Biocompatibility Assessment of Zinc Alloys as a New Potential Material for Bioabsorbable Implants for Osteosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Characterization
2.1.1. Three-Dimensional Laser Scanning Microscopy
2.1.2. Energy Dispersive X-ray (EDX) Spectroscopy and Environmental Scanning Electron Microscope (ESEM)
2.1.3. Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES)
2.1.4. Atomic Absorption Spectroscopy (AAS)
2.2. Biocompatibility
2.2.1. Live/Dead Assay
2.2.2. Cell Proliferation (WST Assay)
2.2.3. Cytotoxicity (LDH Assay)
2.2.4. pH-Measurements
2.3. Statistics
3. Results
3.1. Sample Characterization
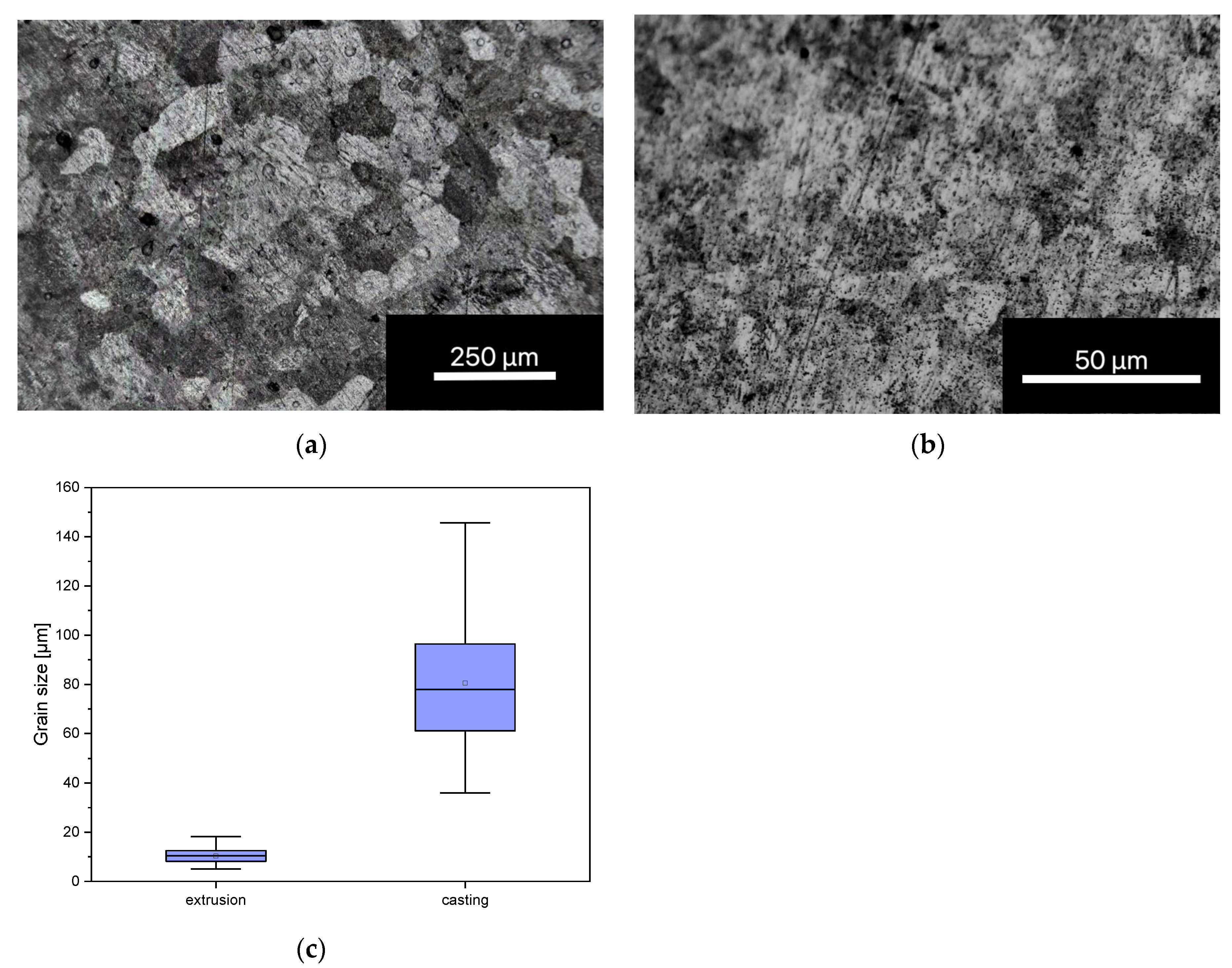
3.1.1. Grain Size Determination
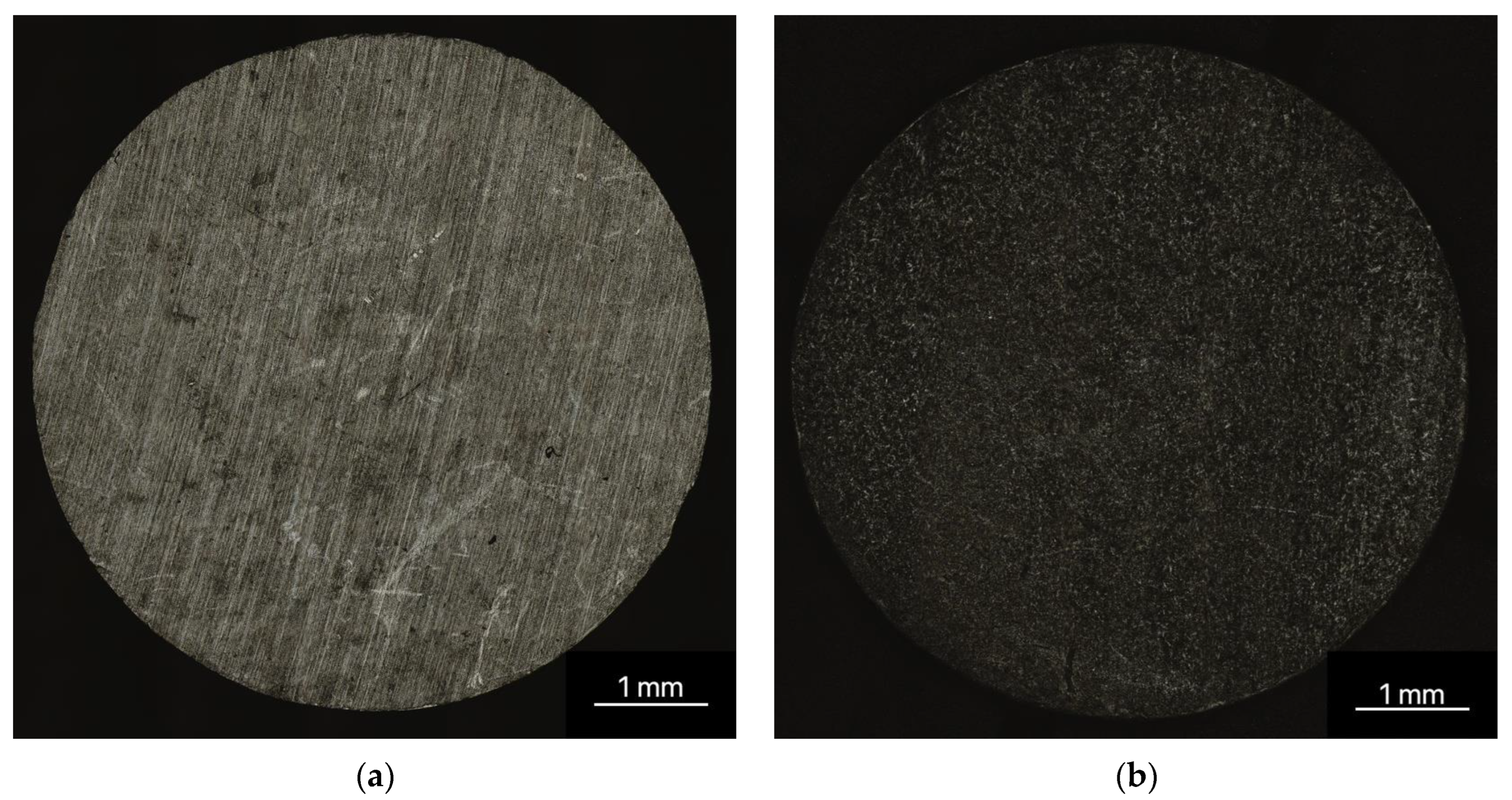
3.1.2. Three-Dimensional Laser Scanning Microscopy
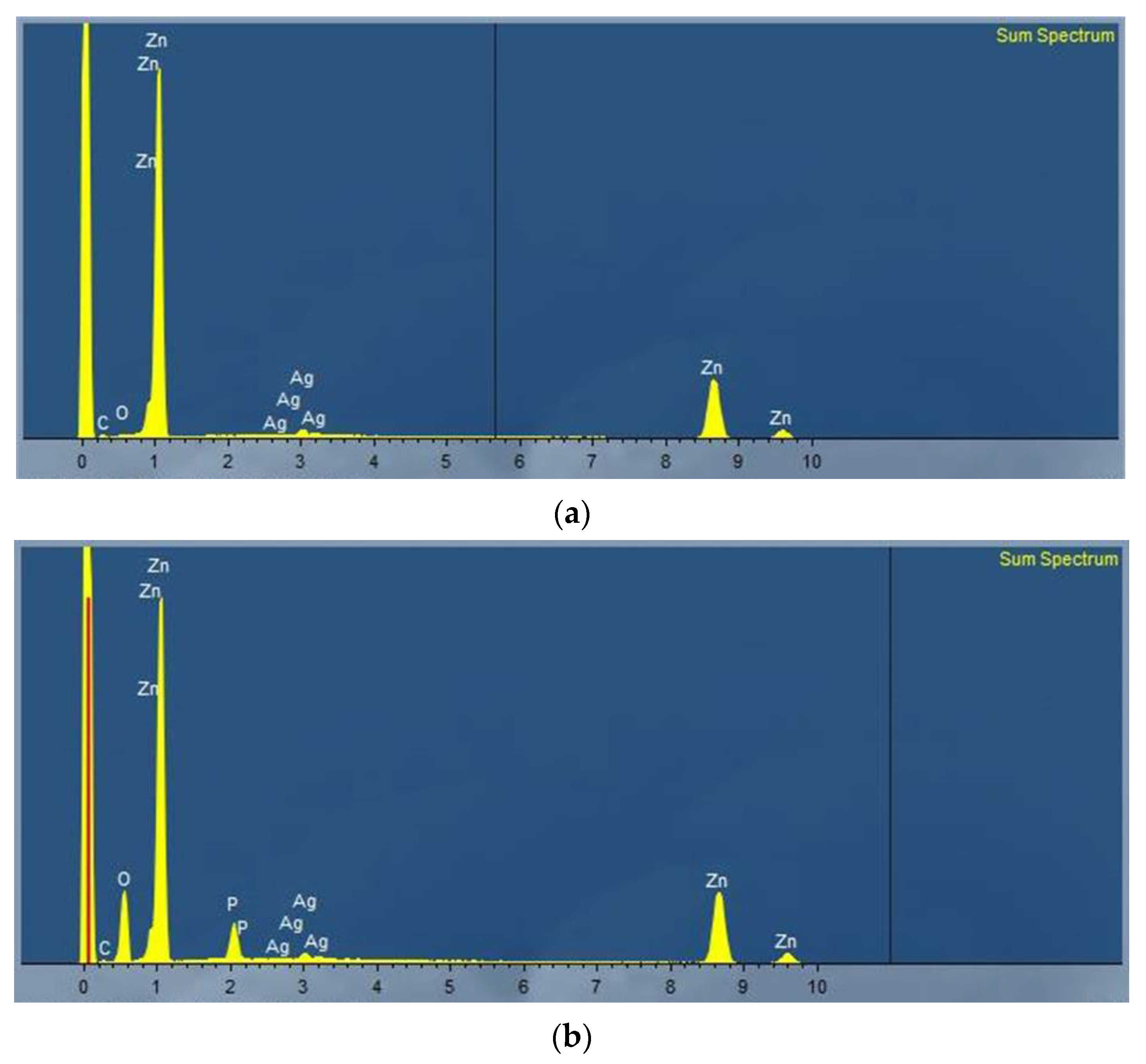
3.1.3. Energy Dispersive X-ray (EDX) Spectroscopy
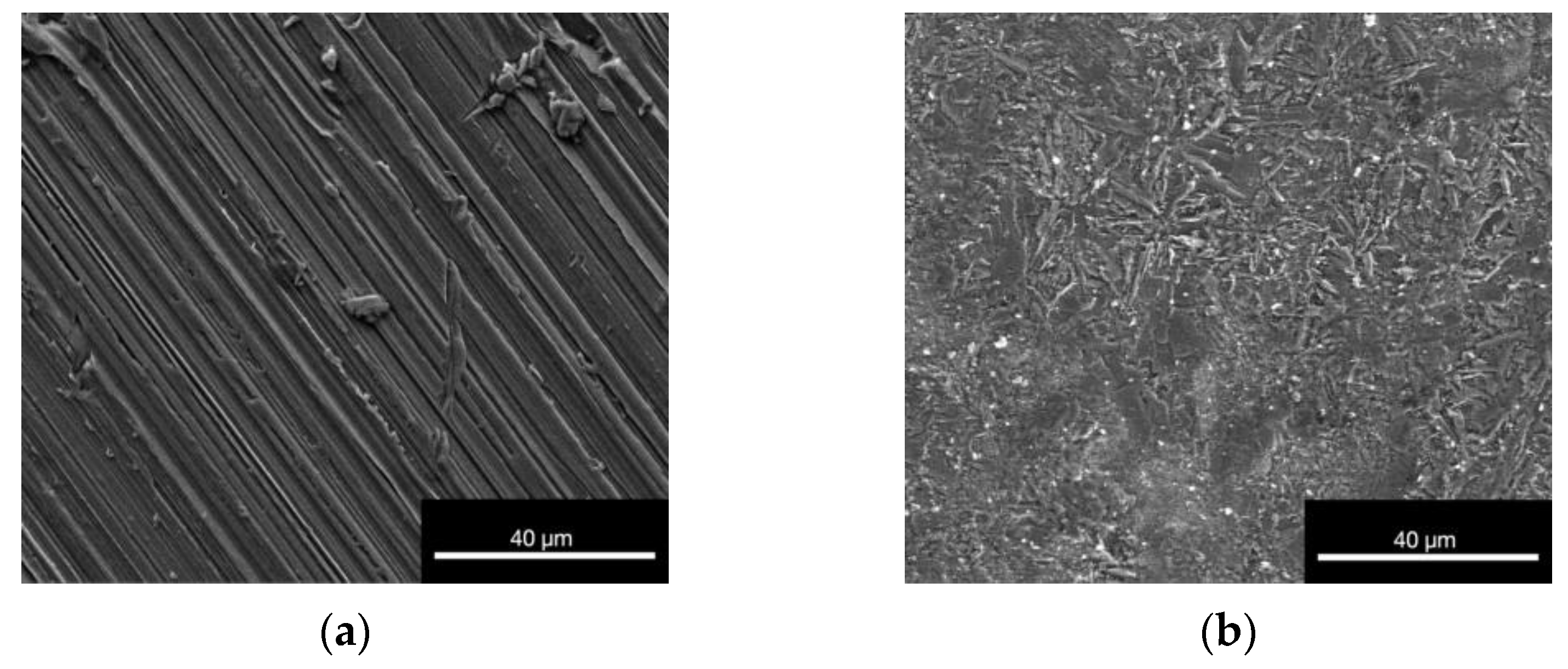
3.1.4. Environmental Scanning Electron Microscope (ESEM)
3.1.5. Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES)
| Element | Concentration mg/L | Percentage % | Index Value % |
| Zn | 13.82 ± 2.44 | 96.70 ± 0.05 | 96.70 |
| Ag | 0.47 ± 0.09 | 3.30 ± 0.05 | 3.30 |
3.1.6. Atomic Absorption Spectroscopy (AAS)
3.2. Biocompatibility
3.2.1. Live/Dead Assay
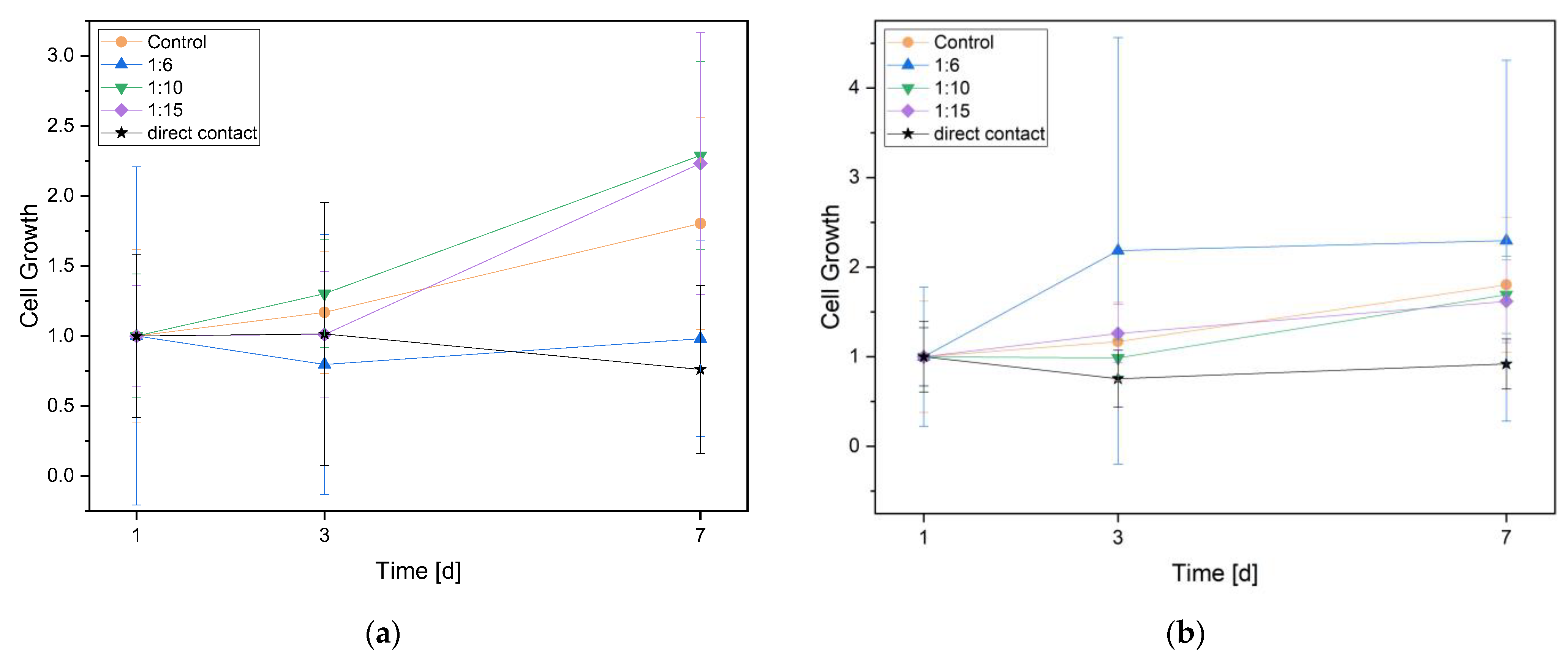
3.2.2. Cell Proliferation (WST Assay)
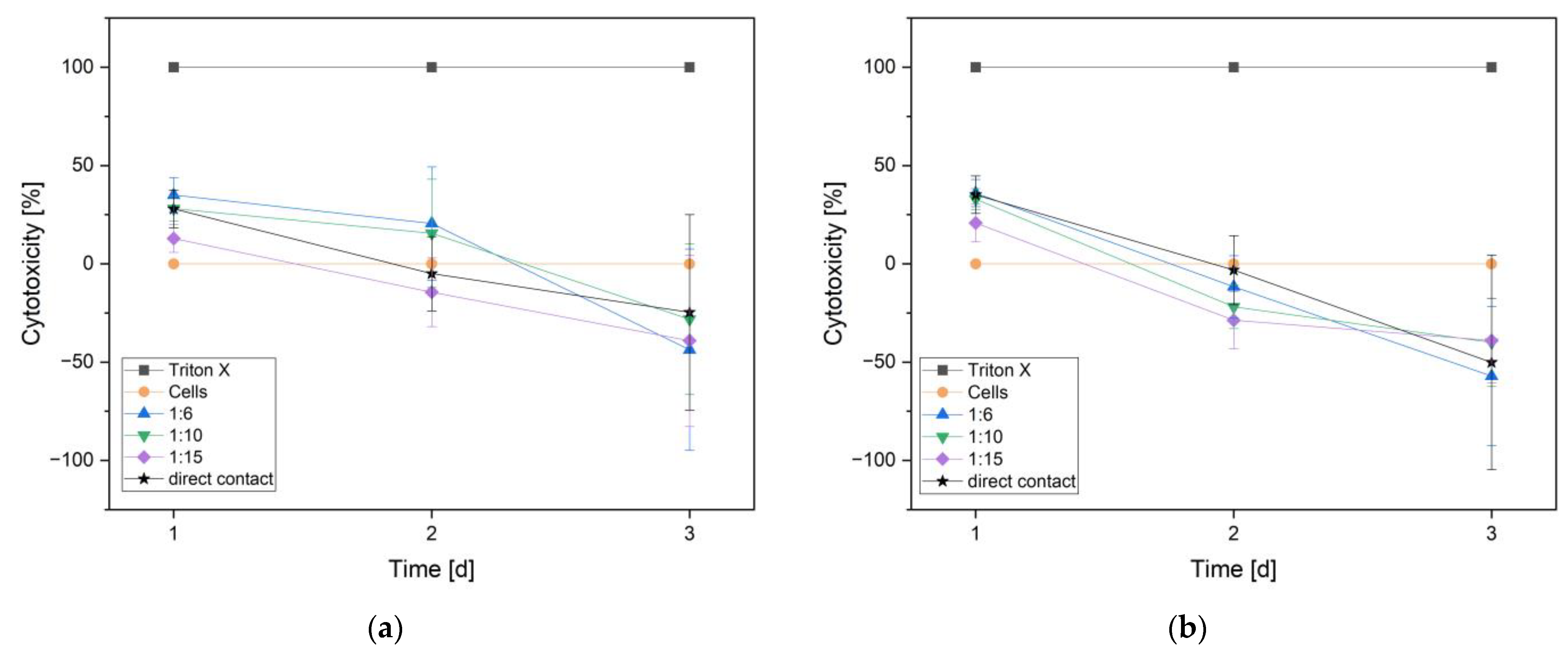
3.2.3. Cytotoxicity (LDH Assay)
3.2.4. pH-Measurements
4. Discussion
4.1. Sample Characterization
4.2. Biocompatibility
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Life-Science-Intelligence. Orthopedic Surgery—Global Trends & Opportunities; Life-Science-Intelligence: Huntington Beach, CA, USA, 2018. [Google Scholar]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the global burden of disease study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Walley, K.C.; Hofmann, K.J.; Velasco, B.T.; Kwon, J.Y. Removal of hardware after syndesmotic screw fixation: A systematic literature review. Foot Ankle Spec. 2016, 10, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.K.; Gamble, J.G.; Kha, S.T.; Hecht, G.G.; Vorhies, J.S.; Lucas, J.F. Indications for and risks associated with implant removal after pediatric trauma. J. Am. Acad. Orthop. Surgeons. Glob. Res. Rev. 2022, 6, e22. [Google Scholar] [CrossRef] [PubMed]
- Driessen, M.L.S.; Goessens, M.L.M.J. Complications of implant removal after healed hip fractures. Arch. Orthop. Trauma Surg. 2020, 140, 1745–1749. [Google Scholar] [CrossRef]
- Gupta, J.; Harkin, E.A.; O’Connor, K.; Enobun, B.; O’Hara, N.N.; O’Toole, R.V. Surgical factors associated with symptomatic implant removal after patella fracture. Injury 2022, 53, 2241–2246. [Google Scholar] [CrossRef]
- Kannan, M.B. 13—Hydroxyapatite coating on biodegradable magnesium and magnesium-based alloys. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 289–306. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y. Biodegradable orthopedic magnesium-calcium (mgca) alloys, processing, and corrosion performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef]
- Paiva, J.C.C.; Oliveira, L.; Vaz, M.F.; Costa-de-Oliveira, S. Biodegradable bone implants as a new hope to reduce device-associated infections—a systematic review. Bioengineering 2022, 9, 409. [Google Scholar] [CrossRef]
- Schlichting, K.; Dahne, M.; Weiler, A. Biodegradable composite implants. Sports Med. Arthrosc. Rev. 2006, 14, 169–176. [Google Scholar] [CrossRef]
- Chandra, G.; Pandey, A. Design approaches and challenges for biodegradable bone implants: A review. Expert Rev. Med. Devices 2021, 18, 629–647. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.-M. Bone–implant interface strength and osseointegration: Biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2011, 7, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, J.; Zhou, W.; Xiong, P.; Wang, P.; Yuan, W.; Zheng, Y.; Cheng, Y. In vitro degradation and biocompatibility evaluation of typical biodegradable metals (mg/zn/fe) for the application of tracheobronchial stenosis. Bioact. Mater. 2019, 4, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, H.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Zhu, D.; Ruan, L.; Takashima, K. Challenges in the use of zinc and its alloys as biodegradable metals: Perspective from biomechanical compatibility. Acta Biomater. 2019, 97, 23–45. [Google Scholar] [CrossRef]
- Torgersen, S.; Gjerdet, N.R.; Erichsen, E.S.; Bang, G. Metal particles and tissue changes adjacent to miniplates. A retrieval study. Acta Odontol. Scand. 1995, 53, 65–71. [Google Scholar] [CrossRef]
- Amano, H.; Miyake, K.; Hinoki, A.; Yokota, K.; Kinoshita, F.; Nakazawa, A.; Tanaka, Y.; Seto, Y.; Uchida, H. Novel zinc alloys for biodegradable surgical staples. World J. Clin. Cases 2020, 8, 504–516. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, E.; Shao, J.; Ge, S. Advances on biodegradable zinc-silver-based alloys for biomedical applications. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211062407. [Google Scholar] [CrossRef]
- Oliver, A.A.; Guillory, R.J., II; Flom, K.L.; Morath, L.M.; Kolesar, T.M.; Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Goldman, J. Analysis of vascular inflammation against bioresorbable zn–ag-based alloys. ACS Appl. Bio Mater. 2020, 3, 6779–6789. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed znag alloys for degradable implant applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual zn/ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef]
- Hehrlein, C.; Schorch, B.; Kress, N.; Arab, A.; von zur Mühlen, C.; Bode, C.; Epting, T.; Haberstroh, J.; Mey, L.; Schwarzbach, H.; et al. Zn-alloy provides a novel platform for mechanically stable bioresorbable vascular stents. PLoS ONE 2019, 14, e0209111. [Google Scholar] [CrossRef]
- Guillory, R.J.; Mostaed, E.; Oliver, A.A.; Morath, L.M.; Earley, E.J.; Flom, K.L.; Kolesar, T.M.; Mostaed, A.; Summers, H.D.; Kwesiga, M.P.; et al. Improved biocompatibility of zn–ag-based stent materials by microstructure refinement. Acta Biomater. 2022, 145, 416–426. [Google Scholar] [CrossRef]
- Shojaei, S.; Shahgholi, M.; Karimipour, A. The effects of atomic percentage and size of zinc nanoparticles, and atomic porosity on thermal and mechanical properties of reinforced calcium phosphate cement by molecular dynamics simulation. J. Mech. Behav. Biomed. Mater. 2023, 141, 105785. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin a, Vitamin k, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Plaass, C.; Ettinger, S.; Sonnow, L.; Koenneker, S.; Noll, Y.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Daniilidis, K.; Stukenborg-Colsman, C.; et al. Early results using a biodegradable magnesium screw for modified chevron osteotomies. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016, 34, 2207–2214. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Vojtìch, D. Comparative mechanical and corrosion studies on magnesium, zinc and iron alloys as biodegradable metals. Mater. Tehnol. 2015, 49, 877–882. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. Mgznca glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yang, H.; Jia, B.; Wang, M.; Yue, B.; Zheng, Y.; Dai, K. Zinc alloy-based bone internal fixation screw with antibacterial and anti-osteolytic properties. Bioact. Mater. 2021, 6, 4607–4624. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Hagelstein, S.; Zankovic, S.; Kovacs, A.; Barkhoff, R.; Seidenstuecker, M. Mechanical analysis and corrosion analysis of zinc alloys for bioabsorbable implants for osteosynthesis. Materials 2022, 15, 421. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Schille, C.; Schweizer, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Mechanical characteristics, in vitro degradation, cytotoxicity, and antibacterial evaluation of zn-4.0ag alloy as a biodegradable material. Int. J. Mol. Sci. 2018, 19, 755. [Google Scholar] [CrossRef]
- Katarivas Levy, G.; Goldman, J.; Aghion, E. The prospects of zinc as a structural material for biodegradable implants—A review paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zheng, Y.F.; Liu, Y. Effect of ag on the corrosion behavior of ti–ag alloys in artificial saliva solutions. Dent. Mater. 2009, 25, 672–677. [Google Scholar] [CrossRef]
- Simchi, A.; Tamjid, E.; Pishbin, F.; Boccaccini, A.R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 22–39. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Flege, S.; Ensinger, W.; Shibata, Y.; Nakashima, J.; Sawase, T.; Morimura, T. Preparation and antibacterial properties of ag-containing diamond-like carbon films prepared by a combination of magnetron sputtering and plasma source ion implantation. Vacuum 2013, 89, 179–184. [Google Scholar] [CrossRef]
- Li, P.; Schille, C.; Schweizer, E.; Kimmerle-Müller, E.; Rupp, F.; Han, X.; Heiss, A.; Richter, A.; Legner, C.; Klotz, U.E.; et al. Evaluation of a zn–2ag–1.8au–0.2v alloy for absorbable biocompatible materials. Materials 2020, 13, 56. [Google Scholar] [CrossRef]
- Zierold, A.A. Reaction of bone to various metals. Arch. Surg. 1924, 9, 365–412. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef]
- Khoshniat, S.; Bourgine, A.; Julien, M.; Weiss, P.; Guicheux, J.; Beck, L. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell. Mol. Life Sci. CMLS 2011, 68, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.M.; Felix, R.; Bisaz, S.; Fleisch, H. Aggregation of hydroxyapatite crystals. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 1976, 451, 549–559. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Seifzadeh, D.; Harrafi, H. Phosphatation of iron powder metallurgical samples for corrosion protection. J. Iran. Chem. Soc. 2007, 4, 72–77. [Google Scholar] [CrossRef]
- ISO 10993-15:2019-11; Biologische Beurteilung von Medizinprodukten—Teil 15: Qualitativer und Quantitativer Nachweis von Abbauprodukten aus Metallen und Legierungen. Beuth Verlag GmbH: Berlin, Germany, 2019.
- DIN EN ISO 10993-1:2021-05; Biologische Beurteilung Von Medizinprodukten—Teil 1: Beurteilung und Prüfungen im Rahmen eines Risikomanagementsystems. Beuth Verlag GmbH: Berlin, Germany, 2020.
- Roche. Cytotoxicity Detection Kit (Ldh); Version 12; Roche: Basel, Switzerland, 2020. [Google Scholar]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Fernandes, A.; Lopes, M.A.; Fernandes, M.H. Hydroxyapatite surface roughness: Complex modulation of the osteoclastogenesis of human precursor cells. Acta Biomater. 2012, 8, 1137–1145. [Google Scholar] [CrossRef]
- Wątroba, M.; Bednarczyk, W.; Szewczyk, P.K.; Kawałko, J.; Mech, K.; Grünewald, A.; Unalan, I.; Taccardi, N.; Boelter, G.; Banzhaf, M.; et al. In vitro cytocompatibility and antibacterial studies on biodegradable zn alloys supplemented by a critical assessment of direct contact cytotoxicity assay. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 241–260. [Google Scholar] [CrossRef]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Kubásek, J.; Vojtěch, D.; Jablonská, E.; Pospíšilová, I.; Lipov, J.; Ruml, T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable zn–mg alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.A. Human osteoblast culture. Methods Mol. Med. 2003, 80, 3–18. [Google Scholar] [CrossRef]
- Holthaus, M.G.; Treccani, L.; Rezwan, K. Osteoblast viability on hydroxyapatite with well-adjusted submicron and micron surface roughness as monitored by the proliferation reagent wst-1. J. Biomater. Appl. 2012, 27, 791–800. [Google Scholar] [CrossRef]
- Bosetti, M.; Massè, A.; Tobin, E.; Cannas, M. Silver coated materials for external fixation devices: In vitro biocompatibility and genotoxicity. Biomaterials 2002, 23, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Gstraunthaler, G.L.T. Zell- und Gwebekultur Allgemeine Grundlagen und Spezielle Anwendungen; Springer: Berlin/Heidelberg, Germany, 2013; Volume 10. [Google Scholar]
- Zhen, Z.; Liu, X.; Huang, T.; Xi, T.; Zheng, Y. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater. Sci. Eng. C 2015, 46, 202–206. [Google Scholar] [CrossRef] [PubMed]
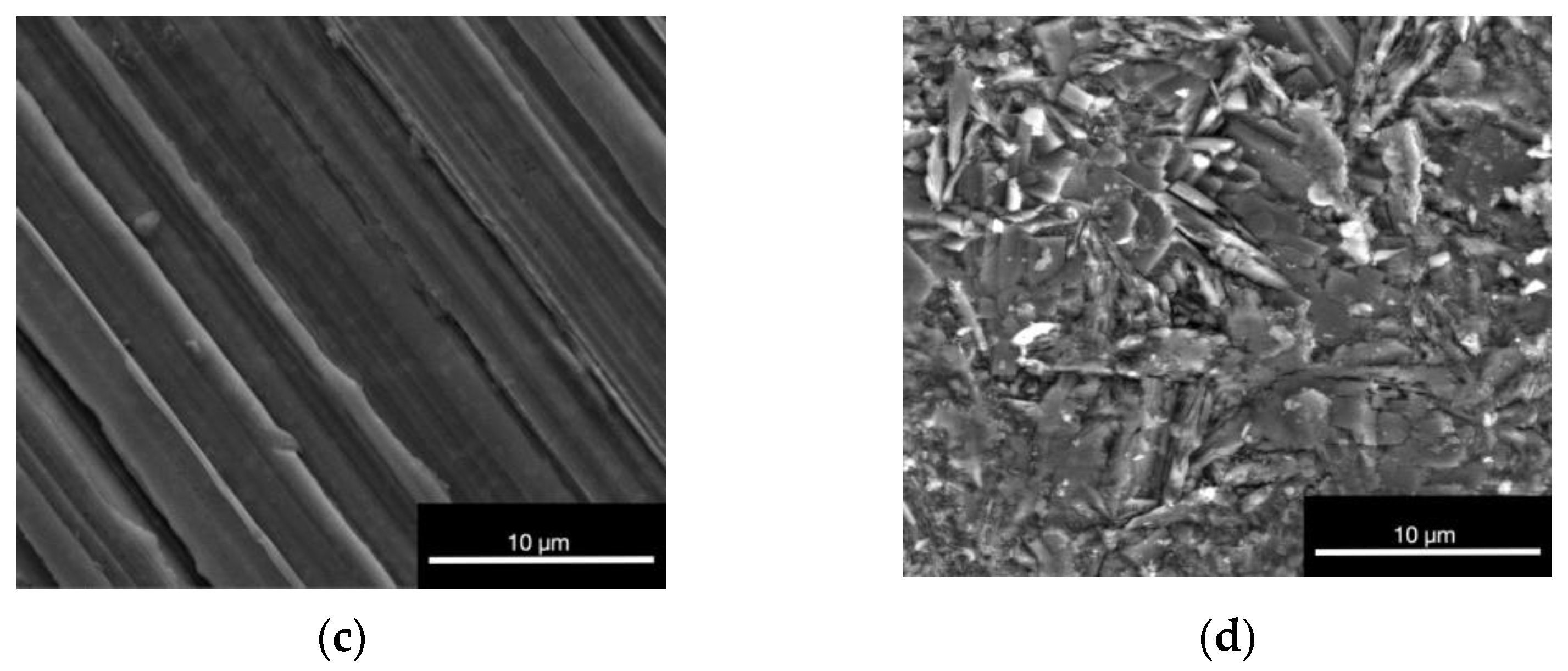
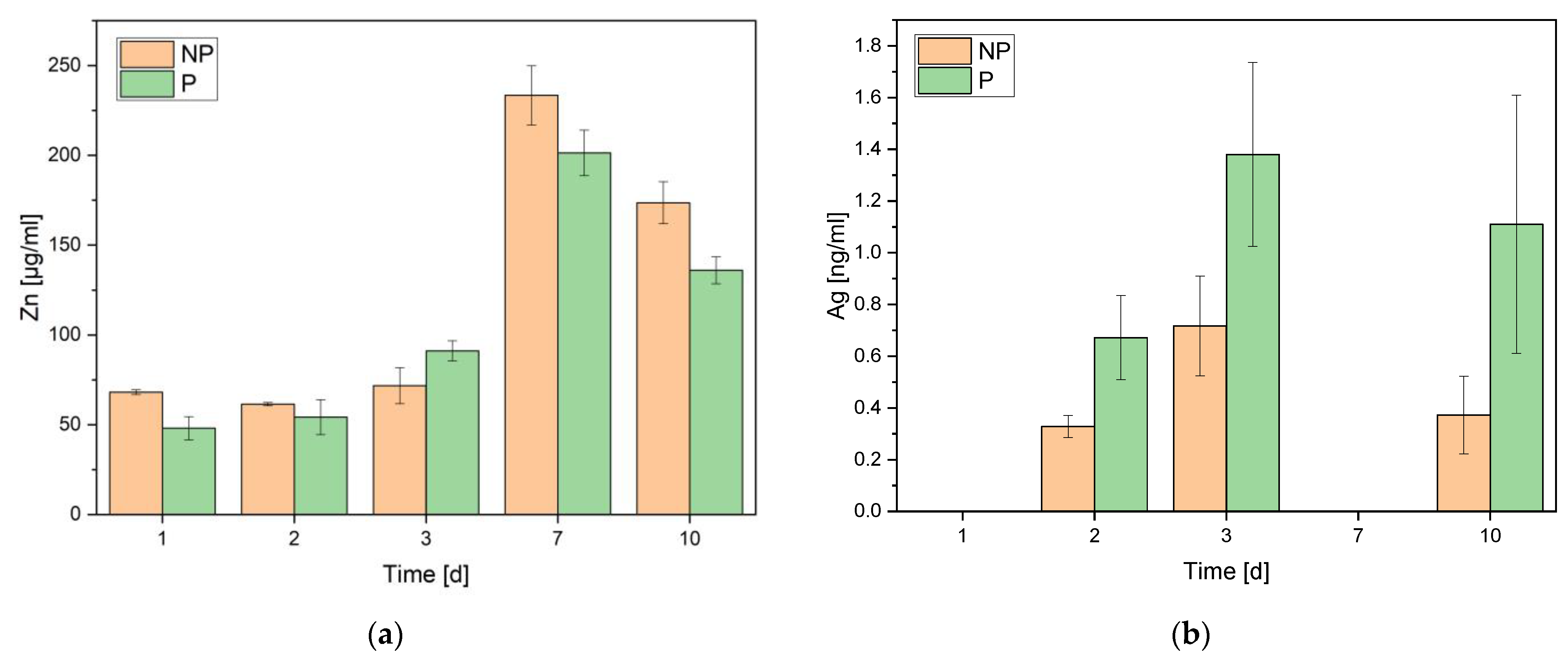
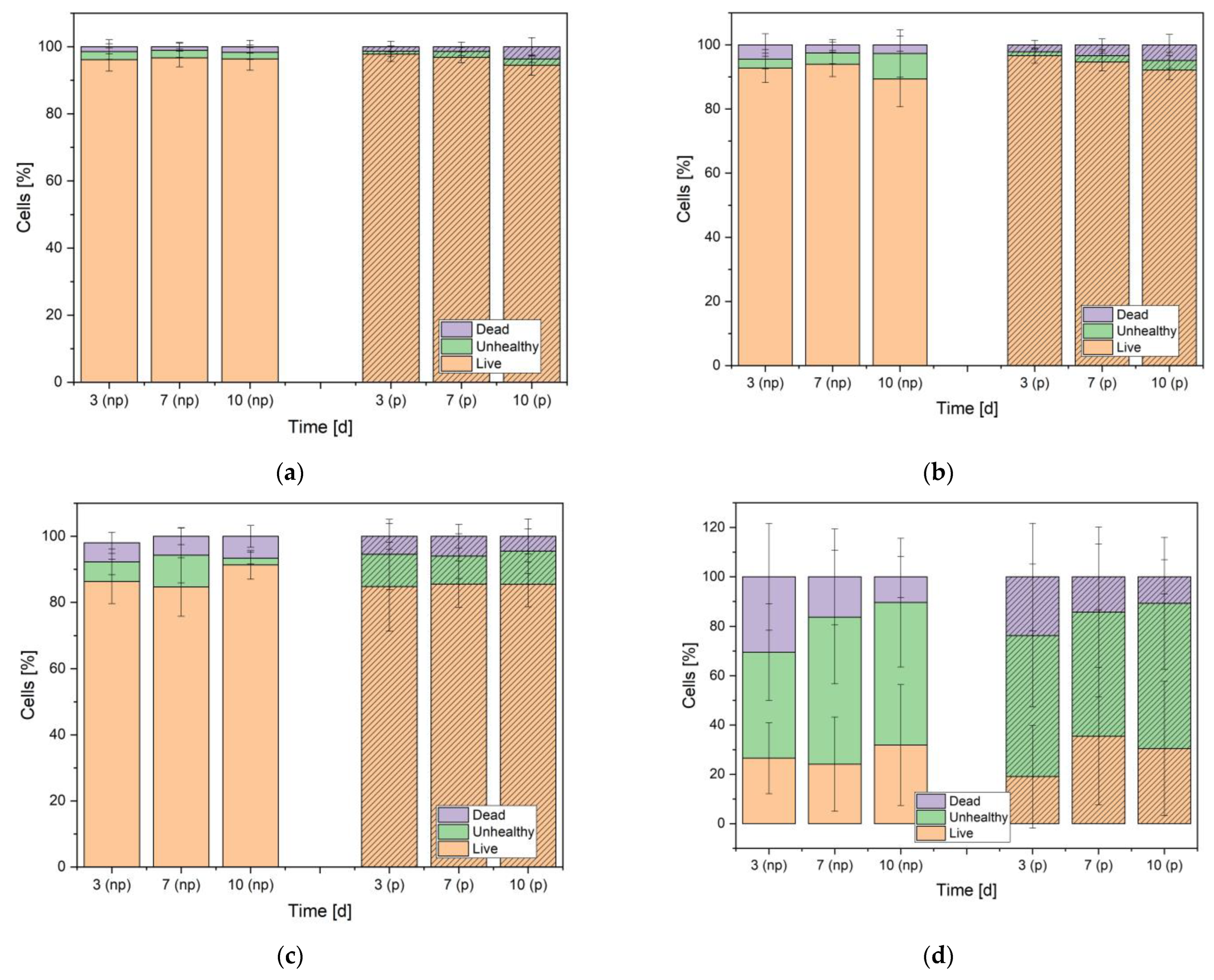
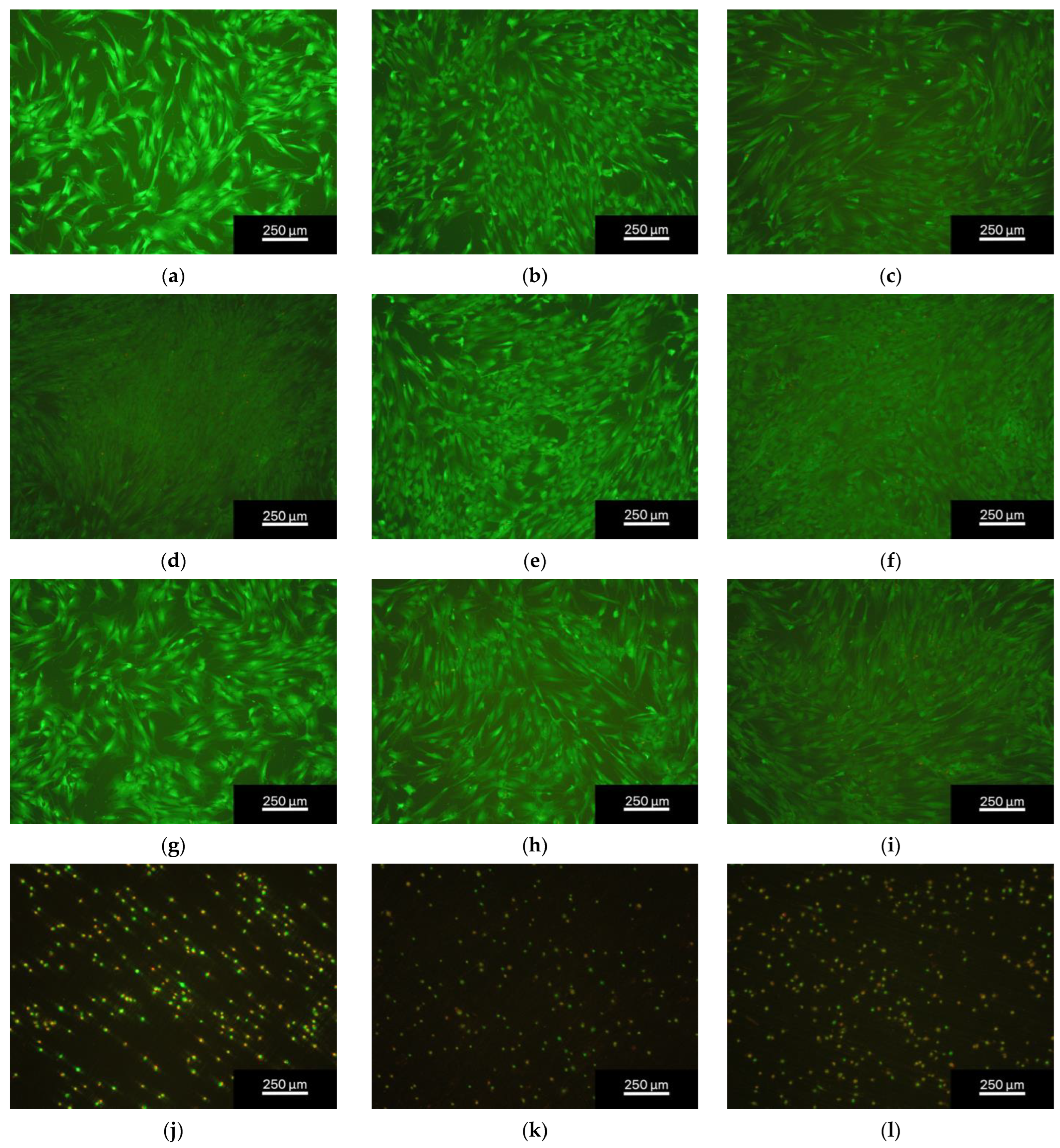

| Element | Weight% np | Weight% p |
|---|---|---|
| Zn | 96.07 ± 0.22 | 96.63 ± 0.52 |
| Ag | 3.93 ± 0.22 | 3.37 ± 0.23 |
| Element | Weight% | Atomic% |
|---|---|---|
| Zn | 67.02 ± 0.36 | 36.77 |
| O | 24.91 ± 0.34 | 55.82 |
| P | 5.73 ± 0.13 | 6.64 |
| Ag | 2.34 ± 0.16 | 0.78 |
| np | p | |
|---|---|---|
| Eluate (medium with phenol red) | 8.48 | 8.50 |
| Eluate (medium without phenol red) | 8.69 | 8.64 |
| Day 3 | 7.77 | 7.87 |
| Day 7 | 7.76 | 7.76 |
| Day 10 | 8.09 | 8.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roesner, M.; Zankovic, S.; Kovacs, A.; Benner, M.; Barkhoff, R.; Seidenstuecker, M. Biocompatibility Assessment of Zinc Alloys as a New Potential Material for Bioabsorbable Implants for Osteosynthesis. Materials 2023, 16, 5224. https://doi.org/10.3390/ma16155224
Roesner M, Zankovic S, Kovacs A, Benner M, Barkhoff R, Seidenstuecker M. Biocompatibility Assessment of Zinc Alloys as a New Potential Material for Bioabsorbable Implants for Osteosynthesis. Materials. 2023; 16(15):5224. https://doi.org/10.3390/ma16155224
Chicago/Turabian StyleRoesner, Maria, Sergej Zankovic, Adalbert Kovacs, Moritz Benner, Roland Barkhoff, and Michael Seidenstuecker. 2023. "Biocompatibility Assessment of Zinc Alloys as a New Potential Material for Bioabsorbable Implants for Osteosynthesis" Materials 16, no. 15: 5224. https://doi.org/10.3390/ma16155224
APA StyleRoesner, M., Zankovic, S., Kovacs, A., Benner, M., Barkhoff, R., & Seidenstuecker, M. (2023). Biocompatibility Assessment of Zinc Alloys as a New Potential Material for Bioabsorbable Implants for Osteosynthesis. Materials, 16(15), 5224. https://doi.org/10.3390/ma16155224







