3D Printing of Habitats on Mars: Effects of Low Temperature and Pressure
Abstract
1. Introduction
2. Differences between 3D Printing on the Earth and Mars
3. Materials and Methods
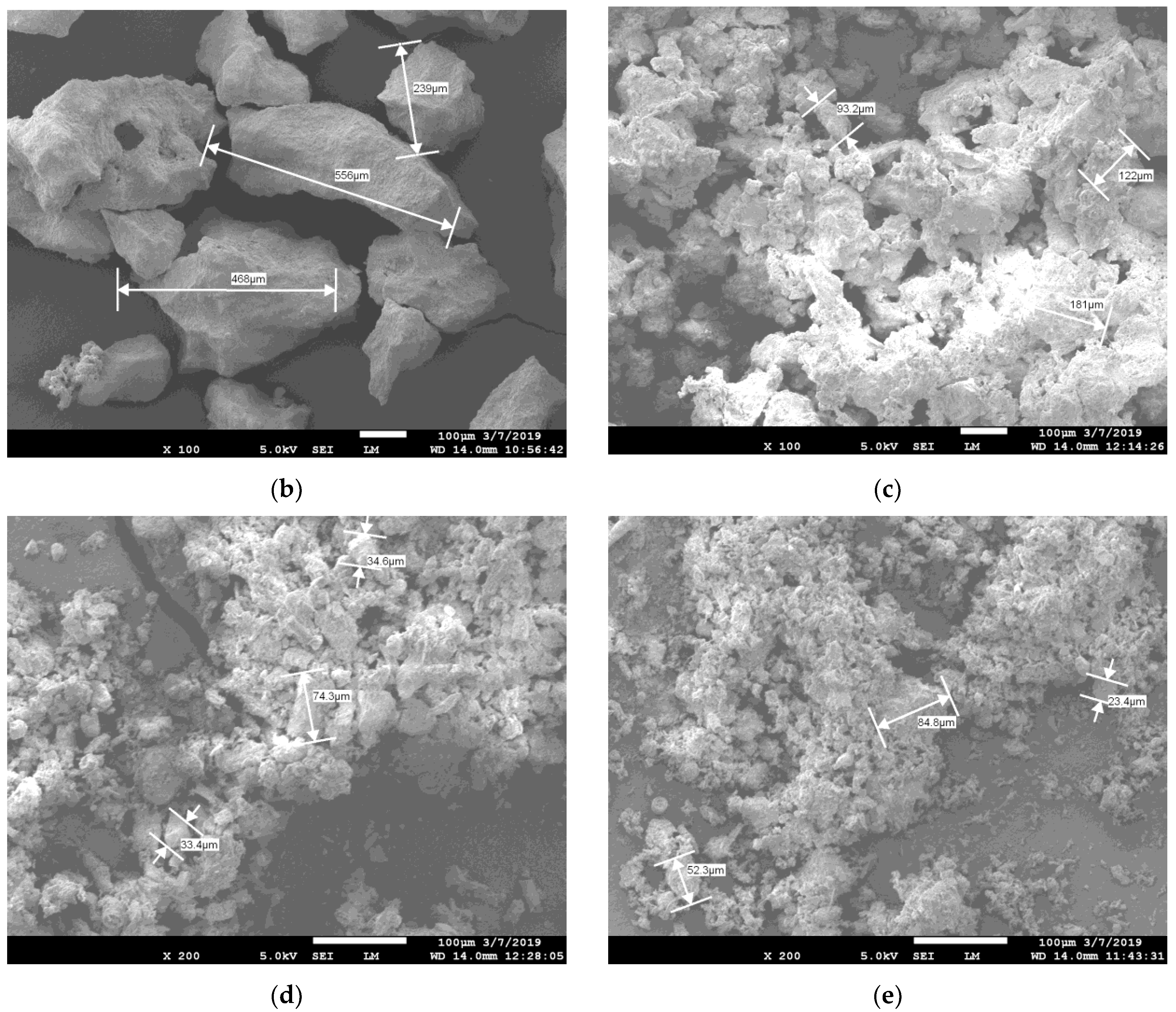
3.1. Regolith Characterization
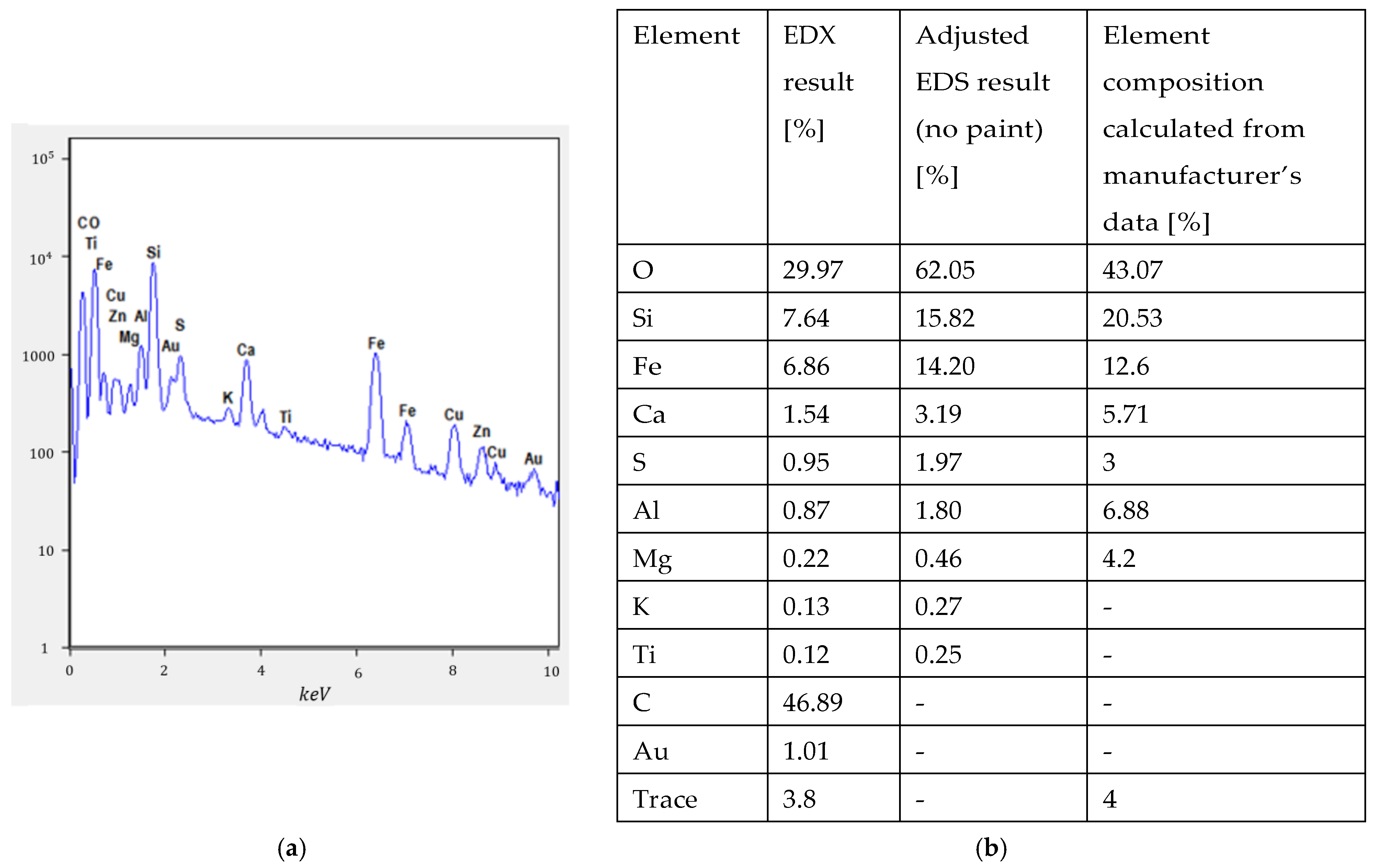
3.2. Binder Selection and Characterization
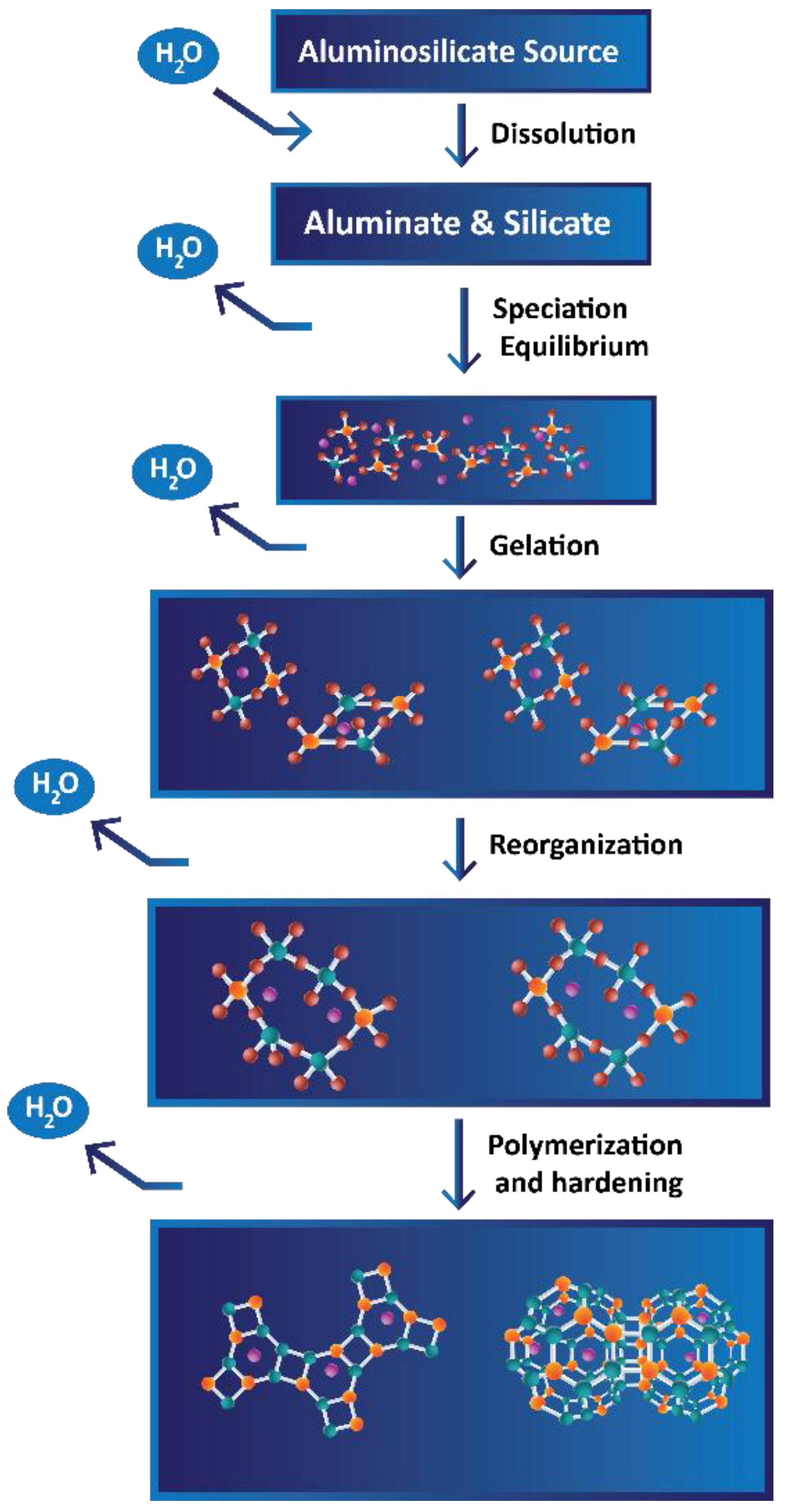
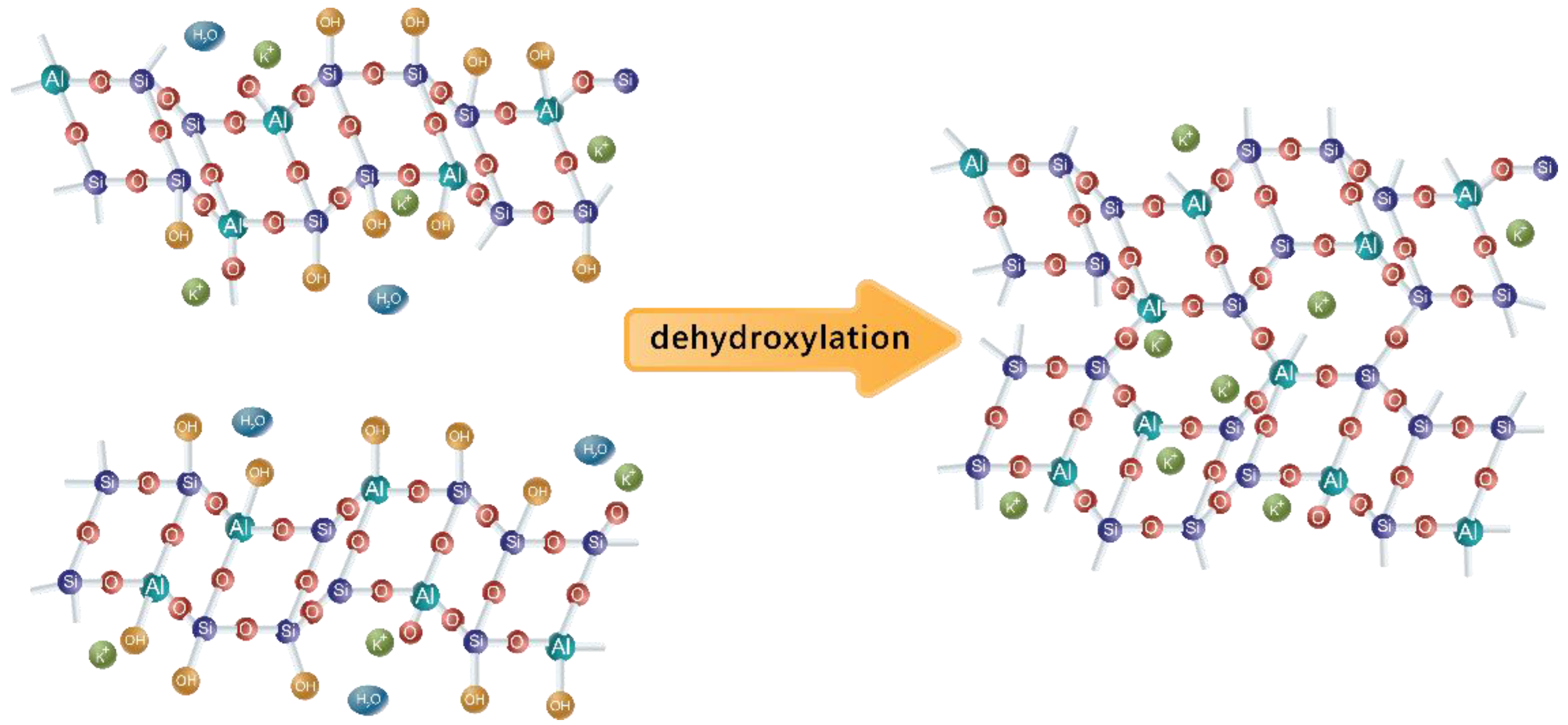
3.2.1. Theory
3.2.2. Measurements
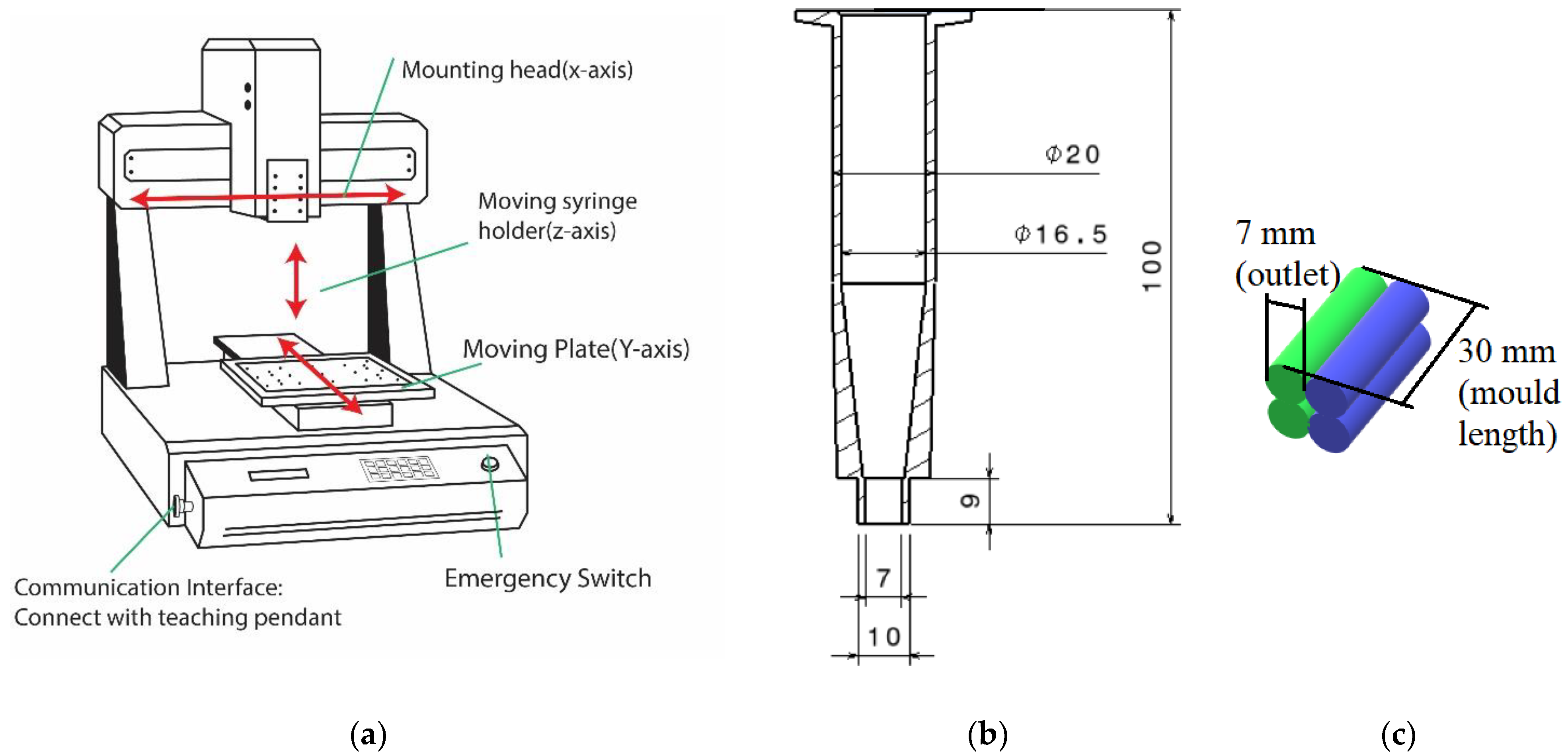
3.3. Molding Process
3.4. 3D Printing Process
3.5. Mechanical Tests
4. Results
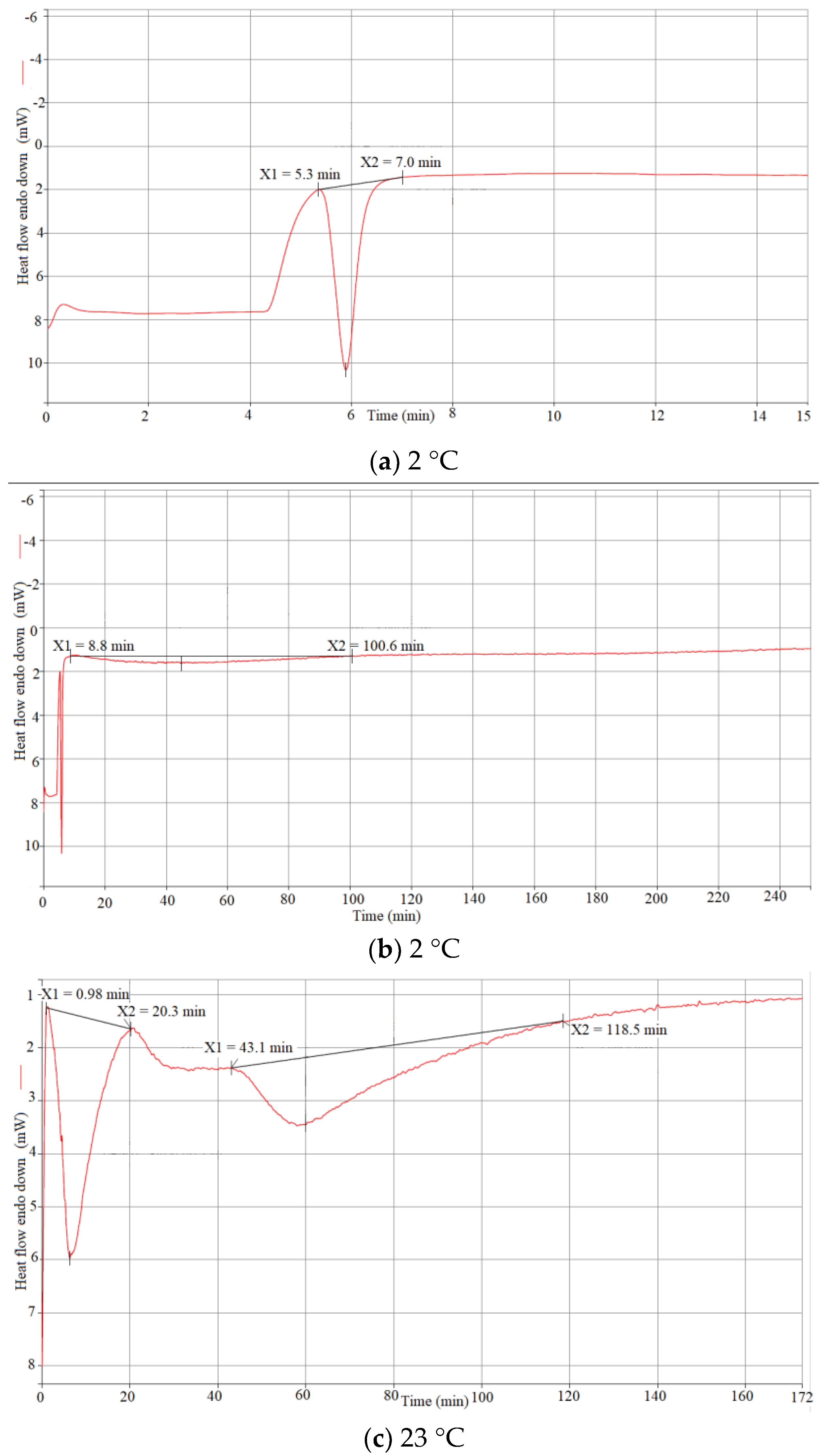
4.1. DSC Results
4.2. Molded Specimens
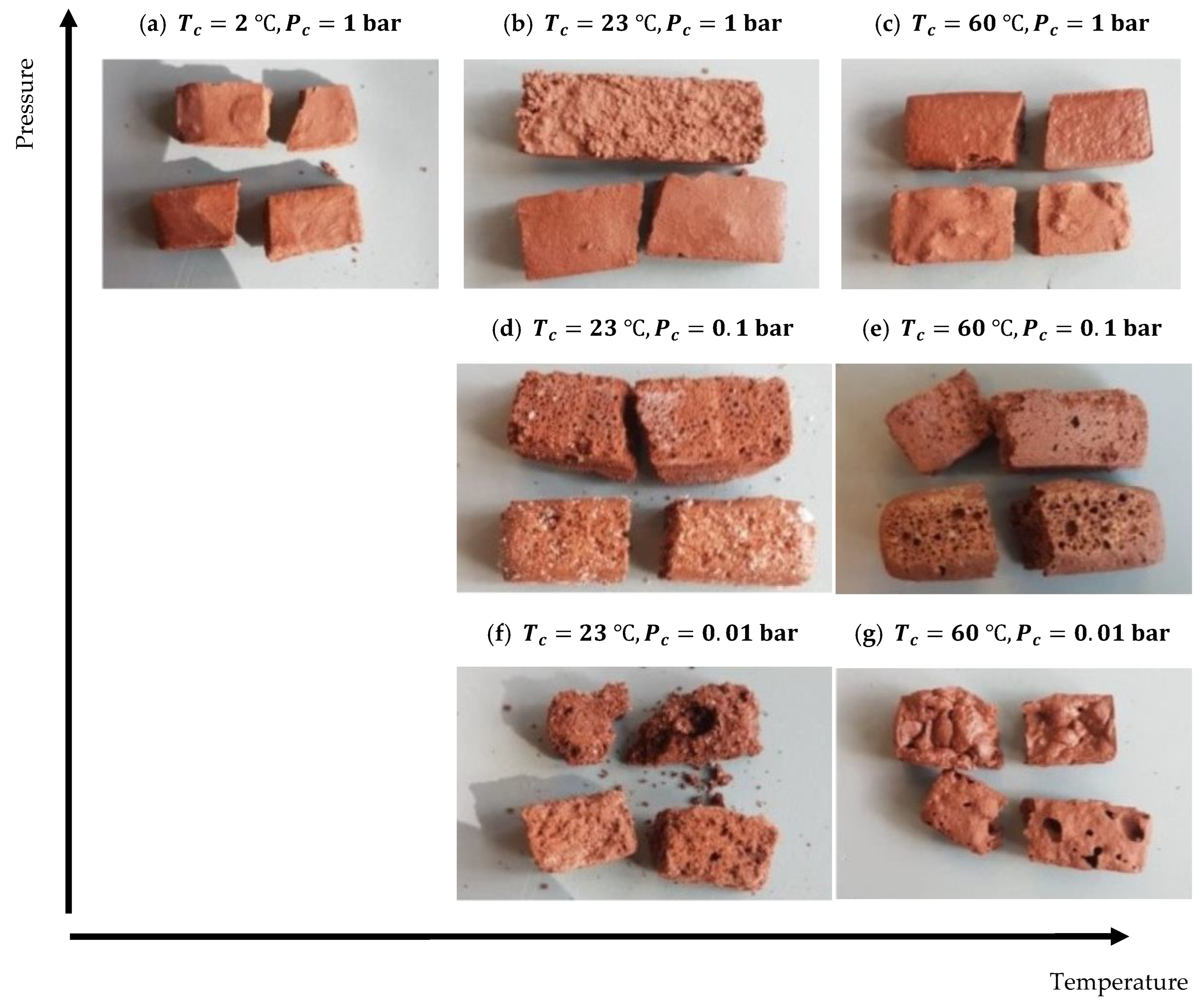
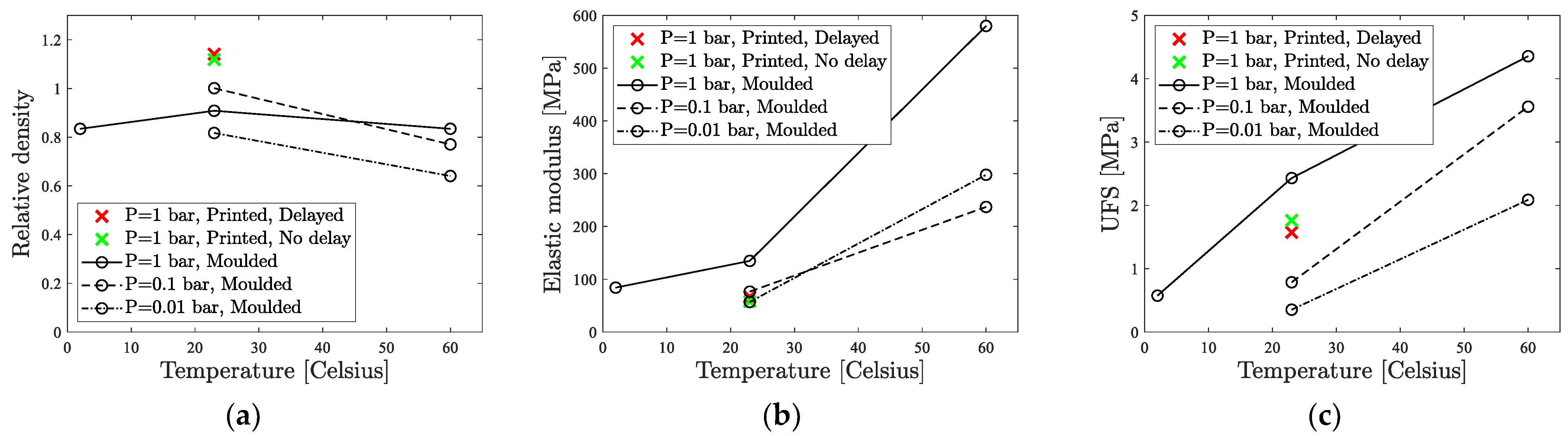
4.2.1. Physical Properties
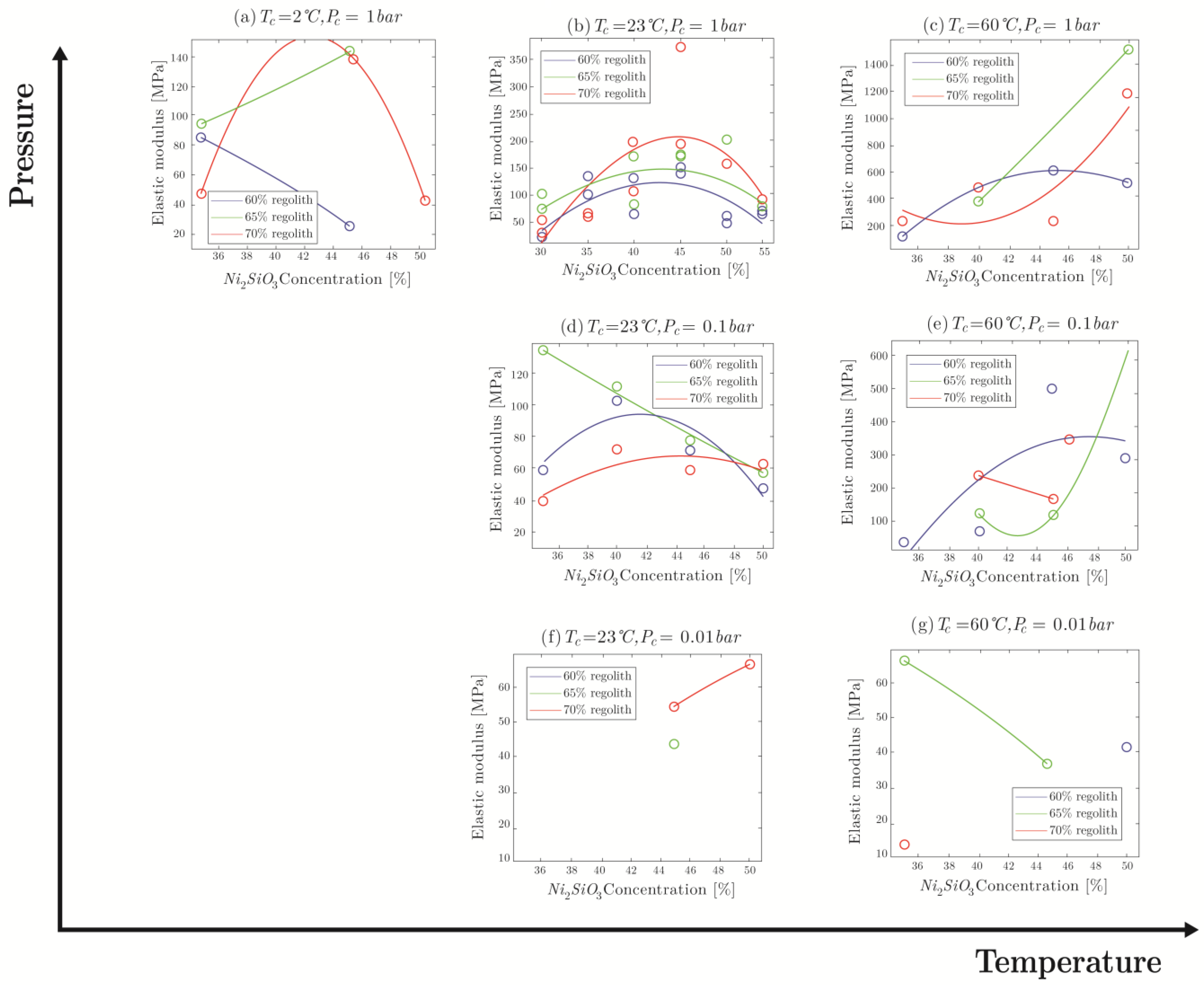
4.2.2. Elastic Modulus
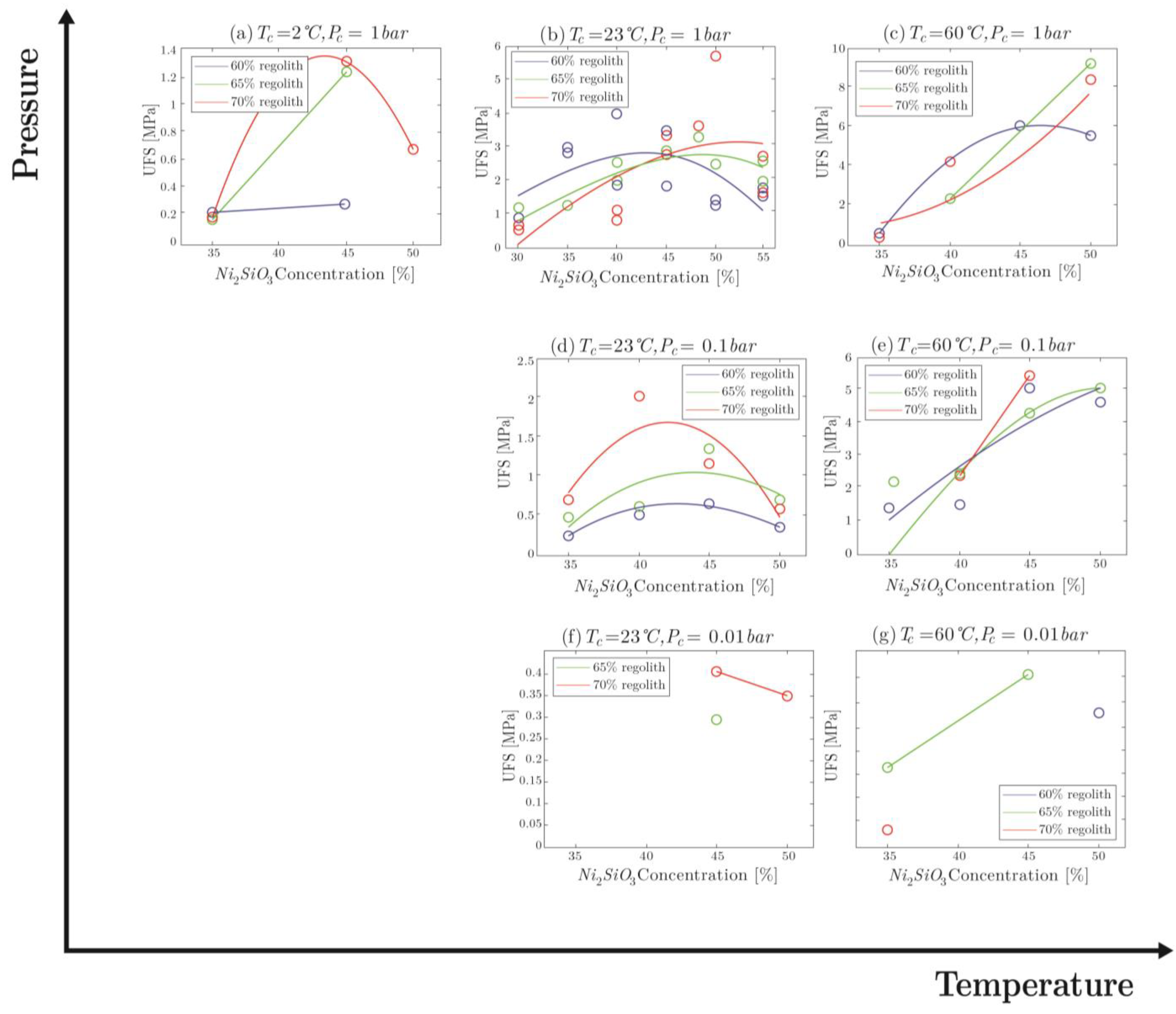
4.2.3. Ultimate Flexural Strength
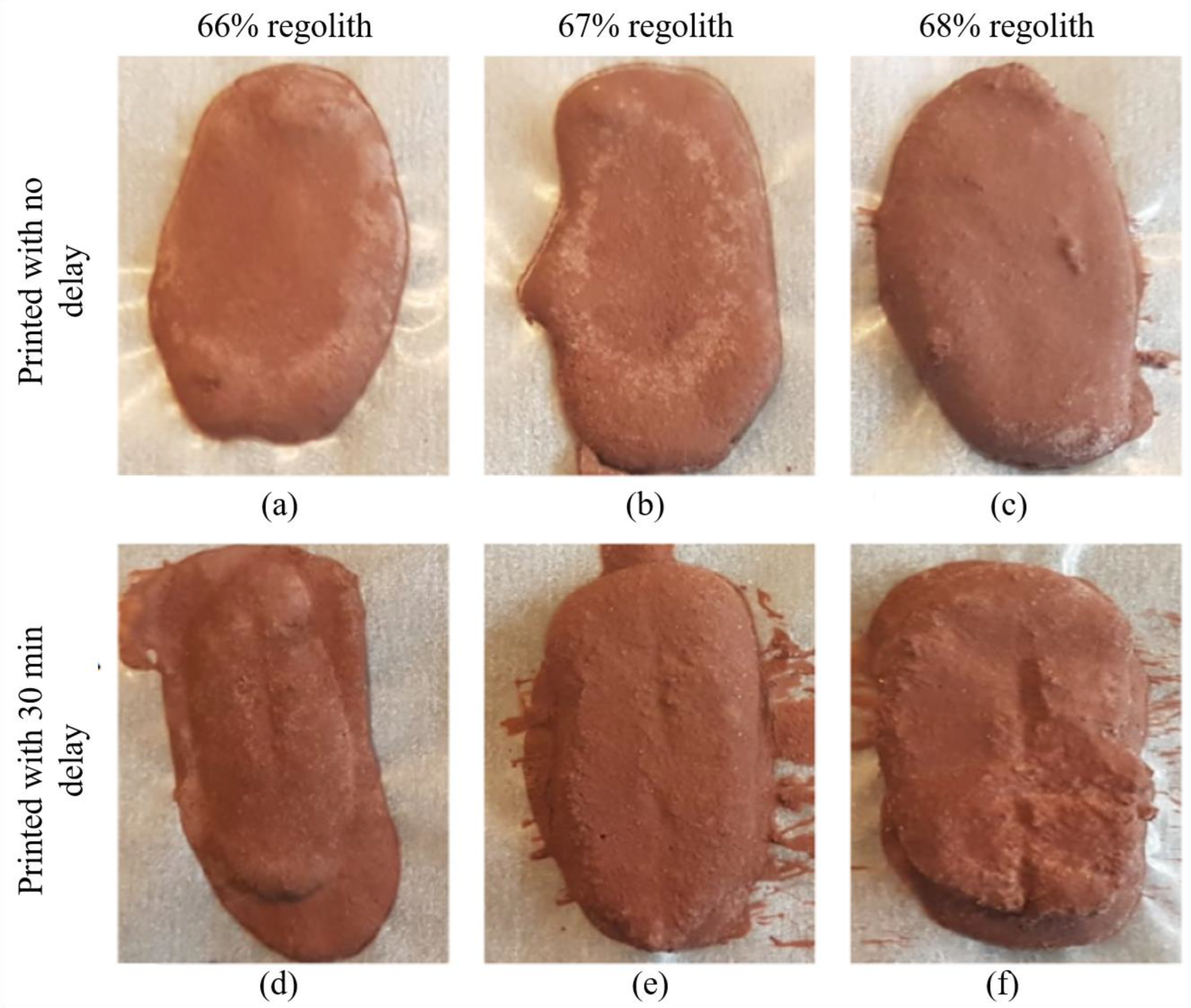
4.3. 3D Printed Specimens
4.3.1. Physical Properties
4.3.2. Elastic Modulus
4.3.3. Flexural Strength
5. Discussions
5.1. Viability of the Products
5.2. Geopolymer Binder Improvement
5.3. Application to Mars
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spudis, P.D. Robots vs. Humans: Who Should Explore Space?-Astronaut explorers can perform science in space that robots cannot. Humans are needed to study planets and moons in detail and to repair scientific. Sci. Am. 1999, 1, 24–31. [Google Scholar]
- Bettiol, L.; De La Torre, A.; Patel, D.; Oluwafemi, F.; Kamaletdinova, G.; Kumar Singh, R.; Sorokin, A. Manned Mars Mission Risks Evaluation. In Proceedings of the 69th International Astronautical Congress (IAC 2018), Bremen, Germany, 1–5 October 2018. [Google Scholar]
- Hellweg, C.E.; Baumstark-Khan, C. Getting ready for the manned mission to Mars: The astronauts’ risk from space radiation. Naturwissenschaften 2007, 94, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Hu, S.; Schwadron, N.A.; Kozarev, K.; Townsend, L.W.; Kim, M.-H.Y. Space radiation risk limits and Earth-Moon-Mars environmental models. Space Weather. 2010, 8. [Google Scholar] [CrossRef]
- LDE Missions. A Study on the Survivability and Adaptation of Humans to Long-Duration Exploratory Missions; European Space Agency: Paris, France, 2003. [Google Scholar]
- Lyne, J.E.; Townsend, L.W. Critical need for a swingby return option for early manned Mars missions. J. Spacecr. Rocket. 1998, 35, 855–856. [Google Scholar] [CrossRef] [PubMed]
- Blüm, V.; Gitelson, J.; Horneck, G.; Kreuzberg, K. Opportunities and constraints of closed man-made ecological systems on the moon. Adv. Space Res. 1994, 14, 271–280. [Google Scholar] [CrossRef]
- Kading, B.; Straub, J. Utilizing in-situ resources and 3D printing structures for a manned Mars mission. Acta Astronaut. 2015, 107, 317–326. [Google Scholar] [CrossRef]
- Gannor, M.G. News, “3D Printer Could Transform Moon Dirt Into Lunar Base”. 2015. Available online: https://www.space.com/18694-moon-dirt-3d-printing-lunar-base.html (accessed on 13 June 2023).
- Ceccanti, F.; Dini, E.; De Kestelier, X.; Colla, V.; Pambaguian, L. 3D printing technology for a moon outpost exploiting lunar soil. In Proceedings of the 61st International Astronautical Congress, Prague, Czech Republic, 27 September–1 October 2010. [Google Scholar]
- Ghavidelnia, N.; Bodaghi, M.; Hedayati, R. Idealized 3D auxetic mechanical metamaterial: An analytical, numerical, and experimental study. Materials 2021, 14, 993. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, R.; Güven, A.; Van Der Zwaag, S. 3D gradient auxetic soft mechanical metamaterials fabricated by additive manufacturing. Appl. Phys. Lett. 2021, 118, 141904. [Google Scholar] [CrossRef]
- Boschetto, A.; Bottini, L.; Eugeni, M.; Cardini, V.; Nisi, G.G.; Veniali, F.; Gaudenzi, P. Selective laser melting of a 1u Cubesat structure. Design for additive manufacturing and assembly. Acta Astronaut. 2019, 159, 377–384. [Google Scholar]
- He, R.; Zhou, N.; Zhang, K.; Zhang, X.; Zhang, L.; Wang, W.; Fang, D. Progress and challenges towards additive manufacturing of SiC ceramic. J. Adv. Ceram. 2021, 10, 637–674. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, Q.; Zhang, K.; Zhu, R.; Qu, Z.; Li, Y.; He, R. 3D-printed bioinspired Al2O3/polyurea dual-phase architecture with high robustness, energy absorption, and cyclic life. Chem. Eng. J. 2023, 463, 142378. [Google Scholar] [CrossRef]
- Raval, S. Exploration colonization resource extraction and utilization of Moon and Mars (ECROMM). In Proceedings of the 62nd International Astronautical Congress IAC, Cape Town, South Africa, 3–7 October 2011. [Google Scholar]
- Jarrah, H.R.; Zolfagharian, A.; Hedayati, R.; Serjouei, A.; Bodaghi, M. Nonlinear Finite Element Modelling of Thermo-Visco-Plastic Styrene and Polyurethane Shape Memory Polymer Foams. Actuators 2021, 10, 46. [Google Scholar] [CrossRef]
- Hedayati, R.; Ghavidelnia, N.; Sadighi, M.; Bodaghi, M. Improving the accuracy of analytical relationships for mechanical properties of permeable metamaterials. Appl. Sci. 2021, 11, 1332. [Google Scholar] [CrossRef]
- Martín-Montal, J.; Pernas-Sánchez, J.; Varas, D. Experimental characterization framework for SLA additive manufacturing materials. Polymers 2021, 13, 1147. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Hedayati, R.; Yousefi, A.; Bodaghi, M. Sandwich structures with repairable cores based on truncated cube cells. Compos. Part B Eng. 2022, 243, 110124. [Google Scholar] [CrossRef]
- Gasser, A.; Backes, G.; Kelbassa, I.; Weisheit, A.; Wissenbach, K. Laser additive manufacturing: Laser Metal Deposition (LMD) and Selective Laser Melting (SLM) in turbo-engine applications. Laser Tech. J. 2010, 7, 58–63. [Google Scholar] [CrossRef]
- Roudbarian, N.; Jebellat, E.; Famouri, S.; Baniasadi, M.; Hedayati, R.; Baghani, M. Shape-memory polymer metamaterials based on triply periodic minimal surfaces. Eur. J. Mech.-A Solids 2022, 96, 104676. [Google Scholar] [CrossRef]
- Roudbarian, N.; Baniasadi, M.; Nayyeri, P.; Ansari, M.; Hedayati, R.; Baghani, M. Enhancing shape memory properties of multi-layered and multi-material polymer composites in 4D printing. Smart Mater. Struct. 2021, 30, 105006. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Hedayati, R.; Yousefi, A.; Dezaki, M.L.; Bodaghi, M. Analytical relationships for 2D Re-entrant auxetic metamaterials: An application to 3D printing flexible implants. J. Mech. Behav. Biomed. Mater. 2023, 143, 105938. [Google Scholar] [CrossRef]
- JeongAhn, Y.; Malhotra, R. The current impact flux on Mars and its seasonal variation. Icarus 2015, 262, 140–153. [Google Scholar] [CrossRef]
- Bland, P.; Smith, T.; Jull, A.T.; Berry, F.; Bevan, A.; Cloudt, S.; Pillinger, C. The flux of meteorites to the Earth over the last 50,000 years. Mon. Not. R. Astron. Soc. 1996, 283, 551–565. [Google Scholar] [CrossRef]
- Toutanji, H.; Glenn-Loper, B.; Schrayshuen, B. Strength and durability performance of waterless lunar concrete. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit, Reno, Nevada, 10–13 January 2005. [Google Scholar]
- Lin, T.; Senseney, J.A.; Arp, L.D.; Lindbergh, C. Concrete lunar base investigation. J. Aerosp. Eng. 1989, 2, 10–19. [Google Scholar] [CrossRef]
- Montes, C.; Broussard, K.; Gongre, M.; Simicevic, N.; Mejia, J.; Tham, J.; Allouche, E.; Davis, G. Evaluation of lunar regolith geopolymer binder as a radioactive shielding material for space exploration applications. Adv. Space Res. 2015, 56, 1212–1221. [Google Scholar] [CrossRef]
- Kim, M.; Thibeault, S.; Wilson, J.; Heilbronn, L.; Kiefer, R.; Weakley, J.; Dueber, J.; Fogarty, T.; Wilkins, R. Radiation protection using Martian surface materials in human exploration of Mars. Phys. Med. 2001, 17, 81–83. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Man-made rock geosynthesis and the resulting development of very early high strength cement. J. Mater. Educ. 1994, 16, 91. [Google Scholar]
- Puertas, F.; González-Fonteboa, B.; González-Taboada, I.; Alonso, M.; Torres-Carrasco, M.; Rojo, G.; Martínez-Abella, F. Alkali-activated slag concrete: Fresh and hardened behaviour. Cem. Concr. Compos. 2018, 85, 22–31. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Effect of elevated temperature curing on properties of alkali-activated slag concrete. Cem. Concr. Res. 1999, 29, 1619–1625. [Google Scholar] [CrossRef]
- Systems, T. Tsr2000 Series 3 Axis Dispensing Robot-Hardware Instruction Manual. 2018. Available online: https://www.techcon.com/wp-content/uploads/2022/06/Techcon-TSR2000-Dispensing-Robot-User-Guide-EN.pdf (accessed on 13 June 2023).
- ASTM C78; Standard Test Method for Flexural Strength of Concrete. ASTM International: West Conshohocken, PA, USA, 2010. [CrossRef]
- Sagoe-Crentsil, K.; Weng, L. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part II. High Si/Al ratio systems. J. Mater. Sci. 2007, 42, 3007–3014. [Google Scholar]
- Hedayati, R.; Sadighi, M.; Mohammadi-Aghdam, M. On the difference of pressure readings from the numerical, experimental and theoretical results in different bird strike studies. Aerosp. Sci. Technol. 2014, 32, 260–266. [Google Scholar] [CrossRef]
- Hedayati, R.; Ziaei-Rad, S. Foam-core effect on the integrity of tailplane leading edge during bird-strike event. J. Aircr. 2011, 48, 2080–2089. [Google Scholar] [CrossRef]
- Hedayati, R.; Ziaei-Rad, S. Effect of Impact Orientation on Bird Strike Analysis. Int. J. Veh. Struct. Syst. 2011, 3, 184–191. [Google Scholar] [CrossRef]
- Silnikov, M.; Guk, I.; Nechunaev, A.; Smirnov, N. Numerical simulation of hypervelocity impact problem for spacecraft shielding elements. Acta Astronaut. 2018, 150, 56–62. [Google Scholar] [CrossRef]
- Calimanescu, I.; Buzbuchi, N.; Dima, A. Explicit dynamic simulation of a space debris projectile impact over a satellite structure. J. Mar. Technol. Environ. 2009, 1, 71–80. [Google Scholar]





| Challenge | Description |
|---|---|
| Material availability | The costs related to transporting payload are currently very high, and the time required to set up and perform a delivery mission can be also high depending upon destination. |
| Meteoroid strikes | Low amount of atmosphere on Mars does not offer enough protection against meteoroids. Meteorite impact has a potential to strike a built habitat or equipment, damaging it and impairing functionality. |
| Temperature variance and near-vacuum conditions | High temperature variance inflicts high thermal stresses, which cause the material to expand and shrink, resulting in high fatigue loads. Low pressure and high temperature cause the binder to evaporate easier, while low temperature does not allow for binder to react. |
| Low gravity | Combination of low gravity and low pressure can interfere with the binder deposition, especially if the distance between a regolith layer and printer nozzle is large. |
| Autonomy | The distance to Mars is large, meaning signal traveling time is 8.6–24 min, thus obstructing continuous communication. Hence, a trade-off has to be made between full autonomy with no humans present, and partial autonomy with either humans present or slow Mars–Earth communication. |
| Sieve Size [µm] | >125 | 63–125 | <63 | Total |
|---|---|---|---|---|
| Mass [g] | 17.38 | 38.86 | 26.52 | 82.76 |
| Mass [%] | 21 | 47 | 32 | 100 |
| Na2SiO3 Concentration [%] | 30 | 35 | 40 | 45 | 50 | 55 | |
|---|---|---|---|---|---|---|---|
| Regolith Concentration [%] | |||||||
| 60 | Two specimens per each condition manufactured (36 specimens in total) | ||||||
| 65 | |||||||
| 70 | |||||||
| Na2SiO3 Concentration [%] | 35 | 40 | 45 | 50 | |
|---|---|---|---|---|---|
| Regolith Concentration [%] | |||||
| 60 | One specimen for each combination (12 specimens in total) | ||||
| 65 | |||||
| 70 | |||||
| Tc [°C] | 2 | 23 | 60 | |
|---|---|---|---|---|
| Pc [bar] | ||||
| 1 |
|
|
| |
| 0.1 | N/A |
|
| |
| 0.01 | N/A |
|
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hedayati, R.; Stulova, V. 3D Printing of Habitats on Mars: Effects of Low Temperature and Pressure. Materials 2023, 16, 5175. https://doi.org/10.3390/ma16145175
Hedayati R, Stulova V. 3D Printing of Habitats on Mars: Effects of Low Temperature and Pressure. Materials. 2023; 16(14):5175. https://doi.org/10.3390/ma16145175
Chicago/Turabian StyleHedayati, Reza, and Victoria Stulova. 2023. "3D Printing of Habitats on Mars: Effects of Low Temperature and Pressure" Materials 16, no. 14: 5175. https://doi.org/10.3390/ma16145175
APA StyleHedayati, R., & Stulova, V. (2023). 3D Printing of Habitats on Mars: Effects of Low Temperature and Pressure. Materials, 16(14), 5175. https://doi.org/10.3390/ma16145175







