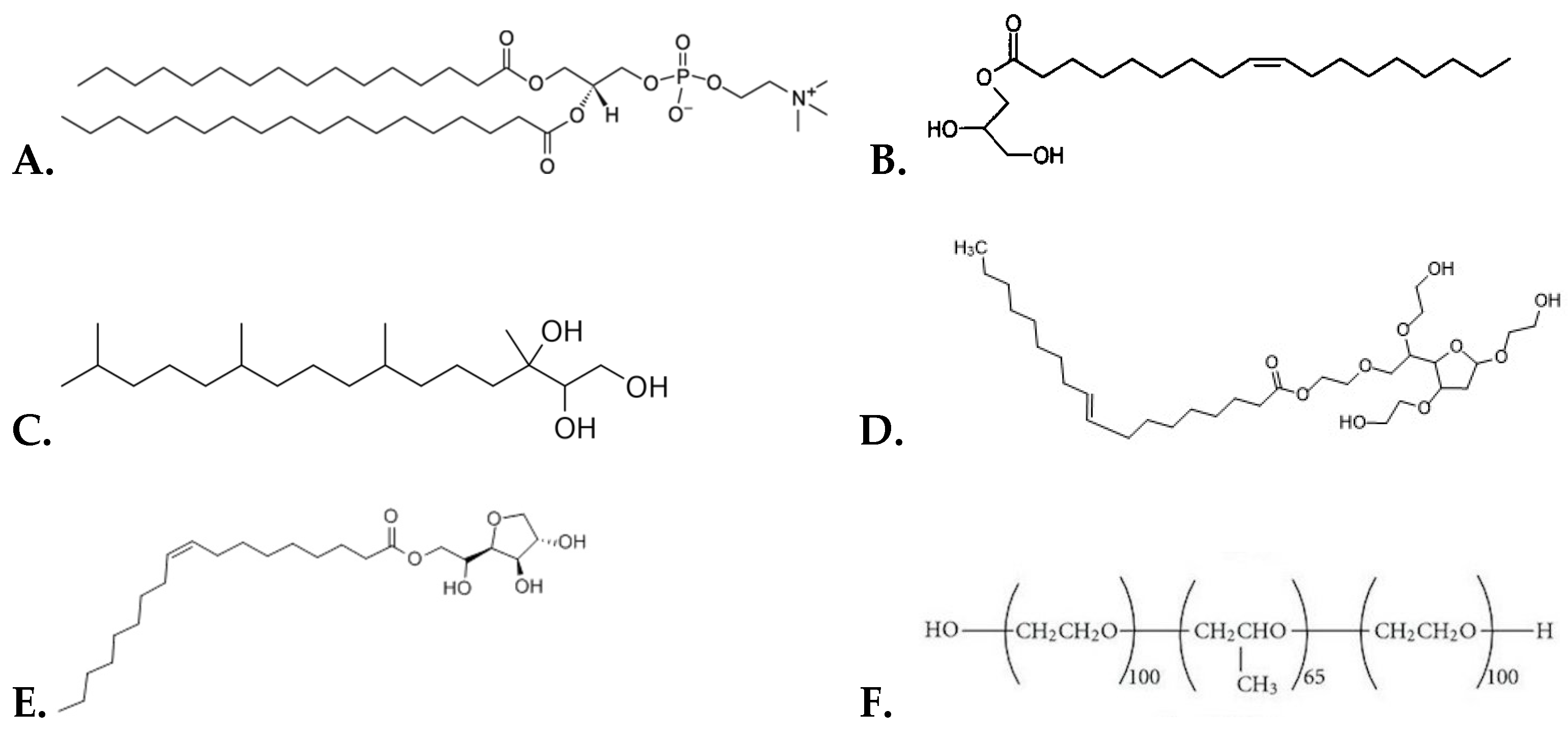
2.1. Materials
2.2. Methods
2.2.1. Preparation of Liposomal Systems
2.2.2. Preparation of Liquid Crystalline Nanoparticles
2.2.3. Dynamic and Electrophoretic Light Scattering
2.2.4. Incubation in Fetal Bovine Serum
2.2.5. Entrapment Efficiency Determination
2.2.6. Cell Culture and Cell Proliferation Assay
3. Results and Discussion
3.1. Physicochemical Characterization of the Prepared Nanoparticles
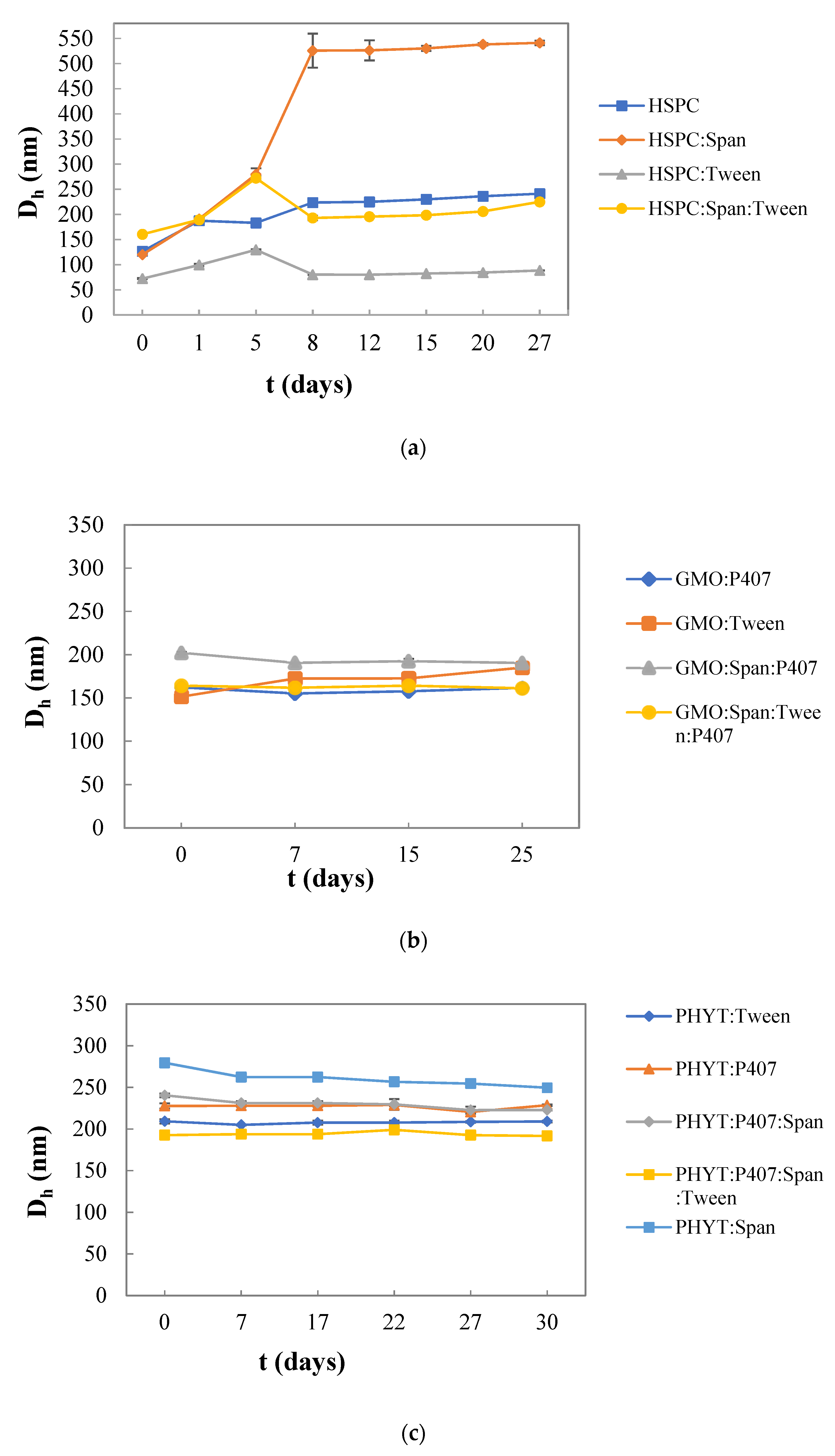
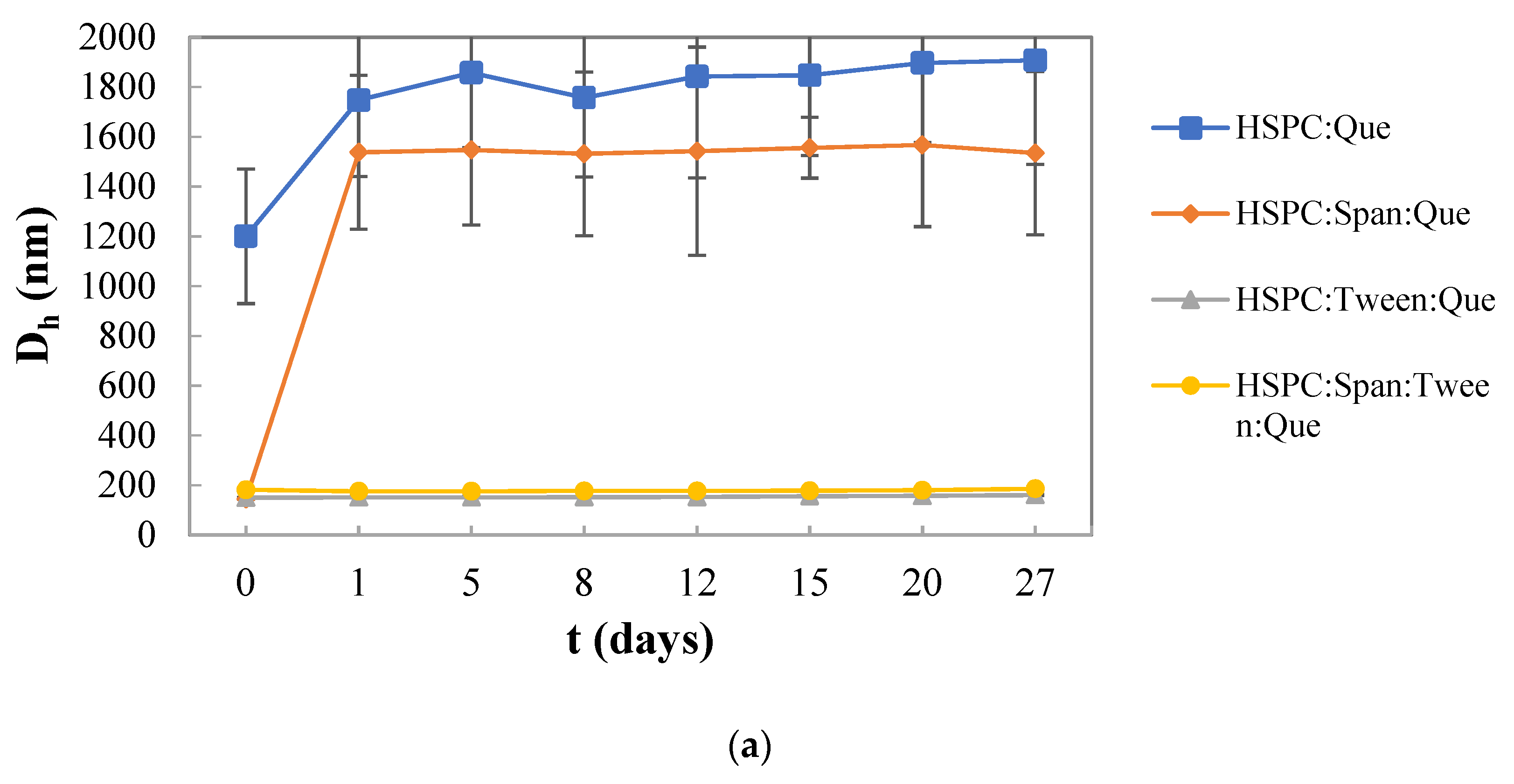
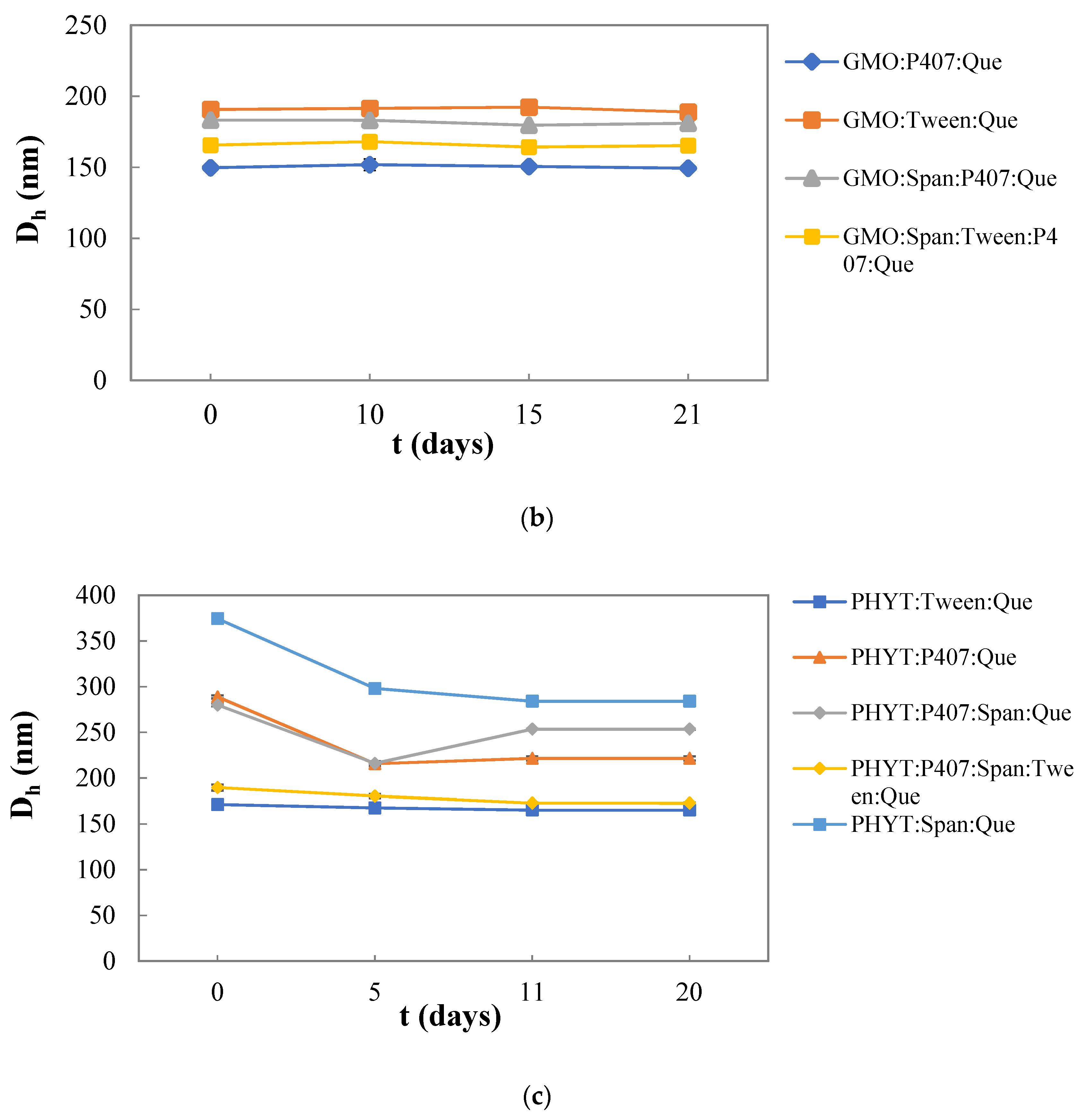
3.2. Colloidal Stability of the Nanosystems over Time
3.3. The Effect of the Serum Proteins on the Physicochemical Behavior of the Prepared Liposomal Nanosystems
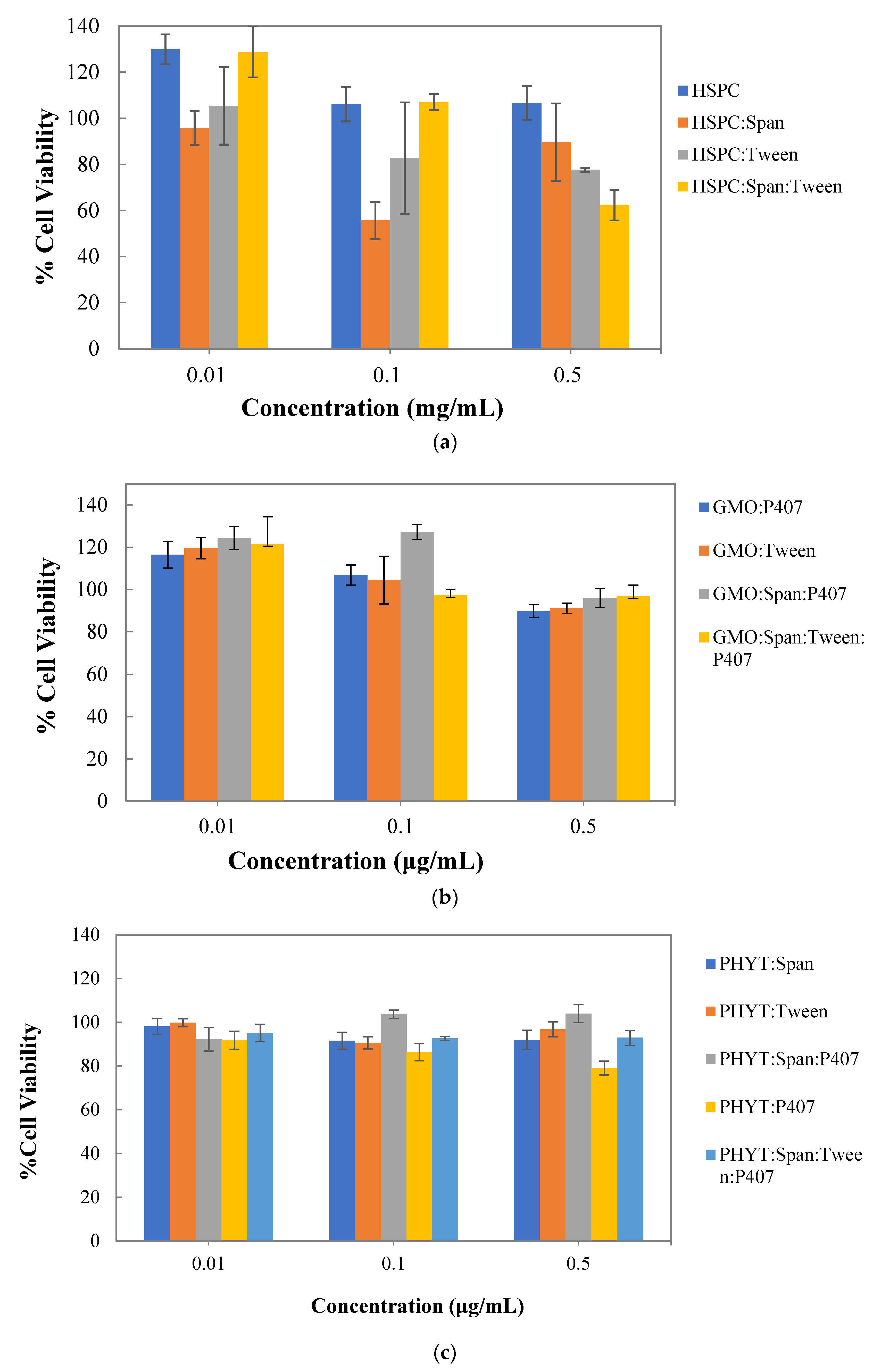
3.4. In Vitro Cytotoxicity Studies
3.5. Quercetin Entrapment Results
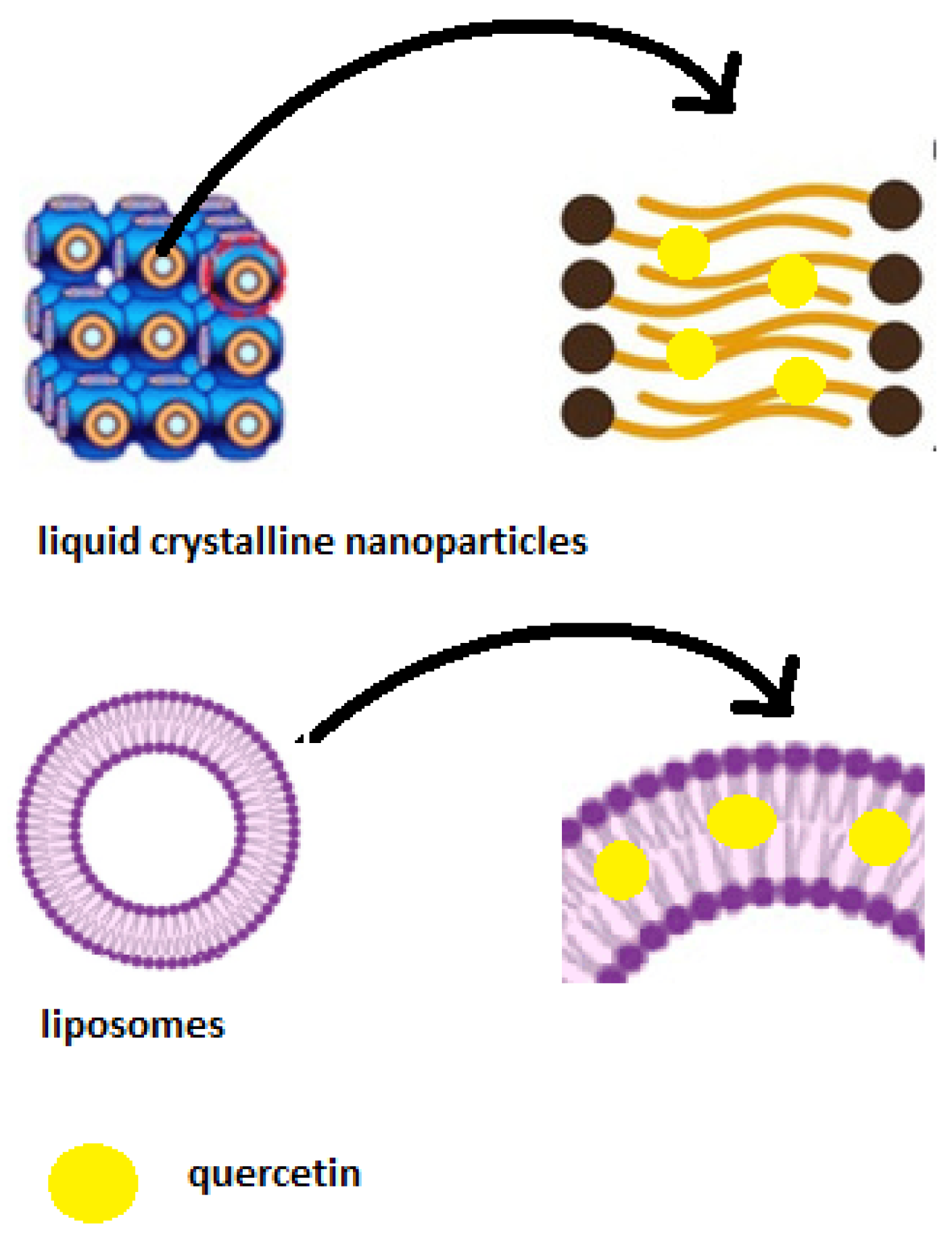
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadhwa, K.; Kadian, V.; Puri, V.; Bhardwaj, B.Y.; Sharma, A.; Pahwa, R.; Rao, R.; Gupta, M.; Singh, I. New Insights into Quercetin Nanoformulations for Topical Delivery. Phytomed. Plus 2022, 2, 100257. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Vitoria Pupo Silvestrini, A.; Wender Debiasi, B.; Garcia Praça, F.; Vitoria Lopes Badra Bentley, M. Progress and Challenges of Lyotropic Liquid Crystalline Nanoparticles for Innovative Therapies. Int. J. Pharm. 2022, 628, 122299. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Papakyriakopoulou, P.; Saitani, E.-M.; Valsami, G.; Pippa, N.; Skaltsa, H. Recent Advances in Nanoformulations for Quercetin Delivery. Pharmaceutics 2023, 15, 1656. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, K.T.; Youn, Y.S.; Lee, E.S. pH-Sensitive Twin Liposomes Containing Quercetin and Laccase for Tumor Therapy. Biomacromolecules 2022, 23, 3688–3697. [Google Scholar] [CrossRef]
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci. Rep. 2020, 10, 2440. [Google Scholar] [CrossRef] [Green Version]
- Caddeo, C.; Gabriele, M.; Fernàndez-Busquets, X.; Valenti, D.; Fadda, A.M.; Pucci, L.; Manconi, M. Antioxidant activity of quercetin in Eudragit-coated liposomes for intestinal delivery. Int. J. Pharm. 2019, 565, 64–69. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.K.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C. Sterically Stabilized Liposomes: Improvements in Pharmacokinetics and Antitumor Therapeutic Efficacy. Proc. Natl. Acad. Sci. USA 1991, 88, 11460–11464. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Sun, M.; Zhang, J.; Bi, Y. Coating Liposomes with Ring-like PEG: The Synthesis and Stealth Effect of Cholesterol–PEG–Cholesterol. Mater. Adv. 2022, 3, 2417–2424. [Google Scholar] [CrossRef]
- Mat Azmi, I.D.; Wu, L.; Wibroe, P.P.; Nilsson, C.; Østergaard, J.; Stürup, S.; Gammelgaard, B.; Urtti, A.; Moghimi, S.M.; Yaghmur, A. Modulatory Effect of Human Plasma on the Internal Nanostructure and Size Characteristics of Liquid-Crystalline Nanocarriers. Langmuir 2015, 31, 5042–5049. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, P.; Gui, S. Cubic and Hexagonal Liquid Crystals as Drug Delivery Systems. BioMed Res. Int. 2014, 2014, 815981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulet, X.; Boyd, B.J.; Drummond, C.J. Advances in Drug Delivery and Medical Imaging Using Colloidal Lyotropic Liquid Crystalline Dispersions. J. Colloid Interface Sci. 2013, 393, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chountoulesi, M.; Pispas, S.; Tseti, I.K.; Demetzos, C. Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms. Pharmaceuticals 2022, 15, 429. [Google Scholar] [CrossRef] [PubMed]
- Kopanichuk, I.V.; Vedenchuk, E.A.; Koneva, A.S.; Vanin, A.A. Structural Properties of Span 80/Tween 80 Reverse Micelles by Molecular Dynamics Simulations. J. Phys. Chem. B 2018, 122, 8047–8055. [Google Scholar] [CrossRef]
- Mahdi, E.S.; Sattar, M.; Sakeena, M.H.F.; Abdulkarim, M.; Noor, A.M.; Abdullah, G. Effect of Surfactant and Surfactant Blends on Pseudoternary Phase Diagram Behavior of Newly Synthesized Palm Kernel Oil Esters. DDDT 2011, 5, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Mitsou, E.; Pletsa, V.; Sotiroudis, G.T.; Panine, P.; Zoumpanioti, M.; Xenakis, A. Development of a Microemulsion for Encapsulation and Delivery of Gallic Acid. The Role of Chitosan. Colloids Surf. B Biointerfaces 2020, 190, 110974. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.G.A.; Ahmed, A.-M.M.; Abdel-Rahman, H.H.; Moustafa, A.H.E. Use of Span 80 and Tween 80 for Blending Gasoline and Alcohol in Spark Ignition Engines. Energy Rep. 2019, 5, 221–230. [Google Scholar] [CrossRef]
- Jiao, J.; Burgess, D.J. Rheology and Stability of Water-in-Oil-in-Water Multiple Emulsions Containing Span 83 and Tween 80. AAPS PharmSci 2003, 5, 62–73. [Google Scholar] [CrossRef]
- Singh, V.K.; Pal, K.; Banerjee, I.; Pramanik, K.; Anis, A.; Al-Zahrani, S.M. Novel Organogel Based Lyotropic Liquid Crystal Physical Gels for Controlled Delivery Applications. Eur. Polym. J. 2015, 68, 326–337. [Google Scholar] [CrossRef]
- Sayyad, N.; Maji, R.; Omolo, C.A.; Ganai, A.M.; Ibrahim, U.H.; Pathan, T.K.; Devnarain, N.; Karpoormath, R.; Dhawan, S.; Obakachi, V.A.; et al. Development of niosomes for encapsulating captopril-quercetin prodrug to combat hypertension. Int. J. Pharm. 2021, 609, 121191. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.; El-Derany, M.O.; Biondo, F.; Tiboni, M.; Casettari, L.; Soliman, M.E. Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant-Hepatoprotective Interplay. Pharmaceutics 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal Nanocarriers for Enhanced Skin Delivery of Quercetin with Functions of Anti-Tyrosinase and Antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javani, R.; Hashemi, F.S.; Ghanbarzadeh, B.; Hamishehkar, H. Quercetin-loaded niosomal nanoparticles prepared by the thin-layer hydration method: Formulation development, colloidal stability, and structural properties. LWT 2021, 141, 110865. [Google Scholar] [CrossRef]
- Wei, X.; Yang, D.; Xing, Z.; Zhao, C.; Wang, L.; Fan, Y.; Nie, H.; Liu, H. Quercetin loaded liposomes modified with galactosylated chitosan prevent LPS/D-GalN induced acute liver injury. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112527. [Google Scholar] [CrossRef]
- Jing, D.; Wu, W.; Chen, X.; Xiao, H.; Zhang, Z.; Chen, F.; Zhang, Z.; Liu, J.; Shao, Z.; Pu, F. Quercetin encapsulated in folic acid-modified liposomes is therapeutic against osteosarcoma by non-covalent binding to the JH2 domain of JAK2 via the JAK2-STAT3-PDL1. Pharmacol. Res. 2022, 182, 106287. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef]
- Mureşan, M.; Olteanu, D.; Filip, G.A.; Clichici, S.; Baldea, I.; Jurca, T.; Pallag, A.; Marian, E.; Frum, A.; Gligor, F.G.; et al. Comparative Study of the Pharmacological Properties and Biological Effects of Polygonum aviculare L. herba Extract-Entrapped Liposomes versus Quercetin-Entrapped Liposomes on Doxorubicin-Induced Toxicity on HUVECs. Pharmaceutics 2021, 13, 1418. [Google Scholar] [CrossRef] [PubMed]
- Zouliati, K.; Stavropoulou, P.; Chountoulesi, M.; Naziris, N.; Demisli, S.; Mitsou, E.; Papadimitriou, V.; Chatzidaki, M.; Xenakis, A.; Demetzos, C. Development and Evaluation of Liposomal Nanoparticles Incorporating Dimethoxycurcumin. In Vitro Toxicity and Permeability Studies. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129223. [Google Scholar] [CrossRef]
- Papageorgiou, F.; Pippa, N.; Naziris, N.; Demetzos, C. Physicochemical Study of the Protein–Liposome Interactions: Influence of Liposome Composition and Concentration on Protein Binding. J. Liposome Res. 2019, 29, 313–321. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Perinelli, D.R.; Forys, A.; Chrysostomou, V.; Kaminari, A.; Bonacucina, G.; Trzebicka, B.; Pispas, S.; Demetzos, C. Development of Stimuli-Responsive Lyotropic Liquid Crystalline Nanoparticles Targeting Lysosomes: Physicochemical, Morphological and Drug Release Studies. Int. J. Pharm. 2023, 630, 122440. [Google Scholar] [CrossRef] [PubMed]
- Tsakiri, M.; Peraki, A.; Chountoulesi, M.; Demetzos, C. Chimeric Liposomes Decorated with P407: An Alternative Biomaterial for Producing Stealth Nano-Therapeutics. J. Liposome Res. 2022, 32, 83–91. [Google Scholar] [CrossRef]
- Ishida, T.; Harashima, H.; Kiwada, H. Interactions of Liposomes with Cells In Vitro and In Vivo: Opsonins and Receptors. CDM 2001, 2, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Chountoulesi, M.; Perinelli, D.R.; Forys, A.; Bonacucina, G.; Trzebicka, B.; Pispas, S.; Demetzos, C. Liquid Crystalline Nanoparticles for Drug Delivery: The Role of Gradient and Block Copolymers on the Morphology, Internal Organisation and Release Profile. Eur. J. Pharm. Biopharm. 2021, 158, 21–34. [Google Scholar] [CrossRef]
- Theochari, I.; Mitsou, E.; Nikolic, I.; Ilic, T.; Dobricic, V.; Pletsa, V.; Savic, S.; Xenakis, A.; Papadimitriou, V. Colloidal Nanodispersions for the Topical Delivery of Ibuprofen: Structure, Dynamics and Bioperformances. J. Mol. Liq. 2021, 334, 116021. [Google Scholar] [CrossRef]
- Theochari, I.; Goulielmaki, M.; Danino, D.; Papadimitriou, V.; Pintzas, A.; Xenakis, A. Drug Nanocarriers for Cancer Chemotherapy Based on Microemulsions: The Case of Vemurafenib Analog PLX4720. Colloids Surf. B Biointerfaces 2017, 154, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Tilley, A.J.; Drummond, C.J.; Boyd, B.J. Disposition and Association of the Steric Stabilizer Pluronic® F127 in Lyotropic Liquid Crystalline Nanostructured Particle Dispersions. J. Colloid Interface Sci. 2013, 392, 288–296. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pippa, N.; Pispas, S.; Chrysina, E.D.; Forys, A.; Trzebicka, B.; Demetzos, C. Cubic Lyotropic Liquid Crystals as Drug Delivery Carriers: Physicochemical and Morphological Studies. Int. J. Pharm. 2018, 550, 57–70. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pippa, N.; Chrysostomou, V.; Pispas, S.; Chrysina, E.D.; Forys, A.; Otulakowski, L.; Trzebicka, B.; Demetzos, C. Stimuli-Responsive Lyotropic Liquid Crystalline Nanosystems with Incorporated Poly(2-Dimethylamino Ethyl Methacrylate)-b-Poly(Lauryl Methacrylate) Amphiphilic Block Copolymer. Polymers 2019, 11, 1400. [Google Scholar] [CrossRef] [Green Version]
- Akbar, S.; Anwar, A.; Ayish, A.; Elliott, J.M.; Squires, A.M. Phytantriol Based Smart Nano-Carriers for Drug Delivery Applications. Eur. J. Pharm. Sci. 2017, 101, 31–42. [Google Scholar] [CrossRef]
- Sinzato, Y.Z.; Sousa Dias, N.J.; Cunha, F.R. An Experimental Investigation of the Interfacial Tension between Liquid-Liquid Mixtures in the Presence of Surfactants. Exp. Therm. Fluid Sci. 2017, 85, 370–378. [Google Scholar] [CrossRef]
- Chatzinikoli, L.; Pippa, N.; Demetzos, C. Preparation and Physicochemical Characterization of Elastic Liposomes: A Road-Map Library for Their Design. J. Liposome Res. 2021, 31, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Al-Hosayni, S.; Amenitsch, H.; Salentinig, S. Structural Investigation of Bulk and Dispersed Inverse Lyotropic Hexagonal Liquid Crystalline Phases of Eicosapentaenoic Acid Monoglyceride. Langmuir 2017, 33, 14045–14057. [Google Scholar] [CrossRef] [PubMed]
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; O’Neill, F.; Roberts, M. Surfactant Effects on Lipid-Based Vesicles Properties. J. Pharm. Sci. 2018, 107, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Submicron Particles of Reversed Lipid Phases in Water Stabilized by a Nonionic Amphiphilic Polymer. Langmuir 1997, 13, 6964–6971. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Larson, I.; Hanley, T.; Boyd, B.J. Bulk and Dispersed Aqueous Phase Behavior of Phytantriol: Effect of Vitamin E Acetate and F127 Polymer on Liquid Crystal Nanostructure. Langmuir 2006, 22, 9512–9518. [Google Scholar] [CrossRef]
- Leesajakul, W.; Nakano, M.; Taniguchi, A.; Handa, T. Interaction of Cubosomes with Plasma Components Resulting in the Destabilization of Cubosomes in Plasma. Colloids Surf. B Biointerfaces 2004, 34, 253–258. [Google Scholar] [CrossRef]
- Valente, F.; Bysell, H.; Simoni, E.; Boge, L.; Eriksson, M.; Martini, A.; Astolfi, L. Evaluation of Toxicity of Glycerol Monooleate Nanoparticles on PC12 Cell Line. Int. J. Pharm. 2018, 539, 23–30. [Google Scholar] [CrossRef]
- Lv, G.; Wang, F.; Cai, W.; Zhang, X. Characterization of the Addition of Lipophilic Span 80 to the Hydrophilic Tween 80-Stabilized Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 447, 8–13. [Google Scholar] [CrossRef]
- Mostafa, M.; Al Fatease, A.; Alany, R.G.; Abdelkader, H. Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases. Pharmaceutics 2023, 15, 1746. [Google Scholar] [CrossRef]
- Abdul Rasool, B.K.; Al Mahri, N.; Alburaimi, N.; Abdallah, F.; Shamma, A.S.B. A Narrative Review of the Potential Roles of Lipid-Based Vesicles (Vesiculosomes) in Burn Management. Sci. Pharm. 2022, 90, 39. [Google Scholar] [CrossRef]
- Azhari, H.; Strauss, M.; Hook, S.; Boyd, B.J.; Rizwan, S.B. Stabilising Cubosomes with Tween 80 as a Step towards Targeting Lipid Nanocarriers to the Blood–Brain Barrier. Eur. J. Pharm. Biopharm. 2016, 104, 148–155. [Google Scholar] [CrossRef] [PubMed]

| HSPC | GMO | PHYT | |
|---|---|---|---|
| Span 80 | ✓ | x | x |
| Tween 80 | ✓ | ✓ | ✓ |
| Span 80:Tween 80 | ✓ | x | x |
| Span 80:P407 | - | ✓ | ✓ |
| Tween 80:P407 | - | - | - |
| Span 80:Tween 80:P407 | - | ✓ | ✓ |
| Sample | Ratio | Dh (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|---|
| HSPC | - | 126.6 ± 0.4 | 0.380 ± 0.008 | −19.6 ± 10.7 |
| HSPC:Span 80 | 9:1 | 119.9 ± 2.1 | 0.345 ± 0.007 | −14.9 ± 2.4 |
| HSPC:Tween 80 | 9:1 | 72.4 ± 0.8 | 0.261 ± 0.010 | −3.8 ± 1.3 |
| HSPC:Span 80:Tween 80 | 9:0.5:0.5 | 160.5 ± 0.4 | 0.320 ± 0.022 | −5.6 ± 1.2 |
| PHYT:P407 | 4:1 | 227.6 ± 3.1 | 0.283 ± 0.003 | −28.9 ± 5.3 |
| PHYT:P407:Span 80 | 4:0.5:0.5 | 240.4 ± 2.3 | 0.379 ± 0.021 | −29.9 ± 9.5 |
| PHYT:P407:Span 80:Tween 80 | 4:0.5:0.5:0.5 | 192.6 ± 2.5 | 0.434 ± 0.031 | −4.9 ± 13.0 |
| PHYT:Span 80 | 4:1 | 279.4 ± 1.3 | 0.413 ± 0.024 | 15.1 ± 16.0 |
| PHYT:Tween 80 | 4:1 | 209.2 ± 2.6 | 0.234 ± 0.015 | −32.8 ± 0.4 |
| GMO:P407 | 4:1 | 162.3 ± 1.0 | 0.214 ± 0.025 | −22.1 ± 0.4 |
| GMO:Tween 80 | 4:1 | 151.5 ± 0.4 | 0.212 ± 0.013 | −26.6 ± 9.0 |
| GMO:Span 80:P407 | 4:1:0.5 | 202.2 ± 1.4 | 0.461 ± 0.026 | −37.3 ± 0.8 |
| GMO:Span 80:Tween 80:P407 | 4:0.5:0.5:0.5 | 164.2 ± 0.6 | 0.327 ± 0.003 | −28.4 ± 1.0 |
| Sample | Ratio | Dh (nm) | PDI | Entrapment Efficiency of Quercetin (%) |
|---|---|---|---|---|
| HSPC:Quercetin | 9:0.1 | 1200.3 ± 270.4 | 1.000 ± 0.000 | 86 |
| HSPC:Span 80:Quercetin | 9:1:0.1 | 144.0 ± 1.2 | 0.307 ± 0.013 | 84 |
| HSPC:Tween 80:Quercetin | 9:1:0.1 | 149.9 ± 0.5 | 0.353 ± 0.020 | 83 |
| HSPC:Span 80:Tween 80:Quercetin | 9:0.5:0.5:0.1 | 181.5 ± 3.7 | 0.298 ± 0.005 | 83 |
| PHYT:P407: Quercetin | 4:1 | 288.8 ± 1.7 | 0.562 ± 0.147 | 80 |
| PHYT:P407:Span 80:Quercetin | 4:0.5:0.5 | 279.9 ± 1.4 | 0.233 ± 0.137 | 85 |
| PHYT:P407:Span 80:Tween 80:Quercetin | 4:0.5:0.5:0.5 | 189.6 ± 3.3 | 0.261 ± 0.002 | 86 |
| PHYT:Span 80:Quercetin | 4:1 | 374.4 ± 4.5 | 0.455 ± 0.130 | 86 |
| PHYT:Tween 80:Quercetin | 4:1 | 171.2 ± 2.6 | 0.278 ± 0.009 | 82 |
| GMO:P407:Quercetin | 4:1 | 149.7 ± 2.5 | 0.272 ± 0.005 | 97 |
| GMO:Tween 80:Quercetin | 4:1 | 190.6 ± 1.8 | 0.272 ± 0.010 | 98 |
| GMO:Span 80:P407:Quercetin | 4:1:0.5 | 183.2 ± 1.0 | 0.307 ± 0.036 | 97 |
| GMO:Span 80:Tween 80:P407:Quercetin | 4:0.5:0.5:0.5 | 165.6 ± 0.2 | 0.265 ± 0.014 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsichlis, I.; Manou, A.-P.; Manolopoulou, V.; Matskou, K.; Chountoulesi, M.; Pletsa, V.; Xenakis, A.; Demetzos, C. Development of Liposomal and Liquid Crystalline Lipidic Nanoparticles with Non-Ionic Surfactants for Quercetin Incorporation. Materials 2023, 16, 5509. https://doi.org/10.3390/ma16165509
Tsichlis I, Manou A-P, Manolopoulou V, Matskou K, Chountoulesi M, Pletsa V, Xenakis A, Demetzos C. Development of Liposomal and Liquid Crystalline Lipidic Nanoparticles with Non-Ionic Surfactants for Quercetin Incorporation. Materials. 2023; 16(16):5509. https://doi.org/10.3390/ma16165509
Chicago/Turabian StyleTsichlis, Ioannis, Athanasia-Paraskevi Manou, Vasiliki Manolopoulou, Konstantina Matskou, Maria Chountoulesi, Vasiliki Pletsa, Aristotelis Xenakis, and Costas Demetzos. 2023. "Development of Liposomal and Liquid Crystalline Lipidic Nanoparticles with Non-Ionic Surfactants for Quercetin Incorporation" Materials 16, no. 16: 5509. https://doi.org/10.3390/ma16165509
APA StyleTsichlis, I., Manou, A.-P., Manolopoulou, V., Matskou, K., Chountoulesi, M., Pletsa, V., Xenakis, A., & Demetzos, C. (2023). Development of Liposomal and Liquid Crystalline Lipidic Nanoparticles with Non-Ionic Surfactants for Quercetin Incorporation. Materials, 16(16), 5509. https://doi.org/10.3390/ma16165509







