Impact of Diverse Parameters on the Physicochemical Characteristics of Green-Synthesized Zinc Oxide–Copper Oxide Nanocomposites Derived from an Aqueous Extract of Garcinia mangostana L. Leaf
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
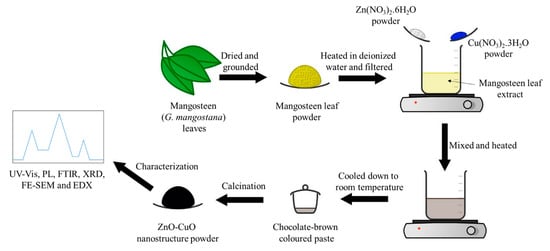
2.3. Preparation of Mangosteen Leaf Aqueous Extract
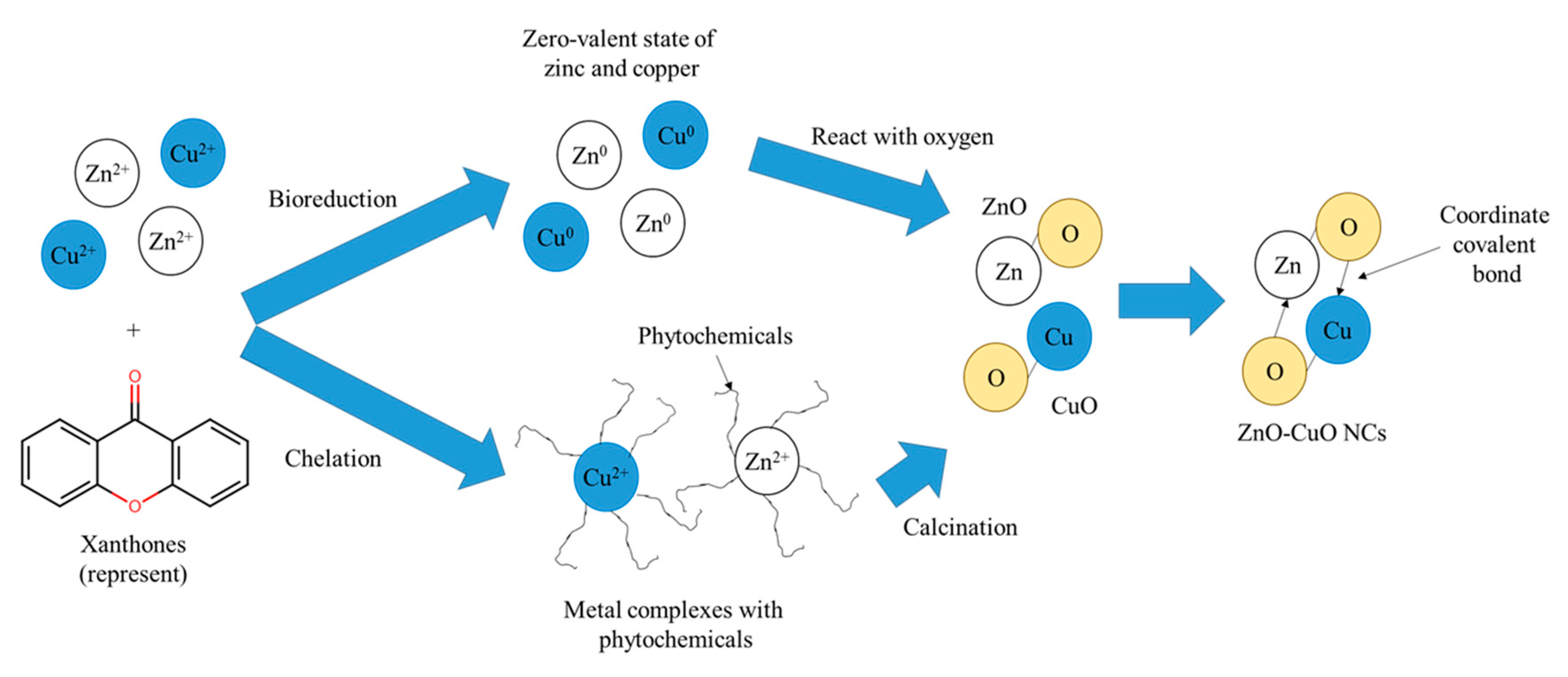
2.4. Synthesis of ZnO-CuO NCs
2.4.1. Leaf Aqueous Extract Optimization
2.4.2. Calcination Temperature Optimization
2.4.3. Precursor Optimization
3. Results
3.1. UV-Vis Spectroscopy Analysis
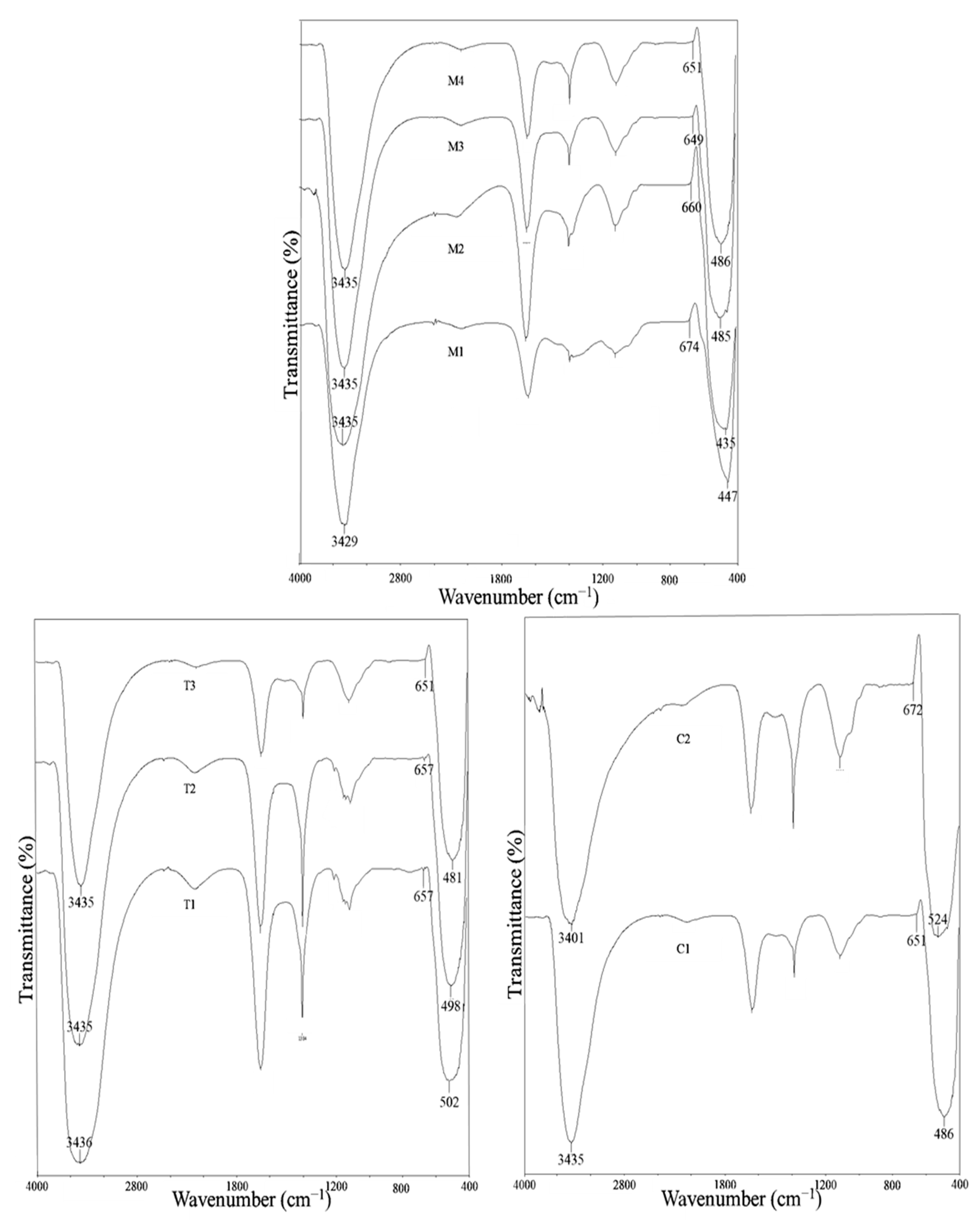
3.2. FTIR Spectroscopy Analysis
3.3. PL Spectroscopy Analysis
3.4. XRD Spectroscopy Analysis
3.5. FE-SEM Spectroscopy Analysis
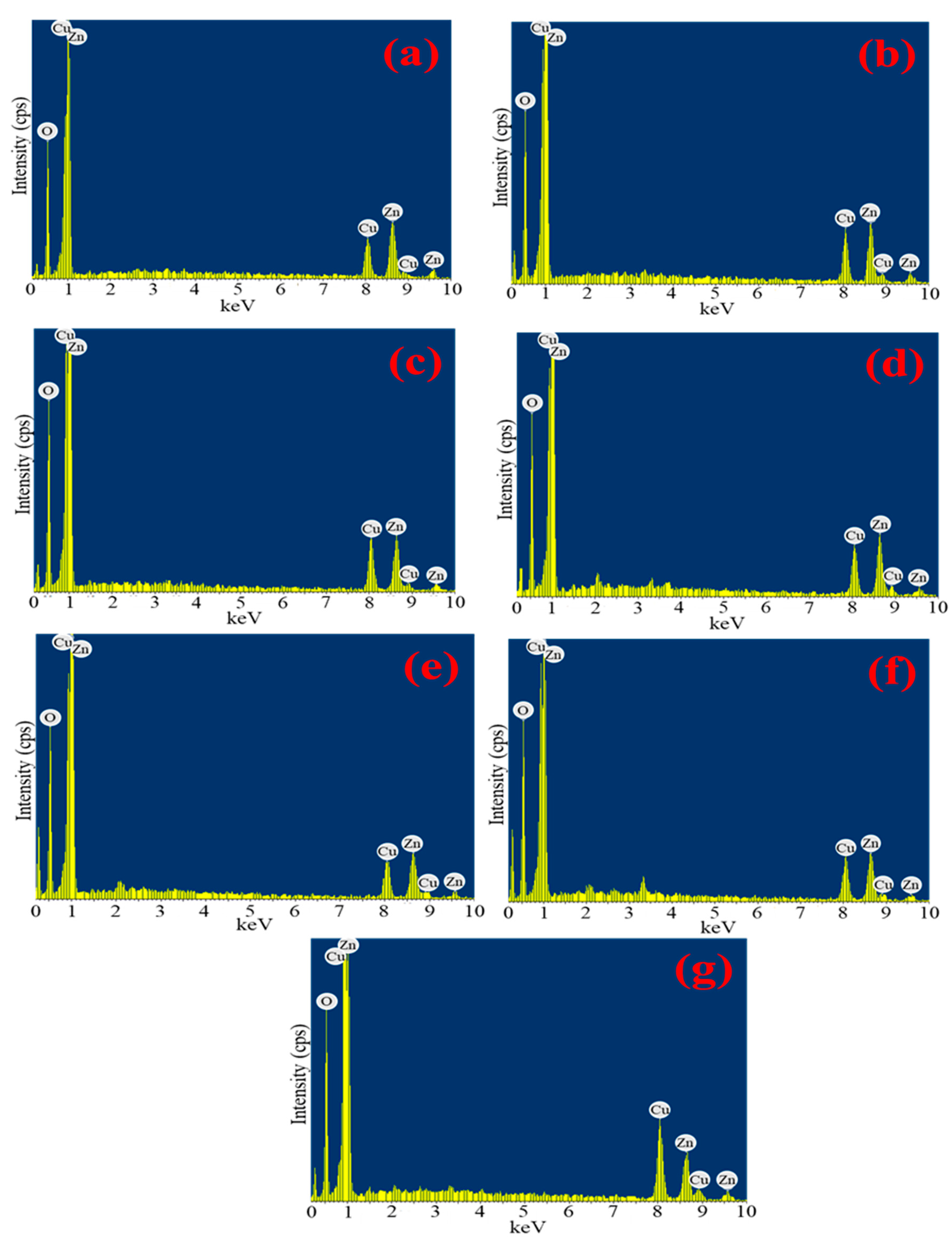
3.6. EDX Spectroscopy Analysis
3.7. Comparison with Other Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, S.; Srivastava, V.C. An overview of the synthesis of CuO-ZnO nanocomposite for environmental and other applications. Nanotechnol. Rev. 2018, 7, 267–282. [Google Scholar] [CrossRef]
- Khan, S.A.; Noreen, F.; Kanwal, S.; Iqbal, A.; Hussain, G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater. Sci. Eng. C 2017, 82, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Vibitha, B.V.; Anitha, B.; Tharayil, N.J. Green synthesis of ZnO:CuO nanocomposites by Aloe barbadansis leaf extract: Structure and photo catalytic properties. In Proceedings of the AIP Conference Proceedings, International Conference on Energy and Environment 2019, Guimaraes, Portugal, 16–17 May 2019. [Google Scholar]
- Rajith Kumar, C.R.; Betageri, V.S.; Nagaraju, G.; Pujar, G.H.; Onkarappa, H.S.; Latha, M.S. One-pot green synthesis of ZnO-CuO nanocomposite and their enhanced photocatalytic and antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 015009. [Google Scholar] [CrossRef]
- Sakib, A.A.M.; Masum, S.M.; Hoinkis, J.; Islam, R.; Molla, M.A.I. Synthesis of CuO/ZnO nanocomposites and their application in photodegradation of toxic textile dye. J. Compos. Sci. 2019, 3, 91–103. [Google Scholar] [CrossRef]
- Yulizar, Y.; Bakri, R.; Apriandanu, D.O.B.; Hidayat, T. ZnO/CuO nanocomposite prepared in one-pot green synthesis using seed bark extract of Theobroma cacao. Nano-Struct. Nano-Objects 2018, 16, 300–305. [Google Scholar] [CrossRef]
- Bano, S.; Pillai, S. Green synthesis of calcium oxide nanoparticles at different calcination temperatures. World J. Sci. Technol. Sustain. Dev. 2020, 17, 283–295. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Fouda, A.; Saied, E.; Farag, M.M.S.; Eid, A.M.; Barghoth, M.G.; Awad, M.A.; Hamza, M.F.; Awad, M.F. Rhizopus oryzae-mediated green synthesis of magnesium oxide nanoparticles (MgO-NPs): A promising tool for antimicrobial, mosquitocidal action, and tanning effluent treatment. J. Fungi 2021, 7, 372–396. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A. Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles. Green. Process Synth. 2020, 9, 386–398. [Google Scholar] [CrossRef]
- Khan, A.; Shabir, D.; Ahmad, P.; Khandaker, M.U.; Faruque, M.R.; Din, I.U. Biosynthesis and antibacterial activity of MgO-NPs produced from Camellia-sinensis leaves extract. Mater. Res. Express 2021, 8, 015402. [Google Scholar] [CrossRef]
- Mazli, S.R.A.; Yusoff, H.M.; Idris, N.H. Synthesis of zinc oxide nanoparticles by using Aloe vera leaf extract as pontential anode material in lithium ion battery. Univ. Malaysia Teren. J. Undergrad. Res. 2020, 2, 1–8. [Google Scholar]
- Xu, J.; Huang, Y.; Zhu, S.; Abbes, N.; Jing, X.; Zhang, L. A review of the green synthesis of ZnO nanoparticles using plant extracts and their prospects for application in antibacterial textiles. J. Eng. Fiber Fabr. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 61, 149–178. [Google Scholar] [CrossRef]
- Prasanth, R.; Dinesh Kumar, S.; Jayalakshmi, A.; Singaravelu, G.; Govindaraju, K.; Ganesh Kumar, V. Green synthesis of magnesium oxide nanoparticles and their antibacterial activity. Indian. J. Geo Mar. Sci. 2019, 48, 1210–1215. [Google Scholar]
- Shammout, M.W.; Awwad, A.M. A novel route for the synthesis of copper oxide nanoparticles using Bougainvillea plant flowers extract and antifungal activity evaluation. Int. Sci. Organ. 2021, 7, 71–78. [Google Scholar]
- Kumar, H.; Bhardwaj, K.; Dhanjal, D.S.; Nepovimova, E.; Șen, F.; Regassa, H.; Singh, R.; Verma, R.; Kumar, V.; Kumar, D.; et al. Fruit extract mediated green synthesis of metallic nanoparticles: A new avenue in pomology applications. Int. J. Mol. Sci. 2020, 21, 8458. [Google Scholar] [CrossRef] [PubMed]
- Kureshi, A.A.; Vaghela, H.M.; Kumar, S.; Singh, R.; Kumari, P. Green synthesis of gold nanoparticles mediated by Garcinia fruits and their biological applications. Pharm. Sci. 2021, 27, 238–250. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Azizi, S.; Sintwa, N.; Mokalane, K.; Mohale, K.C.; Mudau, F.N.; Maaza, M. Effect of optimized precursor concentration, temperature, and doping on optical properties of ZnO nanoparticles synthesized via a green route using bush tea (Athrixia phylicoides DC.) leaf extracts. ACS Omega 2022, 7, 31658–31666. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.; Mondal, M.; Sakthivel, N. Green synthesis of phytogenic nanoparticles. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 37–73. [Google Scholar]
- Adeyemi, J.O.; Onwudiwe, D.C.; Oyedeji, A.O. Biogenic synthesis of CuO, ZnO, and CuO–ZnO nanoparticles using leaf extracts of Dovyalis caffra and their biological properties. Molecules 2022, 27, 3206. [Google Scholar] [CrossRef]
- Bekru, A.G.; Tufa, L.T.; Zelekew, O.A.; Goddati, M.; Lee, J.; Sabir, F.K. Green synthesis of a CuO–ZnO nanocomposite for efficient photodegradation of methylene blue and reduction of 4-nitrophenol. ACS Omega 2022, 7, 30908–30919. [Google Scholar] [CrossRef]
- Cao, Y.; Dhahad, H.A.; El-Shorbagy, M.A.; Alijani, H.Q.; Zakeri, M.; Heydari, A.; Bahonar, E.; Slouf, M.; Khatami, M.; Naderifar, M.; et al. Green synthesis of bimetallic ZnO–CuO nanoparticles and their cytotoxicity properties. Sci. Rep. 2021, 11, 23479. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Singh, M. Eco-friendly synthesis of copper oxide, zinc oxide and copper oxide–zinc oxide nanocomposites, and their anticancer applications. J. Inorg. Organomet. Polym. Mater. 2019, 30, 400–409. [Google Scholar] [CrossRef]
- Govindasamy, G.A.; Mydin, R.B.S.M.N.; Harun, N.H.; Sreekantan, S. Calcination temperatures, compositions and antimicrobial properties of heterostructural ZnO–CuO nanocomposites from Calotropis gigantea targeted for skin ulcer pathogens. Sci. Rep. 2020, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Hiew, C.W.; Lee, L.J.; Junus, S.; Tan, Y.N.; Chai, T.T.; Ee, K.Y. Optimization of microwave-assisted extraction and the effect of microencapsulation on mangosteen (Garcinia mangostana L.) rind extract. Food Sci. Technol. 2021, 42, e35521. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, X.; Dai, Q.; Qin, Z. Antibacterial, antioxidation, UV-blocking, and biodegradable soy protein isolate food packaging film with mangosteen peel extract and ZnO nanoparticles. Nanomaterials 2021, 11, 3337. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Ren, B.L.M.; Talib, R.A.; Zakaria, R.; Kamarudin, S.H. Novel mangosteen-leaves-based marker ink color lightness, viscosity, optimized composition, and microstructural analysis. Polymers 2021, 13, 1581. [Google Scholar] [CrossRef]
- Mulyono, D.; Irawati, Y.; Syah, M.J.A. Identification morphological variability of six mangosteen (Garcinia mangostana L.) as a conservation strategy for local varieties. IOP Conf. Ser. Earth Environ. Sci. 2021, 739, 012076. [Google Scholar] [CrossRef]
- Syahputra, M.R.; Setiado, H.; Siregar, L.A.M.; Damanik, R.I. Morphological characteristics of mangosteen plants (Garcinia mangostana L.) in Langkat District, North Sumatera, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 782, 042056. [Google Scholar] [CrossRef]
- Andani, R.; Fajrina, A.; Asra, R.; Eriadi, A. Antibacterial activity test of mangosteen plants (Garcinia mangostana L.): A review. Asian J. Pharm. Res. Dev. 2021, 9, 164–171. [Google Scholar] [CrossRef]
- Anggraeni, R.S. Antibacterial (Staphylococcus aureus and Escherichia coli) and Antifungal (Saccharomyces cerevisiae) activity assay on nanoemulsion formulation of ethanol extract of mangosteen leaves (Garcinia mangostana L). J. Food Pharm. Sci. 2021, 9, 351–365. [Google Scholar]
- Jassim, A.M.N.; Shafy, G.M.; Mohammed, M.T.; Farhan, S.A.; Noori, O.M. Antioxidant, anti-inflammatory and wound healing of biosynthetic gold nanoparticles using mangosteen (G. mangostona). Iraqi J. Ind. Res. 2021, 8, 59–74. [Google Scholar] [CrossRef]
- Tran, V.A.; Thi Vo, T.-T.; Thi Nguyen, M.-N.; Duy, N.D.; Doan, V.-D.; Nguyen, T.-Q.; Vu, Q.H.; Le, V.T.; Tong, T.D. Novel α-mangostin derivatives from mangosteen (Garcinia mangostana L.) peel extract with antioxidant and anticancer potential. J. Chem. 2021, 2021, 9985604. [Google Scholar] [CrossRef]
- Chan, Y.B.; Selvanathan, V.; Tey, L.-H.; Akhtaruzzaman, M.; Anur, F.H.; Djearamane, S.; Watanabe, A.; Aminuzzaman, M. Effect of calcination temperature on structural, morphological and optical properties of copper oxide nanostructures derived from Garcinia mangostana L. leaf extract. Nanometerials 2022, 12, 3589. [Google Scholar] [CrossRef] [PubMed]
- Hitkari, G.; Chowdhary, P.; Kumar, V.; Singh, S.; Motghare, A. Potential of copper-zinc oxide nanocomposite for photocatalytic degradation of congo red dye. Clean. Chem. Eng. 2022, 1, 100003–100009. [Google Scholar] [CrossRef]
- Rajendran, N.K.; George, B.P.; Houreld, N.N.; Abrahamse, H. Synthesis of zinc oxide nanoparticles using Rubus fairholmianus root extract and their activity against pathogenic bacteria. Molecules 2021, 26, 3029. [Google Scholar] [CrossRef]
- Sivakavinesan, M.; Vanaja, M.; Annadurai, G. Dyeing of cotton fabric materials with biogenic gold nanoparticles. Sci. Rep. 2021, 1, 13249. [Google Scholar] [CrossRef] [PubMed]
- Trang, N.L.N.; Hoang, V.T.; Dinh, N.X.; Tam, L.T.; Le, V.P.; Linh, D.T.; Cuong, D.M.; Khi, N.T.; Anh, N.H.; Nhung, P.T.; et al. Novel eco-friendly synthesis of biosilver nanoparticles as a colorimetric probe for highly selective detection of Fe (III) ions in aqueous solution. J. Nanomater. 2021, 2021, 5527519. [Google Scholar] [CrossRef]
- Mansoor Al-Saeedi, A.M.; Mohamad, F.K.; Ridha, N.J. Synthesis and characterization CuO-ZnO binary nanoparticles. J. Nanostructures 2022, 12, 86–96. [Google Scholar]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, e04896–e04908. [Google Scholar] [CrossRef]
- Rao, G.T.; Ravikumar, R.V.S.S.N. Novel Fe-doped ZnO-CdS nanocomposite with enhanced visible light-driven photocatalytic performance. Mater. Res. Innov. 2020, 25, 215–220. [Google Scholar] [CrossRef]
- Siddiqui, V.U.; Ansari, A.; Ansari, M.T.; Akram, M.K.; Siddiqi, W.A.; Alosaimi, A.M.; Hussein, M.A.; Rafatullah, M. Optimization of facile synthesized ZnO/CuO nanophotocatalyst for organic dye degradation by visible light irradiation using response surface methodology. Catalysts 2021, 11, 1509. [Google Scholar] [CrossRef]
- Khan, M.I.; Fatima, N.; Shakil, M.; Tahir, M.B.; Riaz, K.N.; Rafique, M.; Iqbal, T.; Mahmood, K. Investigation of in-vitro antibacterial and seed germination properties of green synthesized pure and nickel doped ZnO nanoparticles. Phys. B Phys. Condens. Matter. 2021, 601, 412563. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Yee, O.S.; Teow, S.Y.; Hedayatnasab, Z.; Jahangirian, H.; Webster, T.J.; Kuca, K. Green synthesis of Fe3O4 nanoparticles stabilized by a garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int. J. Nanomed. 2021, 16, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Aloucheh, R.; Habibi-Yangjeh, A.; Bayrami, A.; Latifi-Navid, S.; Asadi, A. Enhanced anti-bacterial activities of ZnO nanoparticles and ZnO/CuO nanocomposites synthesized using Vaccinium arctostaphylos L. fruit extract. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Aloucheh, R.; Habibi-Yangjeh, A.; Bayrami, A.; Latifi-Navid, S.; Asadi, A. Green synthesis of ZnO and ZnO/CuO nanocomposites in Mentha longifolia leaf extract: Characterization and their application as anti-bacterial agents. J. Mater. Sci. Mater. Electron. 2018, 29, 13596–13605. [Google Scholar] [CrossRef]
- Haneefa, M.M.; Jayandran, M.; Balasubramanian, V. Green synthesis characterization and antimicrobial activity evaluation of manganese oxide nanoparticles and comparative studies with salicylalchitosan functionalized nanoform. Asian J. Pharm. 2017, 11, 65–74. [Google Scholar]
- Demissie, M.G.; Sabir, F.K.; Edossa, G.D.; Gonfa, B.A. Synthesis of zinc oxide nanoparticles using leaf extract of Lippia adoensis (Koseret) and evaluation of its antibacterial activity. J. Chem. 2020, 2020, 7459042. [Google Scholar] [CrossRef]
- Siddiqui, V.U.; Ansari, A.; Chauhan, R.; Siddiqi, W.A. Green synthesis of copper oxide (CuO) nanoparticles by Punica granatum peel extract. Mater. Today Proc. 2021, 36, 751–755. [Google Scholar] [CrossRef]
- Phang, Y.-K.; Aminuzzaman, M.; Akhtaruzzaman, M.; Muhammad, G.; Ogawa, S.; Watanabe, A.; Tey, L.-H. Green synthesis and characterization of CuO nanoparticles derived from papaya peel extract for the photocatalytic degradation of palm oil mill effluent (POME). Sustainability 2021, 13, 796. [Google Scholar] [CrossRef]
- Sajjad, A.; Bhatti, S.H.; Ali, Z.; Jaffari, G.H.; Khan, N.A.; Rizvi, Z.F.; Zia, M. Photoinduced fabrication of zinc oxide nanoparticles: Transformation of morphological and biological response on light irradiance. ACS Omega 2021, 6, 11783–11793. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Yadav, D.K.; Chawla, K.; Lai, N.; Lai, C. Synthesis and characterization of CuO nanoparticles by Aloe barbadensis leaves. Quantum J. Eng. Sci. Technol. 2021, 2, 1–9. [Google Scholar]
- You, W.; Ahn, J.C.; Boopathi, V.; Arunkumar, L.; Rupa, E.J.; Akter, R.; Kong, B.M.; Lee, G.S.; Yang, D.C.; Kang, S.C.; et al. Enhanced antiobesity efficacy of tryptophan using the nanoformulation of Dendropanax morbifera extract mediated with ZnO nanoparticle. Materials 2021, 14, 824. [Google Scholar] [CrossRef] [PubMed]
- Aminuzzaman, M.; Chong, C.-Y.; Goh, W.S.; Phang, Y.-K.; Tey, L.-H.; Chee, S.-Y.; Akhtaruzzaman, M.; Ogawa, S.; Watanabe, A. Biosynthesis of NiO nanoparticles using soursop (Annona muricata L.) fruit peel green waste and their photocatalytic performance on crystal violet dye. J. Clust. Sci. 2021, 32, 949–958. [Google Scholar] [CrossRef]
- Ramzan, M.; Obodo, R.M.; Mukhtar, S.; Ilyas, S.Z.; Aziz, F.; Thovhogi, N. Green synthesis of copper oxide nanoparticles using Cedrus deodara aqueous extract for antibacterial activity. Mater. Today Proc. 2021, 36, 576–581. [Google Scholar] [CrossRef]
- Baharudin, K.B.; Abdullah, N.; Derawi, D. Effect of calcination temperature on the physicochemical properties of zinc oxide nanoparticles synthesized by coprecipitation. Mater. Res. Express 2018, 5, 125018. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize zinc oxide nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of metal and metal oxide nanoparticles. ChemBioEng Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phyther Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef]
- Selvanathan, V.; Aminuzzaman, M.; Tan, L.X.; Yip, F.W.; Eddy Cheah, S.G.; Heng, M.H.; Tey, L.-H.; Arullappan, S.; Algethami, N.; Alharthi, S.S.; et al. Synthesis, characterization, and preliminary in vitro antibacterial evaluation of ZnO nanoparticles derived from soursop (Annona muricata L.) leaf extract as a green reducing agent. J. Mater. Res. Technol. 2022, 20, 2931–2941. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J. Nanosci. 2017, 2017, 8013850. [Google Scholar] [CrossRef]
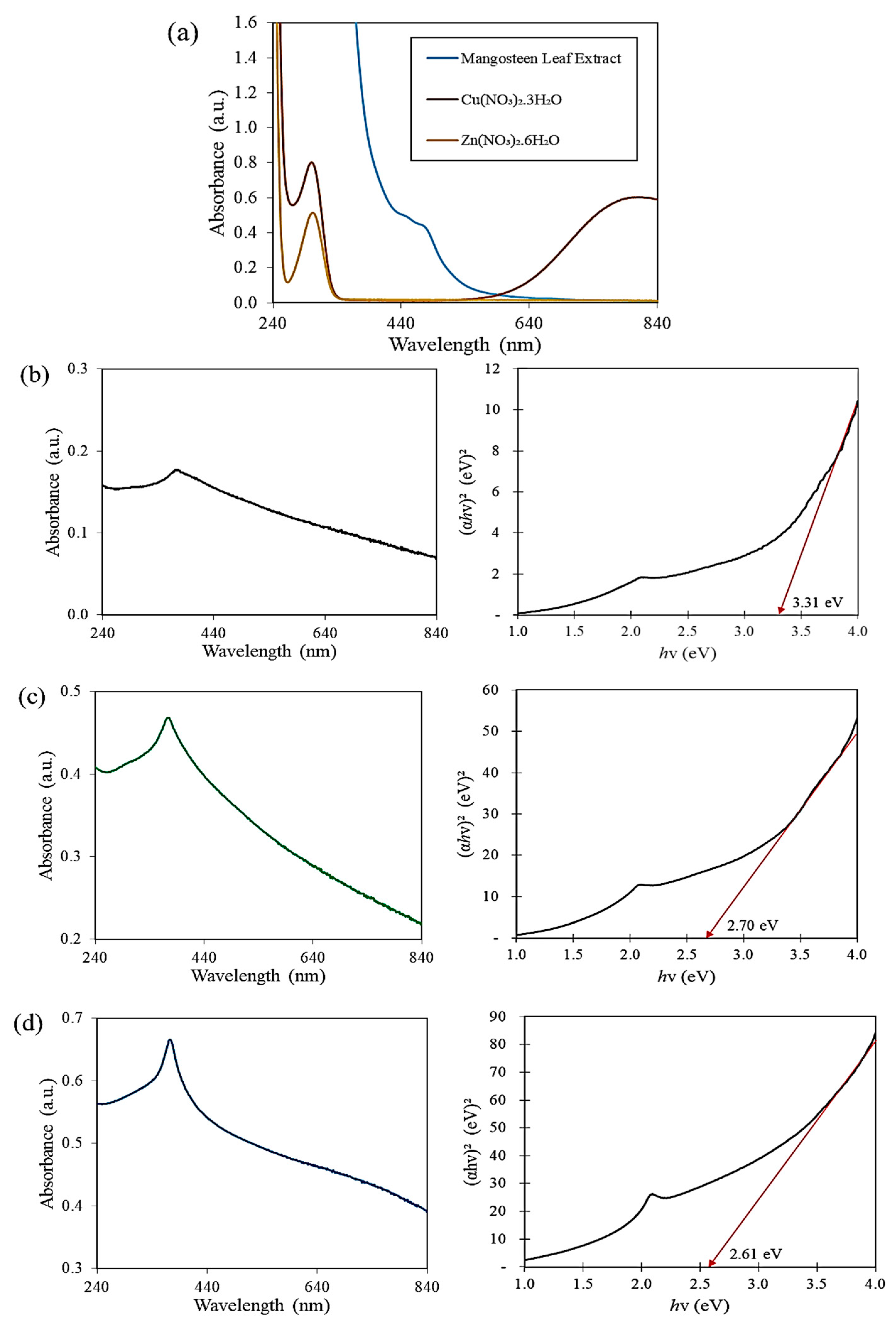
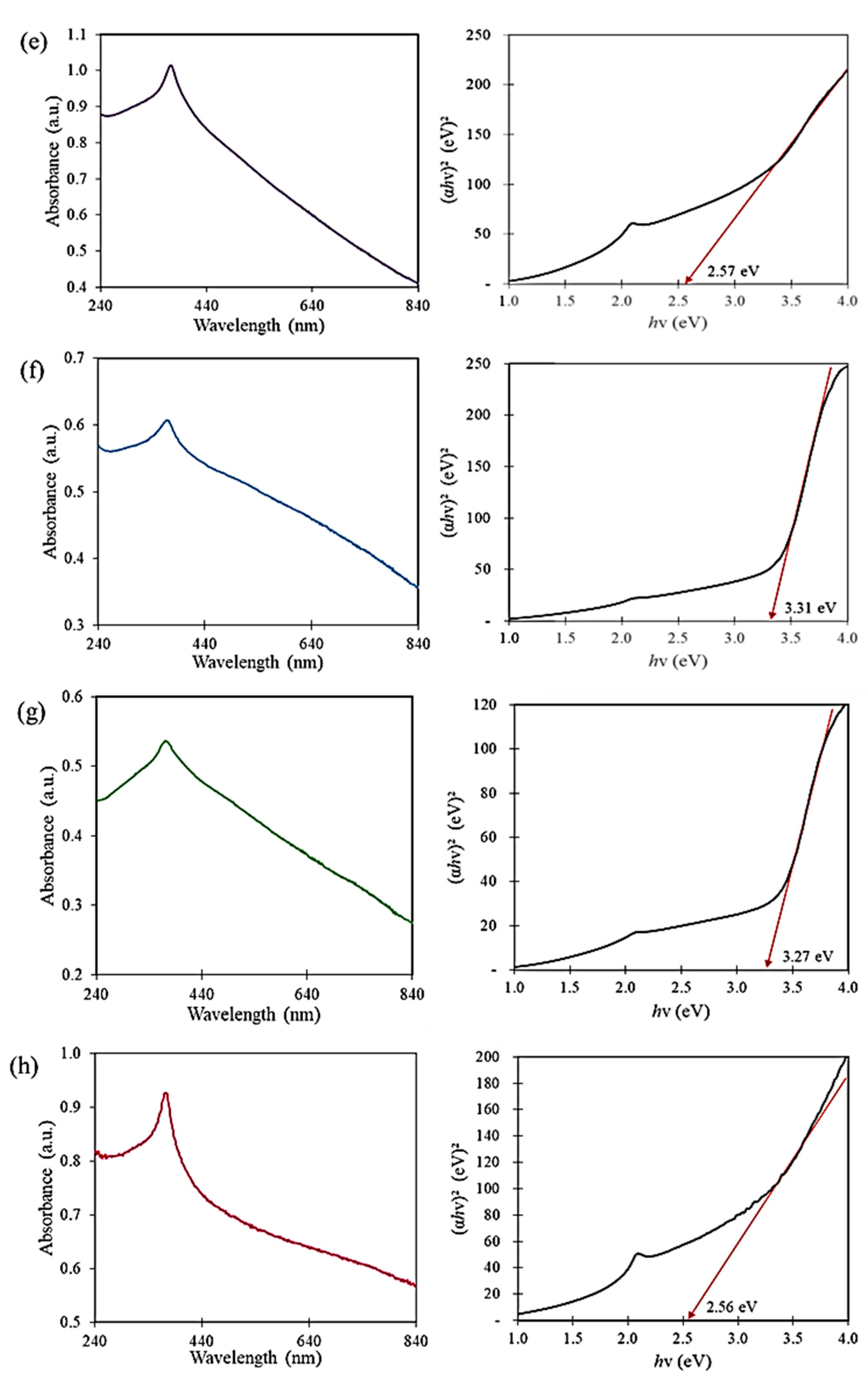
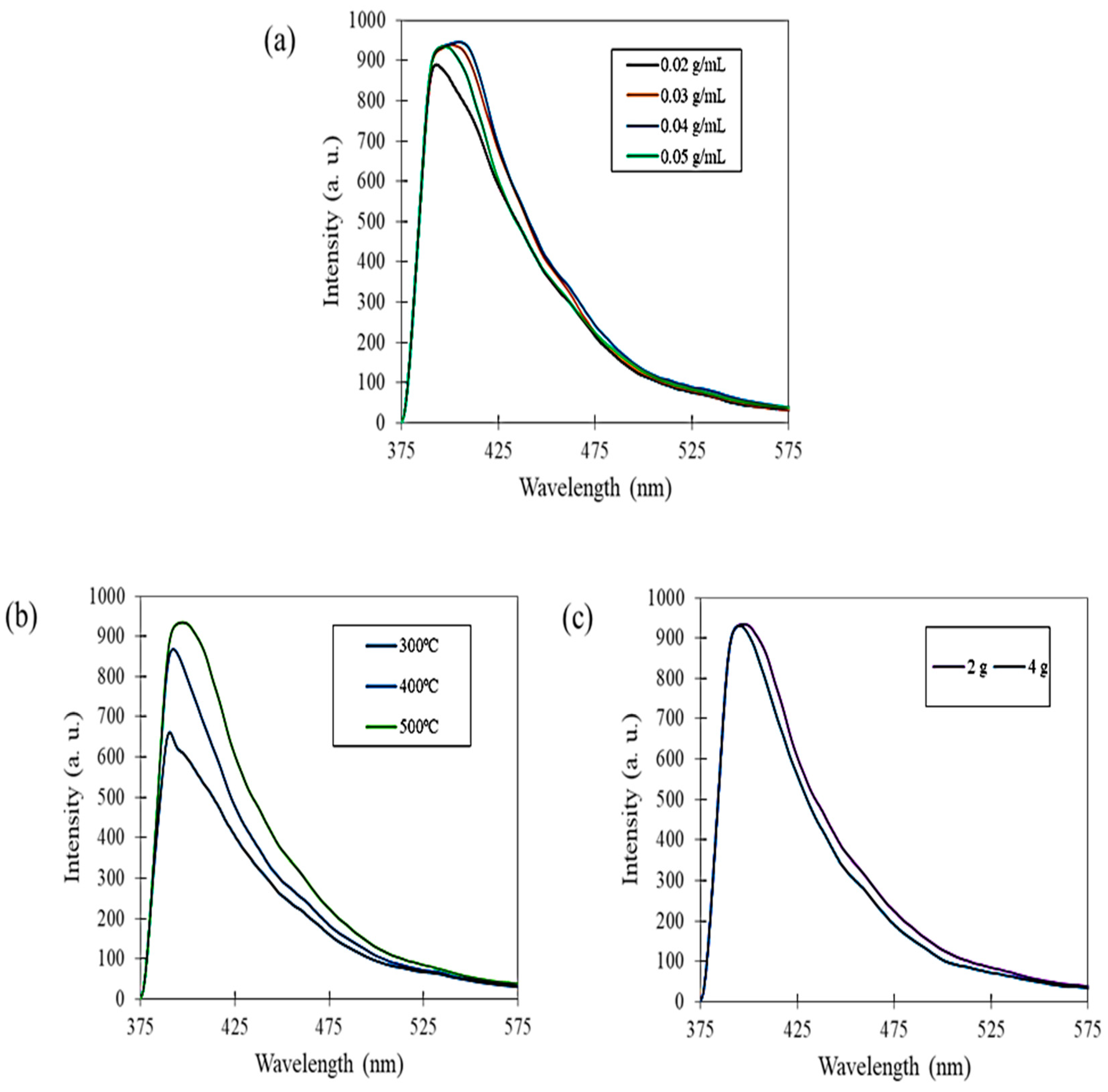
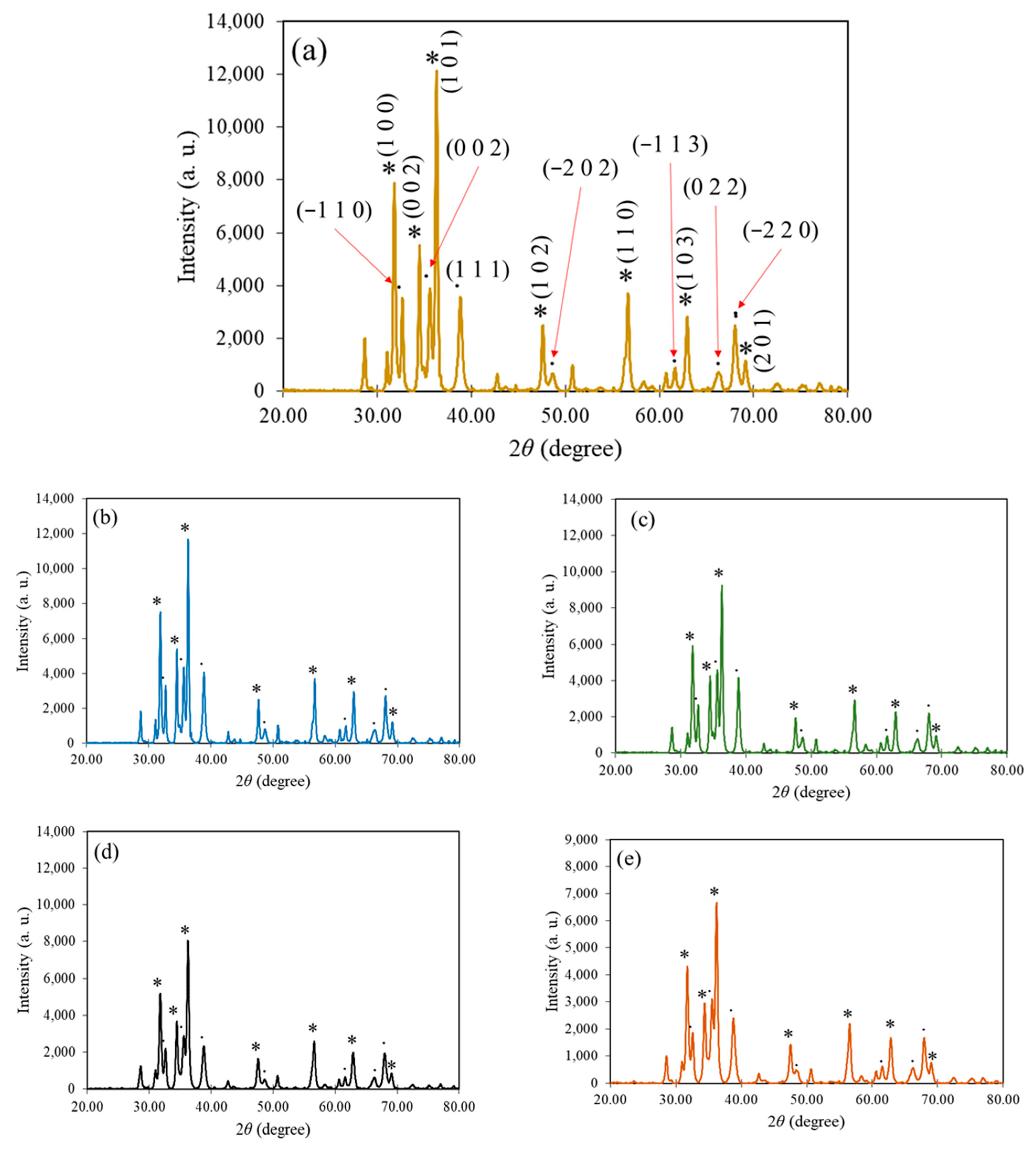
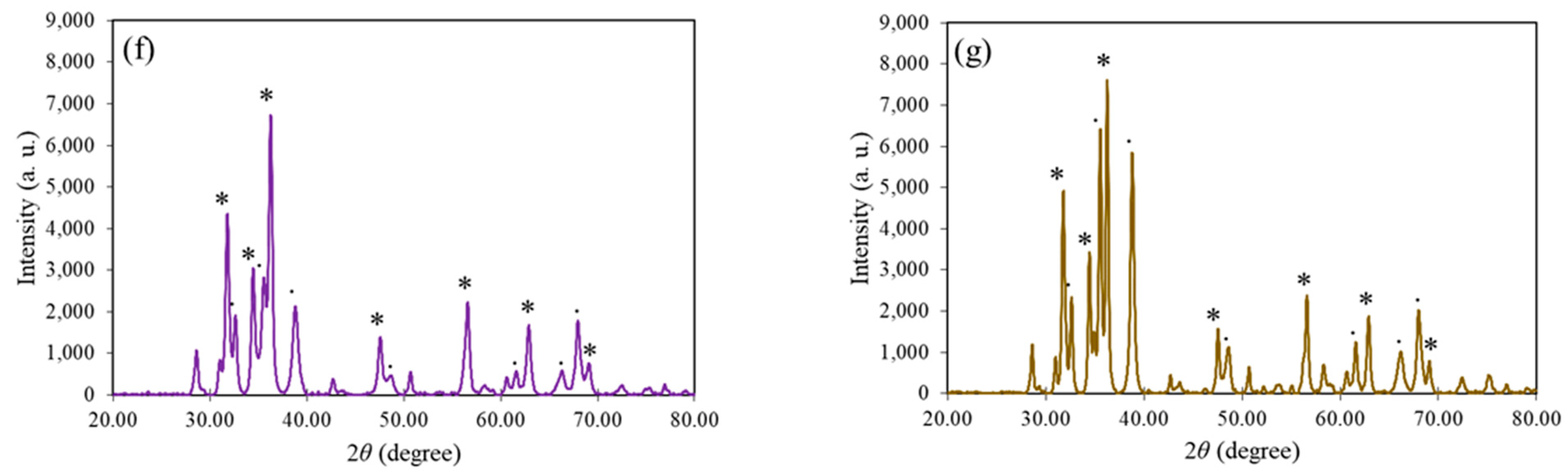
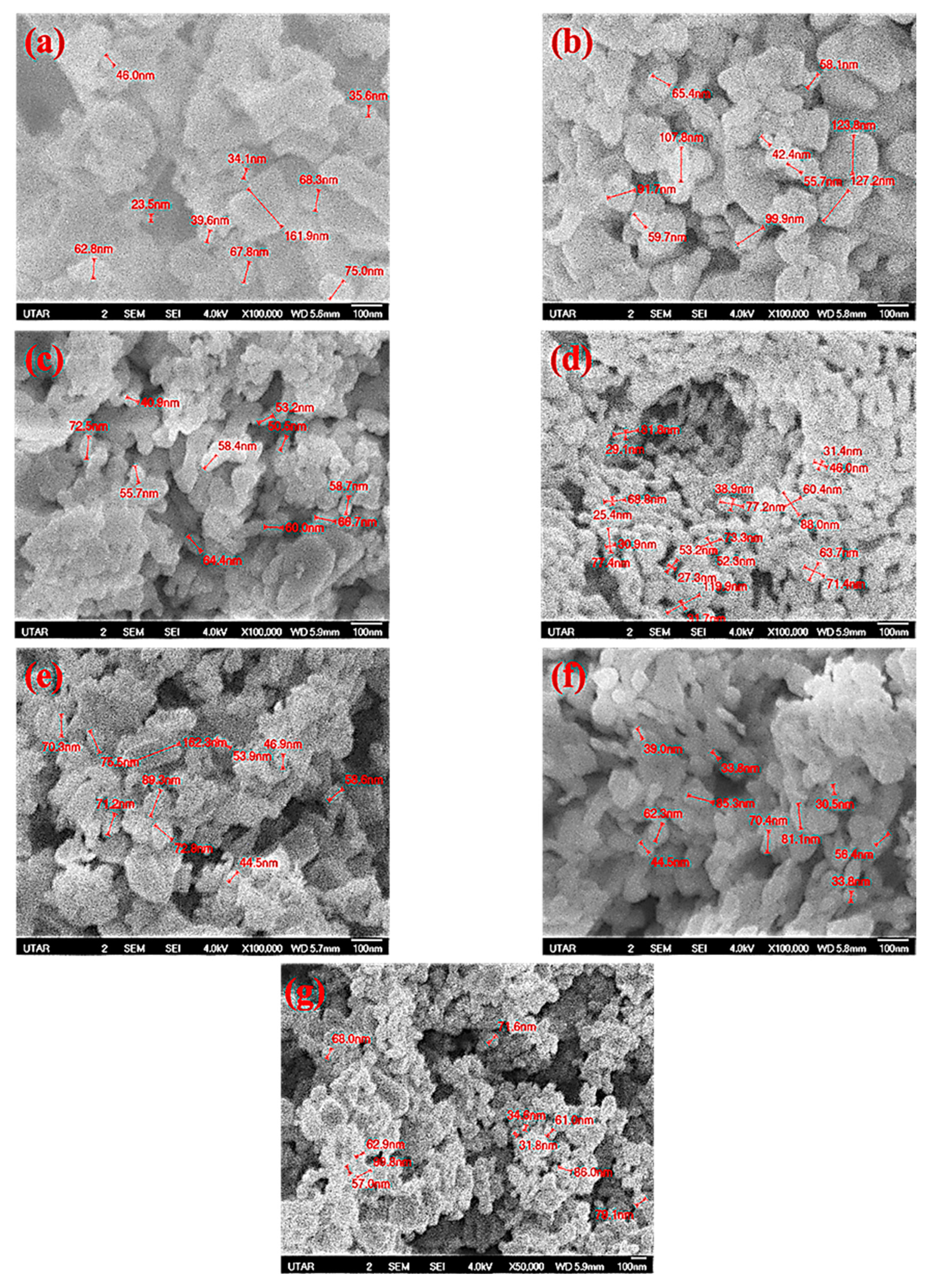
| Mangosteen Leaf Aqueous Extract Concentration (g/mL) | Calcination Temperature (°C) | Weight of Zn(NO3)2·6H2O (g) | Weight of Cu(NO3)2·3H2O (g) | Energy Bandgap (eV) |
|---|---|---|---|---|
| 0.02 | 500 | 4.0 | 2.0 | 3.31 |
| 0.03 | 500 | 4.0 | 2.0 | 2.70 |
| 0.04 | 500 | 4.0 | 2.0 | 2.61 |
| 0.05 | 500 | 4.0 | 2.0 | 2.57 |
| 0.05 | 300 | 4.0 | 2.0 | 3.31 |
| 0.05 | 400 | 4.0 | 2.0 | 3.27 |
| 0.05 | 500 | 4.0 | 4.0 | 2.56 |
| Functional Groups | Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mangosteen Leaf Aqueous Extract Concentration (g/mL) | Calcination Temperature (°C) | Weight of Cu(NO3)2·3H2O (g) | |||||||
| 0.02 | 0.03 | 0.04 | 0.05 * | 300 | 400 | 500 * | 2 * | 4 | |
| v(O-H) | 3429 | 3435 | 3435 | 3435 | 3436 | 3435 | 3435 | 3435 | 3401 |
| v(C=O), v(C=C) | 1629 | 1636 | 1636 | 1636 | 1633 | 1635 | 1636 | 1636 | 1635 |
| v(C-C aromatic) | 1384 | 1384 | 1384 | 1384 | 1384 | 1384 | 1384 | 1384 | 1384 |
| v(C-O) | 1114 | 1108 | 1108 | 1108 | 1102 | 1099 | 1108 | 1108 | 1106 |
| v(Cu-O) | 674 | 660 | 649 | 651 | 657 | 657 | 651 | 651 | 672 |
| v(Zn-O) | 447 | 455 | 485 | 486 | 502 | 498 | 486 | 486 | 524 |
| Mangosteen Leaf Aqueous Extract Concentration (g/mL) | Calcination Temperature (°C) | Weight of Zn(NO3)2·6H2O (g) | Weight of Cu(NO3)2·3H2O (g) | ZnO Crystalline Size (nm) | CuO Crystalline Size (nm) | Crystalline Size (nm) | Dislocation Density (1014 cm−1) | Micro Strain (10−4) |
|---|---|---|---|---|---|---|---|---|
| 0.02 | 500 | 4.0 | 2.0 | 31.98 | 25.03 | 28.51 | 12.31 | 1.35 |
| 0.03 | 500 | 4.0 | 2.0 | 28.67 | 25.23 | 26.95 | 13.77 | 1.32 |
| 0.04 | 500 | 4.0 | 2.0 | 27.42 | 23.23 | 25.32 | 15.59 | 1.40 |
| 0.05 | 500 | 4.0 | 2.0 | 21.10 | 15.23 | 18.17 | 30.30 | 2.77 |
| 0.05 | 300 | 4.0 | 2.0 | 23.13 | 21.37 | 22.25 | 20.19 | 1.69 |
| 0.05 | 400 | 4.0 | 2.0 | 21.94 | 18.52 | 20.23 | 24.42 | 1.86 |
| 0.05 | 500 | 4.0 | 4.0 | 20.47 | 20.31 | 22.29 | 20.13 | 1.63 |
| Mangosteen Leaf Aqueous Extract Concentration (g/mL) | Calcination Temperature (°C) | Weight of Zn(NO3)2·6H2O (g) | Weight of Cu(NO3)2·3H2O (g) | Particle Size (nm) | Morphology |
|---|---|---|---|---|---|
| 0.02 | 500 | 4.0 | 2.0 | 61.46 | Agglomerated with irregular nanostructure |
| 0.03 | 500 | 4.0 | 2.0 | 56.27 | Agglomerated with quasi-spherical nanostructure |
| 0.04 | 500 | 4.0 | 2.0 | 55.23 | Agglomerated with lobed nanostructure |
| 0.05 | 500 | 4.0 | 2.0 | 39.10 | Agglomerated and uniformly distributed with spherical nanostructure |
| 0.05 | 300 | 4.0 | 2.0 | 74.53 | Highly agglomerated with quasi-spherical nanostructure |
| 0.05 | 400 | 4.0 | 2.0 | 53.71 | Agglomerated with spherical nanostructure |
| 0.05 | 500 | 4.0 | 4.0 | 65.18 | Agglomerated with spherical nanostructure |
| Mangosteen Leaf Aqueous ExtractConcentration (g/mL) | Calcination Temperature (°C) | Weight of Zn(NO3)2·6H2O (g) | Weight of Cu(NO3)2·3H2O (g) | Oxygen Atomic Percentage | Copper Atomic Percentage | Zinc Atomic Percentage |
|---|---|---|---|---|---|---|
| 0.02 | 500 | 4.0 | 2.0 | 62.28 | 13.03 | 24.68 |
| 0.03 | 500 | 4.0 | 2.0 | 60.16 | 12.86 | 26.98 |
| 0.04 | 500 | 4.0 | 2.0 | 63.25 | 13.10 | 23.65 |
| 0.05 | 500 | 4.0 | 2.0 | 62.41 | 12.55 | 25.03 |
| 0.05 | 300 | 4.0 | 2.0 | 66.25 | 12.28 | 21.47 |
| 0.05 | 400 | 4.0 | 2.0 | 66.20 | 11.64 | 22.16 |
| 0.05 | 500 | 4.0 | 4.0 | 61.45 | 18.44 | 20.10 |
| Plant Extract | Plant Extract Concentration (g/mL) | Calcination Temperature (°C) | Zinc Salt Added (g) | Copper Salt Added (g) | Energy Bandgap (eV) | Crystalline Size (nm) | Particle Size (nm) | Reference |
|---|---|---|---|---|---|---|---|---|
| Theobroma cacao seed bark | 0.20 1 | 400 | 0.03 M | 0.01 M | - | 10.00 | 20.0–50.0 | [6] |
| D. caffra leaf | 0.10 | 400 | 18.35 2 | 3.19 3 | - | 23.21 | 20.0–32.0 | [20] |
| V. sinaiticum Benth | 0.10 | 500 | 5.0 | 1.25 | 2.74 | 18.00 | - | [21] |
| S. nigra L. shoot | 0.14 | 400 | 1.6 | 0.8 | - | - | 20.0–130.0 | [22] |
| G. mangostana L. leaf | 0.05 | 500 | 4.0 | 2.0 | 2.57 | 18.17 | 25.4–60.4 | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, Y.B.; Aminuzzaman, M.; Tey, L.-H.; Win, Y.F.; Watanabe, A.; Djearamame, S.; Akhtaruzzaman, M. Impact of Diverse Parameters on the Physicochemical Characteristics of Green-Synthesized Zinc Oxide–Copper Oxide Nanocomposites Derived from an Aqueous Extract of Garcinia mangostana L. Leaf. Materials 2023, 16, 5421. https://doi.org/10.3390/ma16155421
Chan YB, Aminuzzaman M, Tey L-H, Win YF, Watanabe A, Djearamame S, Akhtaruzzaman M. Impact of Diverse Parameters on the Physicochemical Characteristics of Green-Synthesized Zinc Oxide–Copper Oxide Nanocomposites Derived from an Aqueous Extract of Garcinia mangostana L. Leaf. Materials. 2023; 16(15):5421. https://doi.org/10.3390/ma16155421
Chicago/Turabian StyleChan, Yu Bin, Mohammod Aminuzzaman, Lai-Hock Tey, Yip Foo Win, Akira Watanabe, Sinouvassane Djearamame, and Md. Akhtaruzzaman. 2023. "Impact of Diverse Parameters on the Physicochemical Characteristics of Green-Synthesized Zinc Oxide–Copper Oxide Nanocomposites Derived from an Aqueous Extract of Garcinia mangostana L. Leaf" Materials 16, no. 15: 5421. https://doi.org/10.3390/ma16155421
APA StyleChan, Y. B., Aminuzzaman, M., Tey, L.-H., Win, Y. F., Watanabe, A., Djearamame, S., & Akhtaruzzaman, M. (2023). Impact of Diverse Parameters on the Physicochemical Characteristics of Green-Synthesized Zinc Oxide–Copper Oxide Nanocomposites Derived from an Aqueous Extract of Garcinia mangostana L. Leaf. Materials, 16(15), 5421. https://doi.org/10.3390/ma16155421