Enhanced Coarse-Grained WC-Co(Ce) Cemented Carbide Prepared through Co-Precipitation
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Powder Preparation and Sintering
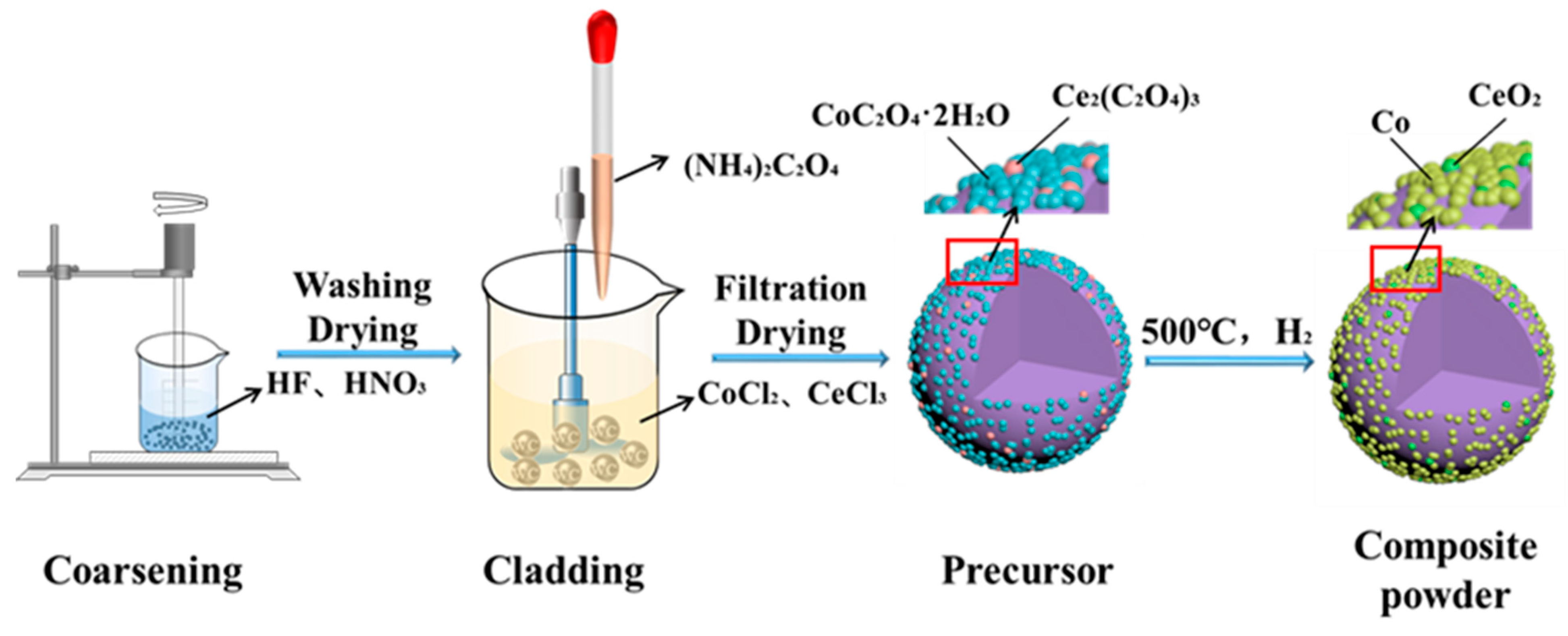
2.1.1. Powder Preparation
2.1.2. Sintering
2.2. Microstructure and Performance Testing
3. Results and Discussion
3.1. Influence of Process Parameters on the Precipitation Rate
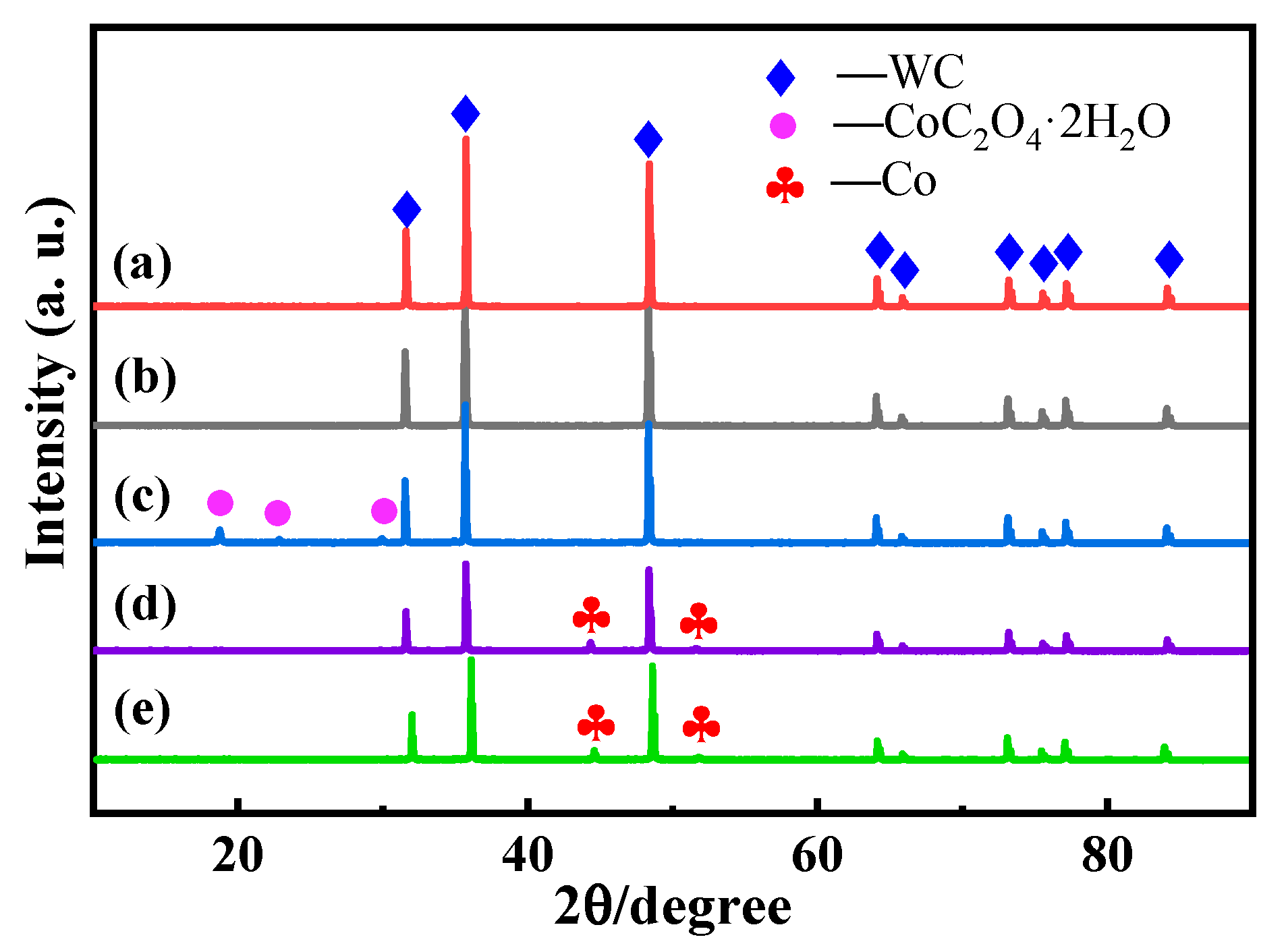
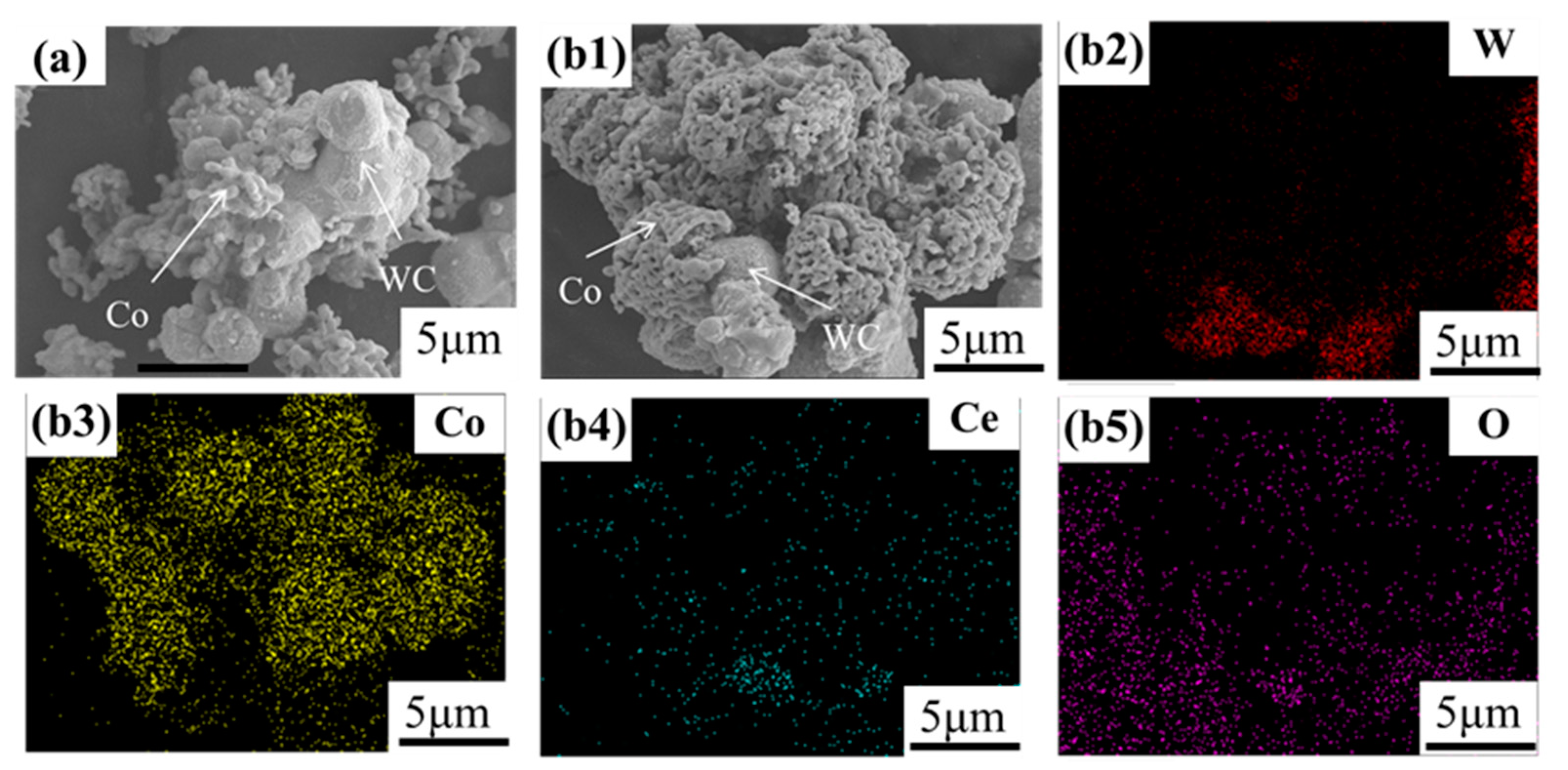
3.2. Phase Composition and Analysis of the Powder Coating Effect
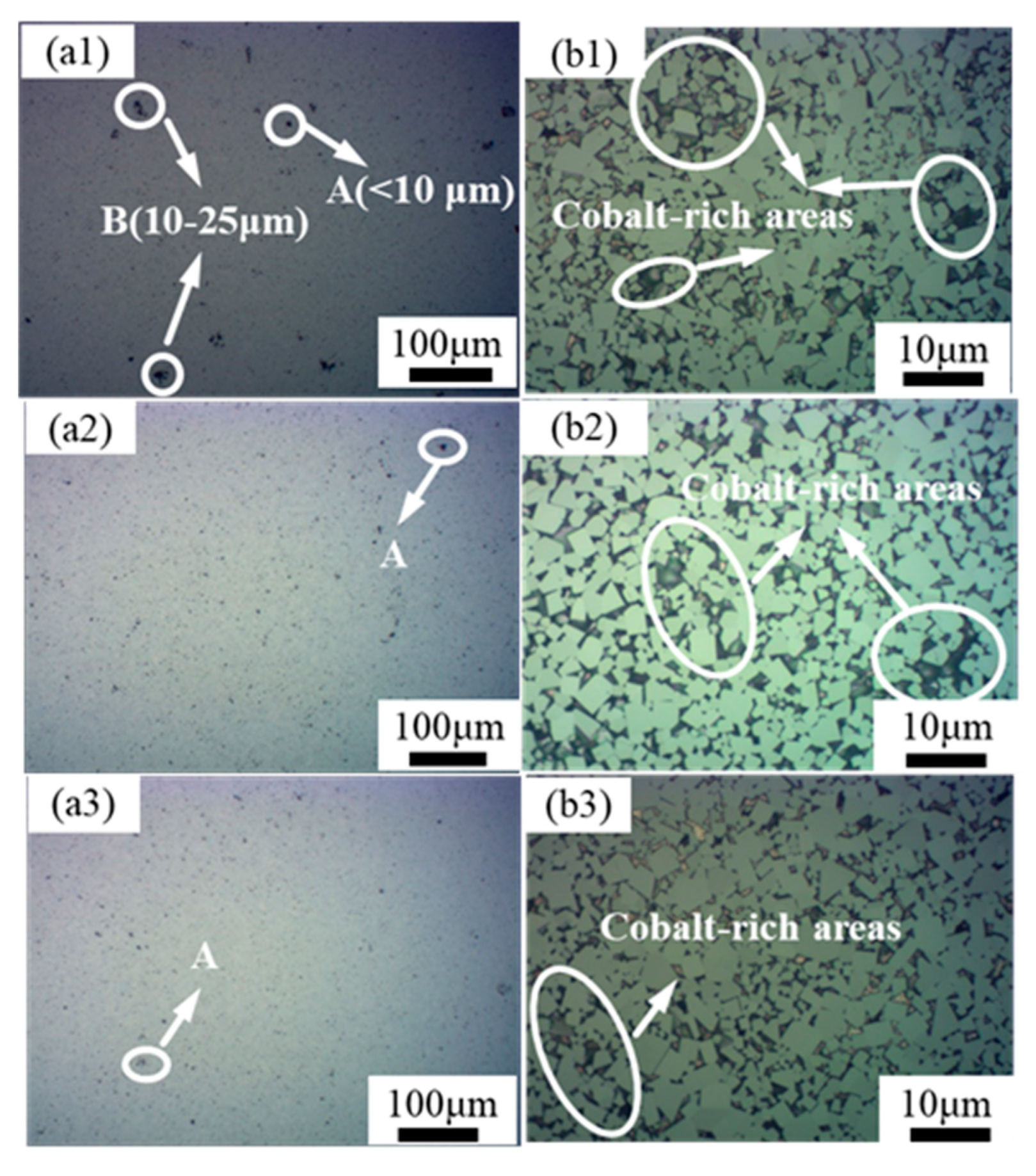
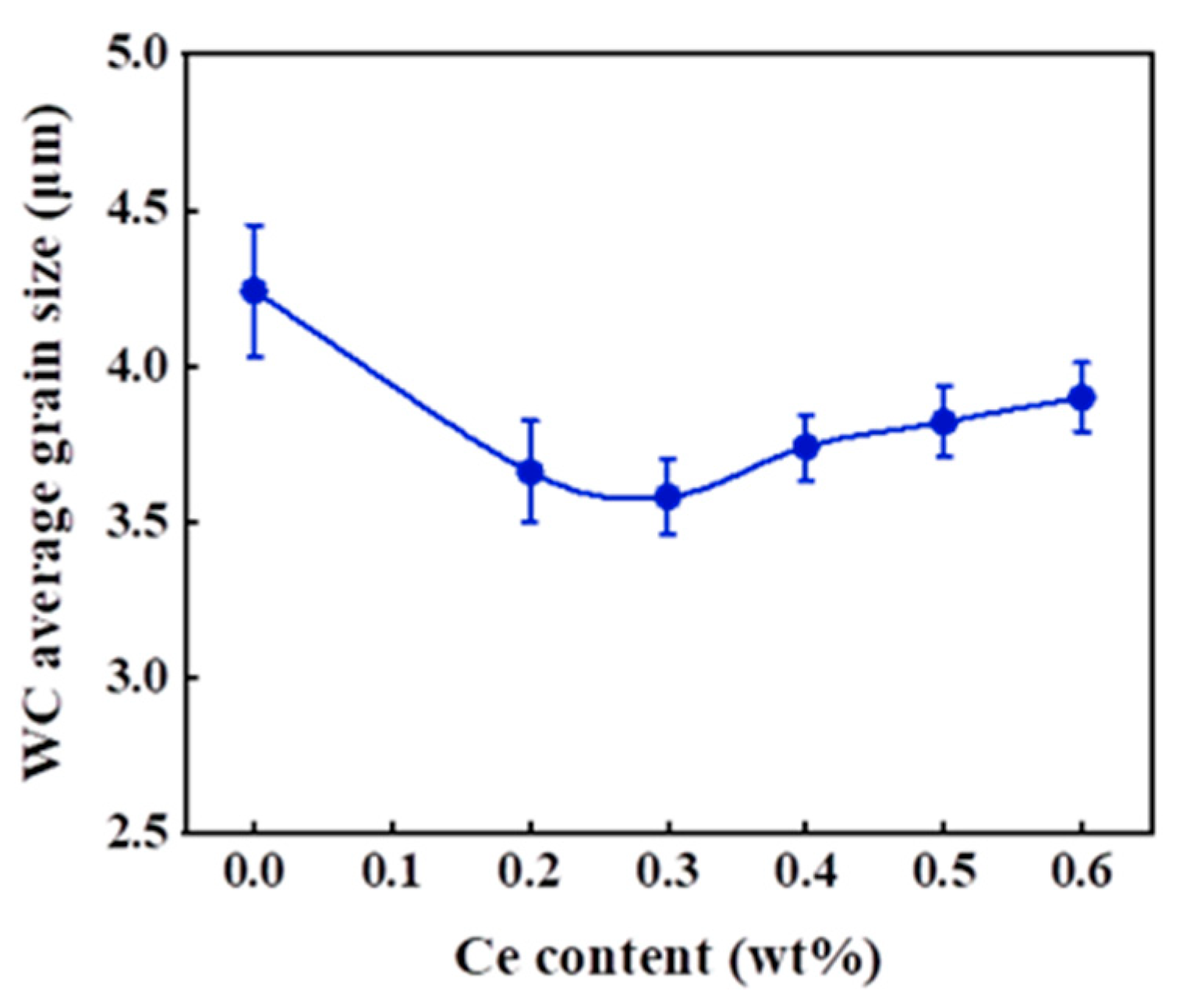
3.3. Cemented Carbide Microstructure
3.4. Mechanical Properties
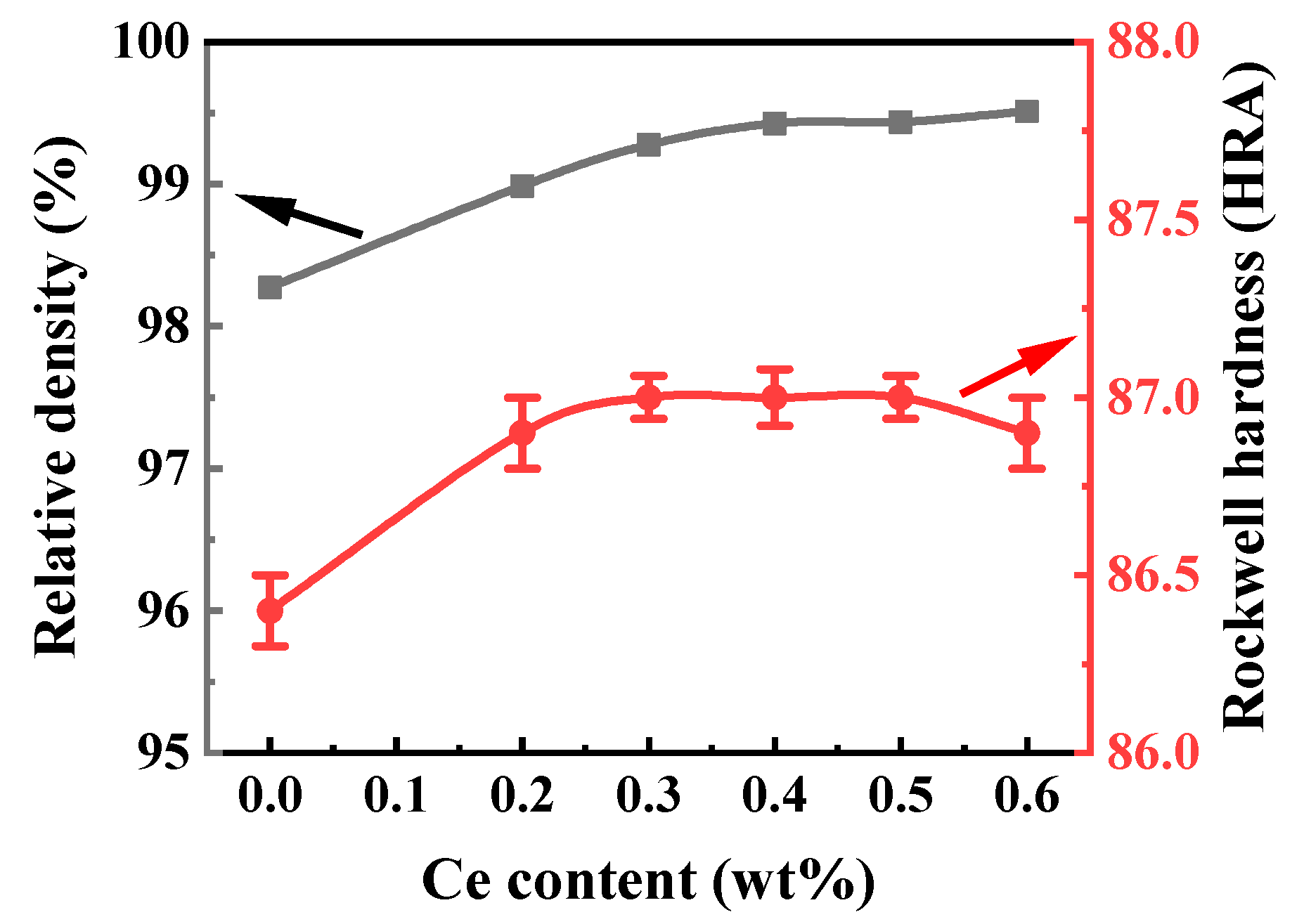
3.4.1. Relative Density and Rockwell Hardness
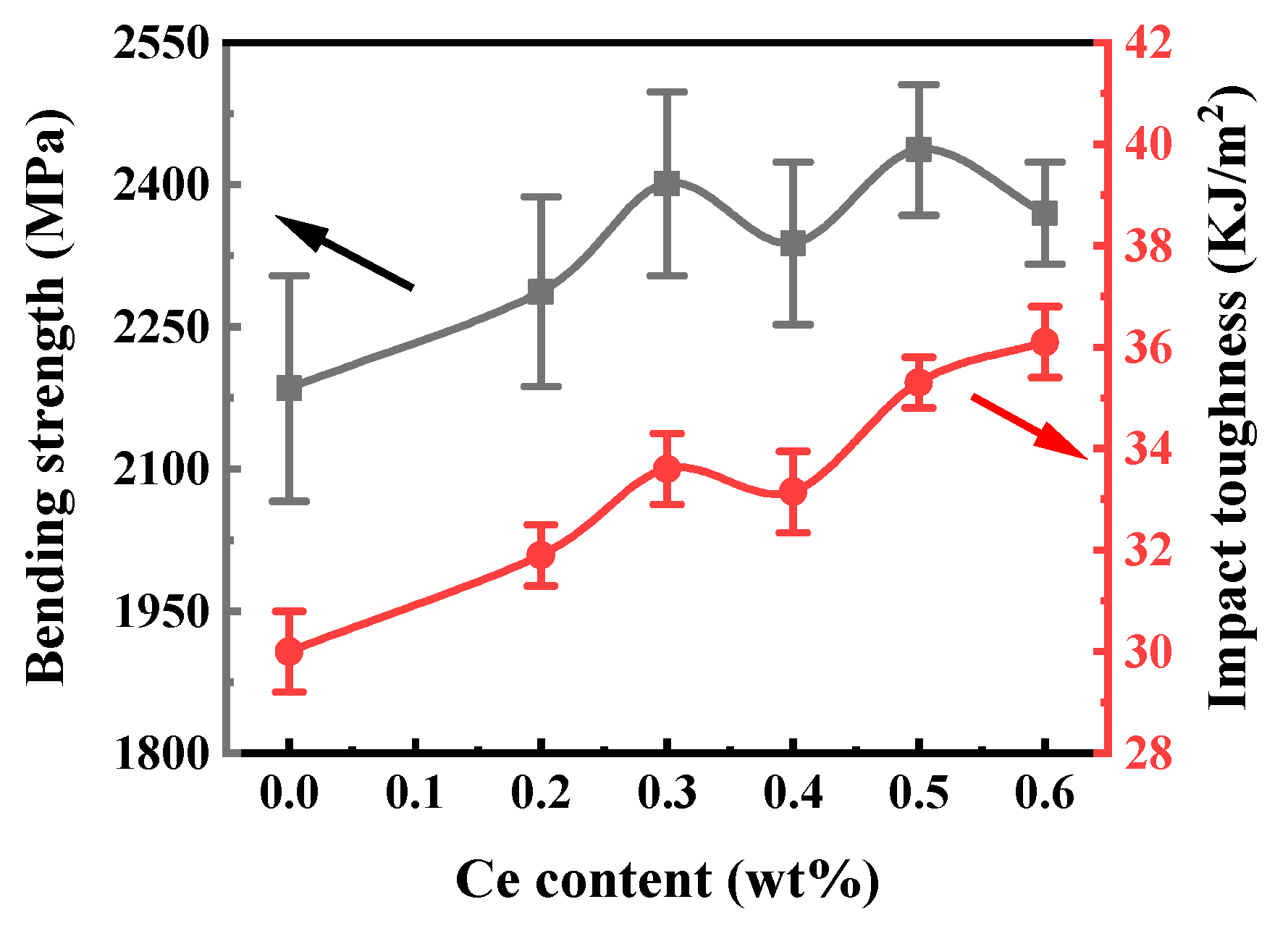
3.4.2. Flexural Strength and Impact Toughness
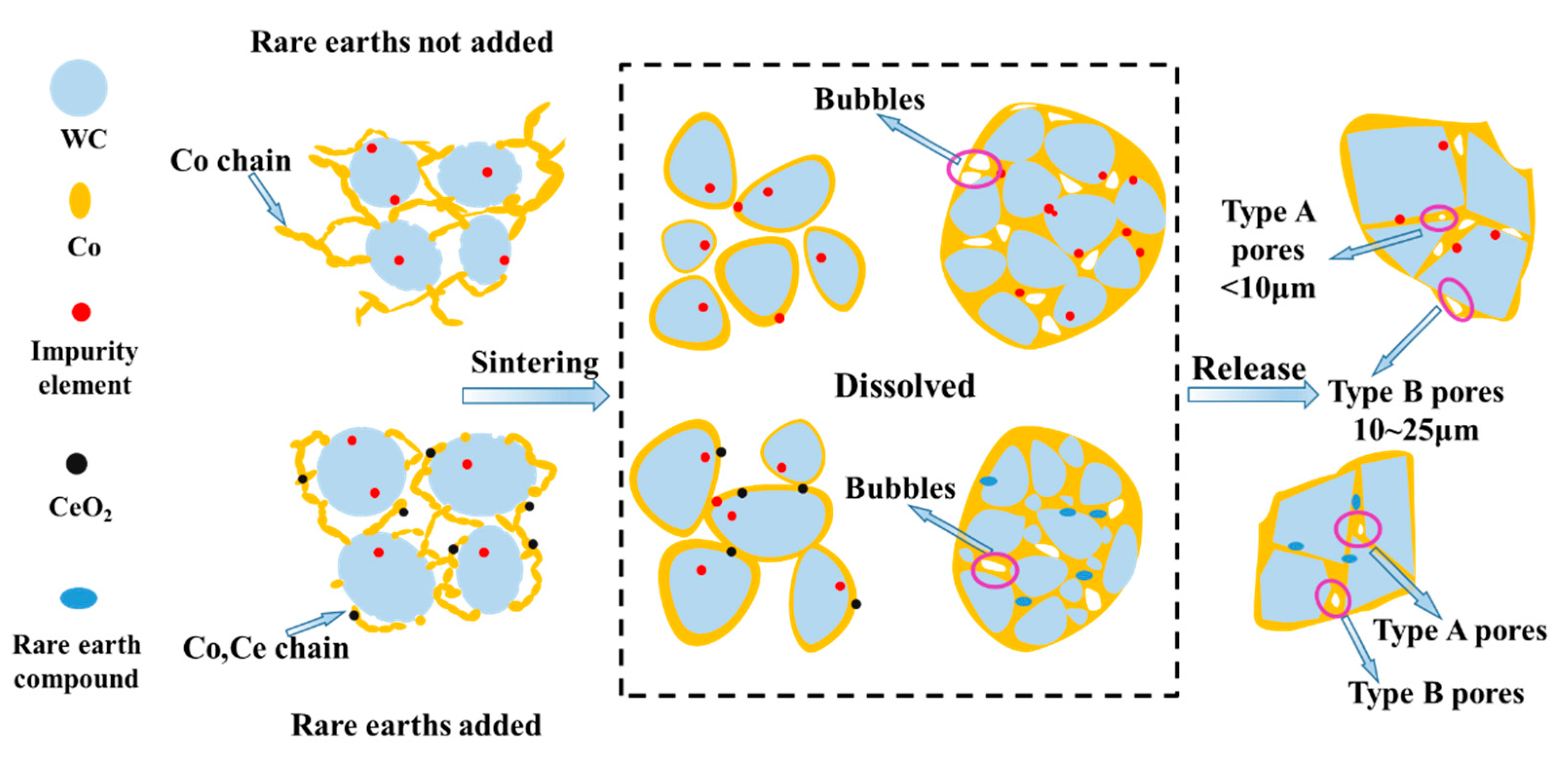
4. Mechanism Analysis
5. Conclusions
- (1)
- The optimum powder precipitation rate can be obtained when the solution temperature, precipitant addition time, and solution concentration are 50 °C, 30 min, and 0.3 mol/L, respectively. Under these process parameters, the precipitation rate of the cobalt–cerium oxalate crystal was appropriate, and the crystals of cobalt and cerium oxalate that formed were relatively small in length and particle size. The overall distribution in the solution was uniform, and the coating effect on the WC particles was good.
- (2)
- When the cobalt content is 12%, with the addition of cerium elements, the mechanical properties of cemented carbide prepared using the chemical co-precipitation method are higher than those of cemented carbide without the addition of cerium elements. The flexural strength and impact toughness were significantly improved by adding cerium elements, which increased to 2487 MPa and 36.1 kJ/m2, respectively.
- (3)
- As the concentration of CoCl2 in the solution increases, the accelerated co-precipitation reaction speed up the formation of cobalt–cerium oxalate nuclei, resulting in the formation of cobalt–cerium oxalate crystals without growth momentum. Then, the cobalt-cerium oxalate crystals generated by the co-precipitation reaction have a relatively small length and particle size, whose overall distribution is uniform. The addition of cerium elements is beneficial to the densification of cemented carbide and the improvement in the mechanical properties because these cerium elements can combine with impurity elements to make the cobalt phase more uniformly distributed and better encapsulated on the surface of the WC particles.
- (4)
- The WC-Co cemented carbide prepared using the method described in this paper was optimized on the basis of the selected materials by adjusting the process parameters and cerium addition. Compared with the nanostructured cemented carbide, a small portion of the hardness and flexural strength are sacrificed to obtain higher impact toughness, while its hardness and flexural strength are stronger than that of the ultra-coarse-crystal cemented carbide, which makes it more wear-resistant and less prone to fracture while satisfying the hardness and flexural strength of the cutting and breaking of rock (such as the cutterheads of the shield tool and the pile-drilling cutterheads).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Yan, M.-Y.; Luo, B.-H.; Ouyang, S.; Chen, W.; Bai, Z.-H.; Jing, H.-B.; Zhang, W.-W. Effects of NbC additions on the microstructure and properties of non-uniform structure WC-Co cemented carbides. Mater. Sci. Eng. A 2017, 687, 259–268. [Google Scholar] [CrossRef]
- Lin, H.; Sun, J.; Li, C.; He, H.; Qin, L.; Li, Q. A facile route to synthesize WC–Co nanocomposite powders and properties of sintered bulk. J. Alloys Compd. 2016, 682, 531–536. [Google Scholar] [CrossRef]
- Liu, W.; Song, X.; Wang, K.; Zhang, J.; Zhang, G.; Liu, X. A novel rapid route for synthesizing WC–Co bulk by in situ reactions in spark plasma sintering. Mater. Sci. Eng. A 2009, 499, 476–481. [Google Scholar] [CrossRef]
- Sugiyama, I.; Mizumukai, Y.; Taniuchi, T.; Okada, K.; Shirase, F.; Tanase, T.; Ikuhara, Y.; Yamamoto, T. Carbon content dependence of grain growth mode in VC-doped WC–Co hardmetals. Int. J. Refract. Met. Hard Mater. 2015, 52, 245–251. [Google Scholar] [CrossRef]
- Beste, U.; Jacobson, S.; Hogmark, S. Rock penetration into cemented carbide drill buttons during rock drilling. Wear 2008, 264, 1142–1151. [Google Scholar] [CrossRef]
- Gille, G.; Szesny, B.; Dreyer, K.; Van Den Berg, H.; Schmidt, J.; Gestrich, T.; Leitner, G. Submicron and ultrafine grained hardmetals for microdrills and metal cutting inserts. Int. J. Refract. Met. Hard Mater. 2002, 20, 3–22. [Google Scholar] [CrossRef]
- Konyashin, I.; Schäfer, F.; Cooper, R.; Ries, B.; Mayer, J.; Weirich, T. Novel ultra-coarse hardmetal grades with reinforced binder for mining and construction. Int. J. Refract. Met. Hard Mater. 2005, 23, 225–232. [Google Scholar] [CrossRef]
- Qian, C.; Li, K.; Guo, X.-Y.; Liu, B.; Long, Z.-Y.; Liu, Y. Effect of WC grain size on mechanical properties and microstructures of cemented carbide with medium entropy alloy Co-Ni-Fe binder. J. Cent. South Univ. 2020, 27, 1146–1157. [Google Scholar] [CrossRef]
- Hou, K.-Z.; Bai, J.-S. Development of Cemented Carbide Inserts for Shield Machine. Powder Metall. Ind. 2009, 19, 36–40. [Google Scholar]
- Yan, M.-Y.; Zhang, W.-B.; Tan, C.-Y.; Long, J.-Z.; Du, Y. Effect of Coarse WC Particles on Microstructure and Properties of Low Cobalt Cemented Carbide. Powder Metall. Mater. Sci. Eng. 2017, 22, 49–55. [Google Scholar]
- Zhu, B.; Bai, Z.-H.; Gao, Y.; Luo, B.-H. Effect of WC Grain Size on Microstructure and Properties of WC-15Fe-5Ni Cemented Carbide. Chin. J. Nonferrous Met. 2016, 26, 1065–1074. [Google Scholar]
- Chivavibul, P.; Watanabe, M.; Kuroda, S.; Shinoda, K. Effects of carbide size and Co content on the microstructure and mechanical properties of HVOF-sprayed WC–Co coatings. Surf. Coat. Technol. 2007, 202, 509–521. [Google Scholar] [CrossRef]
- Saito, H.; Iwabuchi, A.; Shimizu, T. Effects of Co content and WC grain size on wear of WC cemented carbide. Wear 2006, 261, 126–132. [Google Scholar] [CrossRef]
- Watanabe, M.; Owada, A.; Kuroda, S.; Gotoh, Y. Effect of WC size on interface fracture toughness of WC–Co HVOF sprayed coatings. Surf. Coat. Technol. 2006, 201, 619–627. [Google Scholar] [CrossRef]
- Adorjan, C.; Bock, A.; Myllymäki, S.; Schubert, W.-D.; Kontturi, K. WC/Co-composite powders via hydrothermal reduction of Co3O4-suspensions. Int. J. Refract. Met. Hard Mater. 2008, 26, 569–574. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Y.; Li, J.; Zheng, Y. Preparation of nickel-coated tungsten carbide powders by room temperature ultrasonic-assisted electroless plating. Surf. Coat. Technol. 2011, 206, 1091–1095. [Google Scholar] [CrossRef]
- Liu, S.; Xu, K.-H.; Wang, M. Preparation of Co powders for cemented carbides in China. Int. J. Refract. Met. Hard Mater. 2006, 24, 405–412. [Google Scholar] [CrossRef]
- Liu, J.; Yi, Z.-Q.; Zhang, L.; Huang, H.-H.; Tan, D.-Q.; Lu, L. Study on Preparation of Ultrafine Ammonium Paratungstate Powder by Chemical Precipitation. Jiangxi Sci. 2019, 37, 405–408. [Google Scholar]
- Sun, Y.-X.; Su, W.; Yang, H.-L.; Ruan, J.-M. Preparation of WC-Co Ultra-coarse Cemented Carbide by One-step Reduction Coated Powder Process. Rare Met. Mater. Eng. 2016, 45, 409–414. [Google Scholar]
- Wang, Y.-X.; Wen, X.-Q.; Zhou, J. Study on Preparation of Ultrafine WC-Co Composite Powder by Chemical Precipitation. China Tungsten Ind. 2012, 27, 24–27. [Google Scholar]
- Zou, Q.; Zhang, M.-L.; Li, Y.-G. Properties and Application of Additive Modified WC Cemented Carbide. Manuf. Technol. Mach. Tools 2021, 7, 9–15. [Google Scholar]
- Sun, X.; Wang, Y.; Li, D.-Y. Mechanical properties and erosion resistance of ceria nano-particle-doped ultrafine WC–12Co composite prepared by spark plasma sintering. Wear 2013, 301, 406–414. [Google Scholar] [CrossRef]
- Yang, S.-Z.; Xu, Y.; Xiao, Y.-Y.; Zhang, F. Effects of Additives on Properties of Ultrafine Grained WC-8Co Cemented Carbide. China Met. Bull. 2020, 1, 240–241+243. [Google Scholar]
- Shi, X.-L.; Yang, K.-H.; Tang, F.-L.; Shao, G.-Q. Effect of Rare Earth Ce Addition on Properties of WC-Co Cemented Carbide. J. Cent. South Univ. (Nat. Sci. Ed.) 2005, 36, 204–208. [Google Scholar]
- Wu, A.-H.; Tang, J.-C.; Qin, D.-Q.; Lei, C.-P. Development Status of Cobalt Powder for Cemented Carbide. Jiangxi Sci. 2014, 32, 433–438. [Google Scholar]
- GB/T3849.1-2015; Hardmetals-Rock Well Hardness (Scale A) Test Method. Standardization Administration of the People's Republic of China: Beijing, China, 2015.
- GB/T31967.2-2015; Test Method for Physical Property of Rare Earth Permanent Magnetic Materials—Part 2: Determination of Bending Strength and Fracture Toughness. National Standardization Technical Committee of Rare Earth: Beijing, China, 2015.
- Wang, B.; Wang, Z.; Yin, Z.; Liu, K.; Yuan, J. Effects of powder preparation and sintering temperature on consolidation of ultrafine WC-8Co tool material produced by spark plasma sintering. Ceram. Int. 2019, 45, 19737–19746. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Ma, H.; Liu, Z.H. Synthesis of Fibrous Cobalt Oxalate by a Double-Jet Process: Morphology and Growth Control. JOM J. Miner. Met. Mater. Soc. 2019, 71, 2884–2890. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wang, Y.; Xie, X.-H.; Chen, L.-F.; Chen, H. Research Progress of Rare Earth Cemented Carbide. Met. Sci. Eng. 2019, 10, 106–112. [Google Scholar]
- Jiang, H.-L.; Su, F.-Y.; Yang, Y.-T.; Zhang, D.; Hong, Y.; Cui, H.-Z.; Min, F.-L.; Wang, C.-X.; Li, G.-Y.; Zhang, J.-F. Coprecipitation of Co and La2O3 coprecipitation on WC for tailoring the grain distribution and boundary of highperformance coarse-grained WC-10Co cemented carbide. Int. J. Ofrefractory Met. Hard Mater. 2023, 115, 106303. [Google Scholar] [CrossRef]
- Li, J.-F.; Cheng, J.-G.; Wei, B.-Z.; Chen, P.-Q. Preparation and performance of ultrafifine grained WC-10Co alloys with added La2O3. Ceram. Int. 2019, 45, 3969–3976. [Google Scholar] [CrossRef]
- Hu, D.-M.; Du, J.; Wang, Y.-F.; Zhang, C.-Y.; Su, G.-S.; Zhang, J.-J.; Sun, Y.-J. Effects of Rare Earth Oxides on Properties of Recycled YG8 Cemented Carbide. Rare Met. Carbide 2020, 48, 69–74. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, F.; Wang, S.; Yu, S.; Yang, H.; Yao, Z.; Ni, J.; Zhang, J. Enhanced Coarse-Grained WC-Co(Ce) Cemented Carbide Prepared through Co-Precipitation. Materials 2023, 16, 5506. https://doi.org/10.3390/ma16165506
Min F, Wang S, Yu S, Yang H, Yao Z, Ni J, Zhang J. Enhanced Coarse-Grained WC-Co(Ce) Cemented Carbide Prepared through Co-Precipitation. Materials. 2023; 16(16):5506. https://doi.org/10.3390/ma16165506
Chicago/Turabian StyleMin, Fanlu, Shiyu Wang, Songbai Yu, Hao Yang, Zhanhu Yao, Jianzhong Ni, and Jianfeng Zhang. 2023. "Enhanced Coarse-Grained WC-Co(Ce) Cemented Carbide Prepared through Co-Precipitation" Materials 16, no. 16: 5506. https://doi.org/10.3390/ma16165506
APA StyleMin, F., Wang, S., Yu, S., Yang, H., Yao, Z., Ni, J., & Zhang, J. (2023). Enhanced Coarse-Grained WC-Co(Ce) Cemented Carbide Prepared through Co-Precipitation. Materials, 16(16), 5506. https://doi.org/10.3390/ma16165506




