Revisiting the High-Pressure Behaviors of Zirconium: Nonhydrostaticity Promoting the Phase Transitions and Absence of the Isostructural Phase Transition in β-Zirconium
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussions
3.1. Phase Transition
3.2. Absence of Isostructural Phase Transition of β-Zr
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olinger, B.; Jamieson, J.C. Zircoinum: Phases and compressibility to 120 kilobars. High Temp. High Press. 1973, 5, 123–131. [Google Scholar]
- Xia, H.; Duclos, S.J.; Ruoff, A.L.; Vohra, Y.K. New high-pressure phase transition in zirconium metal. Phys. Rev. Lett. 1990, 64, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Akahama, Y.; Kobayashi, M.; Kawamura, H. Studies on the pressure-induced phase transition in zirconium. High Press. Res. 1992, 10, 711–715. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Pentea, C.; Qian, J.; Daemen, L.L.; Rigg, P.A.; Hixson, R.S.; Greeff, C.W.; Gray, G.T., III; Yang, Y.P.; et al. Experimental constraints on the phase diagram of elemental zirconium. J. Phys. Chem. Solids 2005, 66, 1213–1219. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Pentea, C.; Qian, J.; Daemen, L.L.; Rigg, P.A.; Hixson, R.S.; Gray, G.T., III; Yang, Y.P.; Wang, L.P.; et al. Thermal equations of state of the α, β, and ω phases of zirconium. Phys. Rev. B 2005, 71, 184119. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J. Enhancement of yield strength in zirconium metal through high-pressure induced structural phase transition. Appl. Phys. Lett. 2007, 91, 201907. [Google Scholar] [CrossRef]
- Trubistib, V.Y.; Dolgusheva, E. Isostructural transition in bcc Zr induced by the peculiarities of the lattice dynamics under pressure. Phys. Rev. B 2008, 77, 172302. [Google Scholar]
- Liu, W.; Li, B.; Wang, L.; Zhang, J.; Zhao, Y. Simultaneous ultrasonic and synchrotron X-ray studies on pressure induced α-ω phase transition in zirconium. J. Appl. Phys. 2008, 104, 076102. [Google Scholar] [CrossRef]
- Wenk, H.R.; Kaercher, P.; Kanitpanyacharoen, W.; Zepeda-Alarcon, E.; Wang, Y. Orientation relations during the α-ω phase transition of zirconium: In situ texture observations at high pressure and temperature. Phys. Rev. Lett. 2013, 111, 195701. [Google Scholar] [CrossRef]
- Stavrou, E.; Yang, L.H.; Soderlind, P.; Aberg, D.; Radoushky, H.B.; Armstrong, M.R.; Belof, J.L.; Kunz, M.; Greenberg, E.; Prakapenka, V.B.; et al. Anharmonicity-induced first-order isostructural phase transition of zirconium under pressure. Phys. Rev. B 2018, 98, 220101. [Google Scholar] [CrossRef]
- Jaworska, L.; Cyboron, J.; Cygan, S.; Zwolinski, A.; Onderka, B.; Skrzekut, T. Zirconium phase transformation under static high pressure and ω-Zr phase stability at high temperatures. Materials 2019, 12, 2244. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.K.; Levitas, V.I. In situ quantitative study of plastic strain-induced phase transformations under high pressure: Example for ultra-pure Zr. Acta Mater. 2020, 196, 338–346. [Google Scholar] [CrossRef]
- Pigott, J.S.; Velisavljevic, N.; Moss, E.K.; Popov, D.; Park, C.; Van Orman, J.A.; Draganic, N.; Vohra, Y.K.; Sturtevant, B.T. Room-temperature compression and equation of state of body-centered cubic zirconium. J. Phys. Condens. Matter 2020, 32, 12LT02. [Google Scholar] [CrossRef]
- Anzellini, S.; Bottin, F.; Bouchet, J.; Dewaele, A. Phase transitions and equation of state of zirconium under high pressure. Phys. Rev. B 2020, 102, 184105. [Google Scholar] [CrossRef]
- Greeff, C.W.; Brown, J.; Velisavljevic, N.; Rigg, P.A. Phase transitions in high-purity zirconium under dynamic compression. Phys. Rev. B 2022, 105, 184102. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Rigg, P.A.; Hixson, R.S.; Gray, G.T., III. Impurity effects on the phase transformations and equations of state of zirconium metals. J. Phys. Chem. Solids 2007, 68, 2297–2302. [Google Scholar] [CrossRef]
- Krisch, M.; Farber, D.L.; Xu, R.; Antonangeli, D.; Aracne, C.M.; Beraud, A.; Chiang, T.C.; Zarestky, J.; Kim, D.Y.; Isaev, E.I.; et al. Phonons of the anomalous element cerium. Proc. Natl. Acad. Sci. USA 2011, 108, 9342–9345. [Google Scholar] [CrossRef]
- Schwarz, U.; Takemura, K.; Hanfland, M.; Syassen, K. Crystal structure of Cesium-V. Phys. Rev. Lett. 1998, 81, 2711–2714. [Google Scholar] [CrossRef]
- McMahon, M.I.; Nelmes, R.J.; Rekhi, S. Complex crystal structure of Cesium-III. Phys. Rev. Lett. 2001, 87, 255502. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P.; Mezouar, M. Equations of state of six metals above 94 GPa. Phys. Rev. B 2004, 70, 094112. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Rigg, P.A.; Greff, C.W.; Knudson, M.D.; Gray, G.T.; Hixson, R.S. Influence of impurities on the α to ω phase transition in zirconium under dynamic loading conditions. J. Appl. Phys. 2009, 106, 123532. [Google Scholar] [CrossRef]
- Singh, A.K.; Balasingh, C.; Mao, H.K.; Hemley, R.J.; Shu, J. Analysis of lattice strains measured under nonhydrostatic pressure. J. Appl. Phys. 1998, 83, 7567–7575. [Google Scholar] [CrossRef]
- Uchida, T.; Funamori, N.; Yagi, T. Lattice strain in crystal under uniaxial stress field. J. Appl. Phys. 1996, 80, 739–746. [Google Scholar] [CrossRef]
- Liu, L.; Song, H.X.; Wang, Z.; Geng, H.Y.; Jing, Q.M.; Zhang, Y.; Liu, S.; Xiang, S.; Bi, Y.; Xu, J.; et al. Strength and equation of state of fluorite phase CeO2 under high pressure. J. Appl. Phys. 2012, 112, 013352. [Google Scholar] [CrossRef]
- Liu, L.; Bi, Y. How far away are accurate equations of state determinations? Some issues on pressure scales and non-hydrostaticity in diamond anvil cells. Matter Radiat. Extrem. 2016, 1, 224–236. [Google Scholar] [CrossRef]
- Jeanloz, R.; Godwal, B.K.; Meade, C. Static strength and equation of state of rhenium at ultra-high pressures. Nature 1991, 349, 687–689. [Google Scholar] [CrossRef]
- Jing, Q.; Bi, Y.; Wu, Q.; Jing, F.Q.; Wang, Z.; Xu, J.; Jiang, S. Yield strength of molybdenum at high pressures. Rev. Sci. Instrum. 2007, 78, 073906. [Google Scholar] [CrossRef]
- Meade, C.; Jeanloz, R. Yield strength of MgO to 40 GPa. J. Geophys. Res. 1988, 93, 3261–3269. [Google Scholar] [CrossRef]
- Vignes, R.M.; Becker, R.; Stolen, J.; Kumar, M. An assessment of diamond anvil cell measurements on material strength. J. Appl. Phys. 2013, 13, 213503. [Google Scholar] [CrossRef]
- Ning, B.Y.; Ning, X.J. Pressure-induced structural phase transition of vanadium: A revisit from the perspective of ensemble theory. J. Phys. Condens. Matter 2022, 34, 425404. [Google Scholar] [CrossRef] [PubMed]
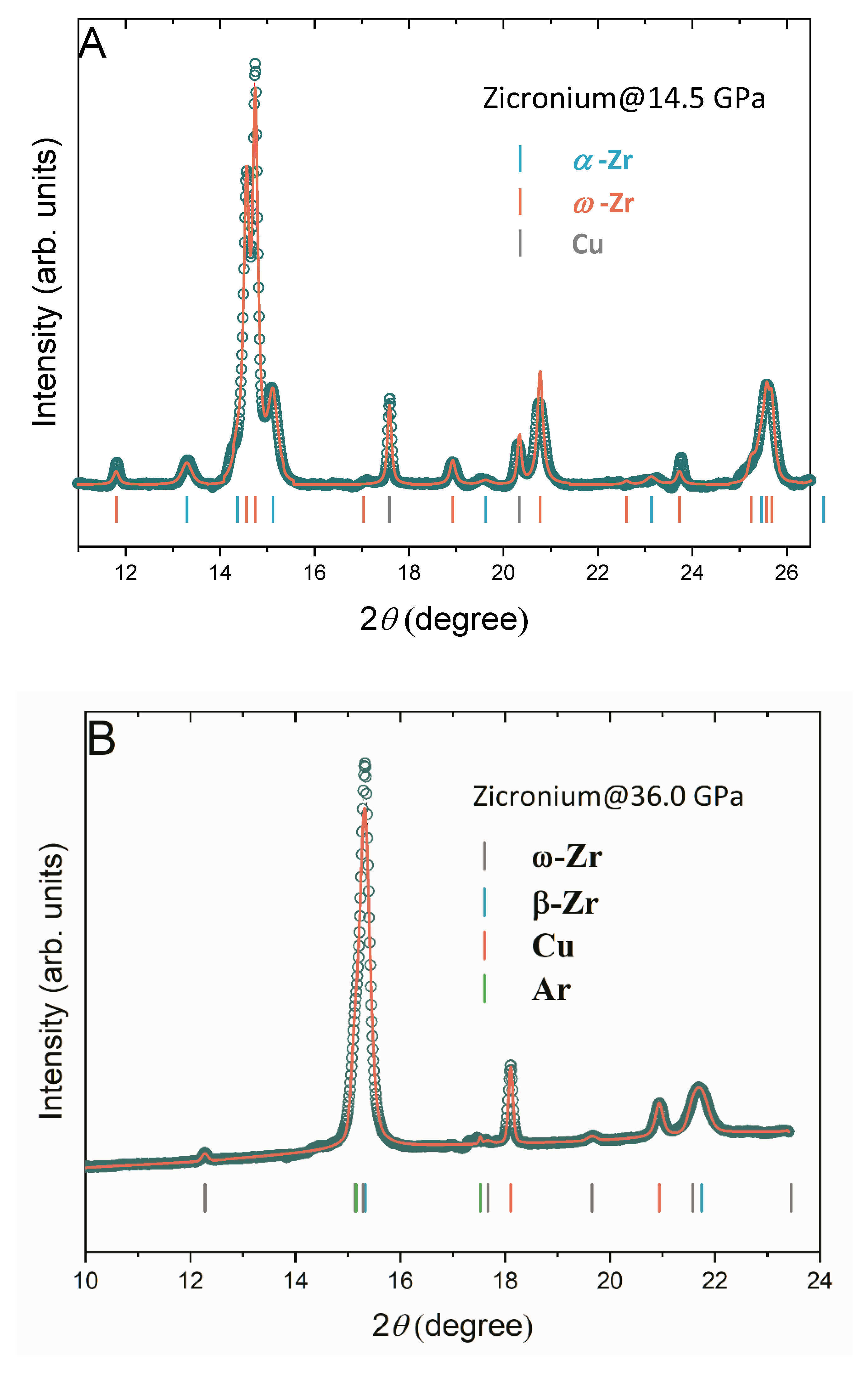

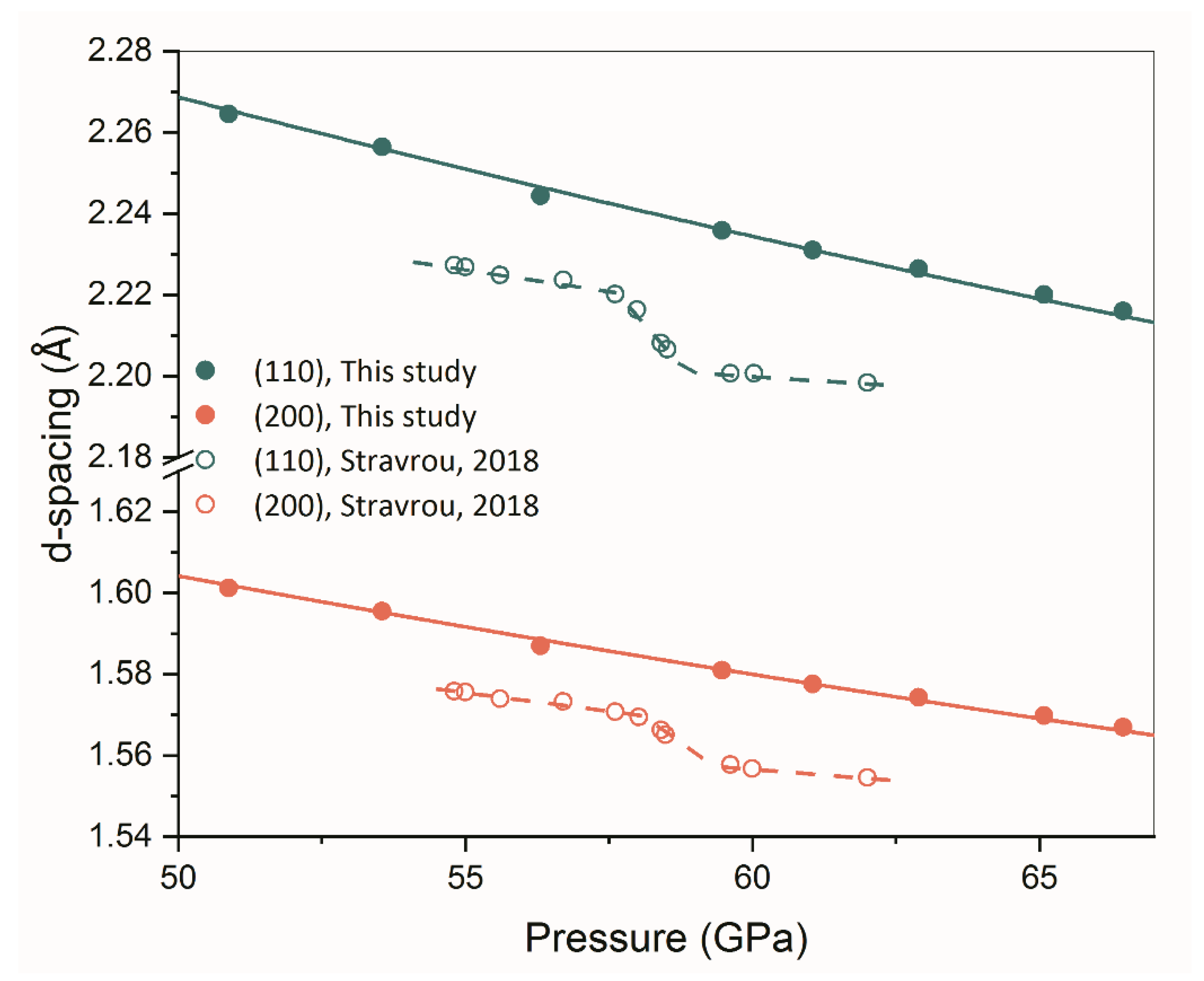
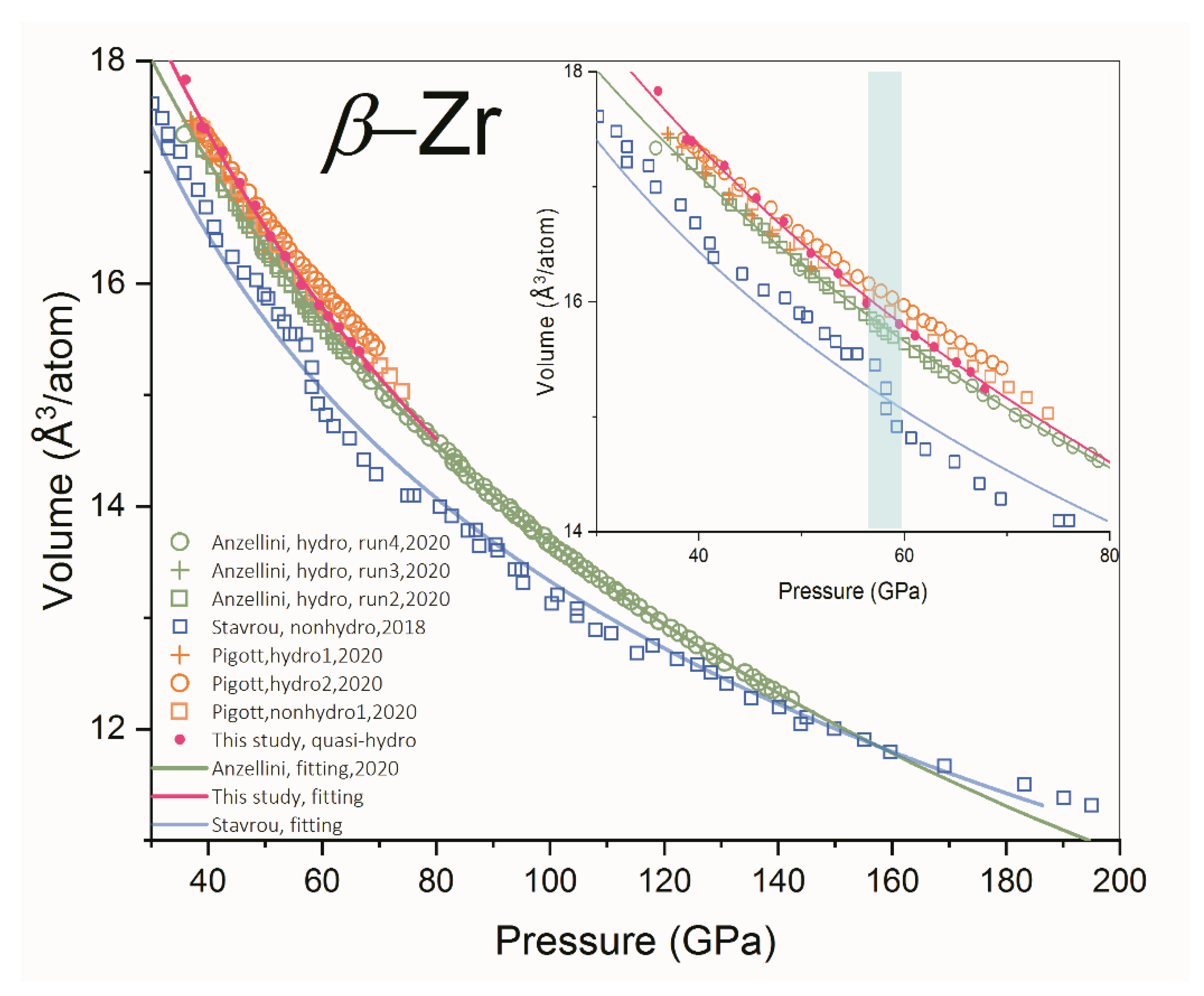
| Reference | Purity | PTM | α-ω (GPa) | ω-β (GPa) | β-β’ (GPa) |
|---|---|---|---|---|---|
| [2] | 99.996% | No | 28 | ||
| ME * | 31 | ||||
| [3] | 99.8% | No | 6.7 | 33 | 53 |
| [5] | 99.989% | NaCl | 5.5 | ||
| [8] | 99.9995% | No | 4.0 | ||
| [9] | 99.9% | No | 3.4–4.2 | ||
| [10] | 99.5% | No | 58 | ||
| [14] | 99.2% | He/Ne | 17 | 35 | |
| This study | 99.99% | Ar | 14.5 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Jing, Q.; Geng, H.Y.; Li, Y.; Zhang, Y.; Li, J.; Li, S.; Chen, X.; Gao, J.; Wu, Q. Revisiting the High-Pressure Behaviors of Zirconium: Nonhydrostaticity Promoting the Phase Transitions and Absence of the Isostructural Phase Transition in β-Zirconium. Materials 2023, 16, 5157. https://doi.org/10.3390/ma16145157
Liu L, Jing Q, Geng HY, Li Y, Zhang Y, Li J, Li S, Chen X, Gao J, Wu Q. Revisiting the High-Pressure Behaviors of Zirconium: Nonhydrostaticity Promoting the Phase Transitions and Absence of the Isostructural Phase Transition in β-Zirconium. Materials. 2023; 16(14):5157. https://doi.org/10.3390/ma16145157
Chicago/Turabian StyleLiu, Lei, Qiumin Jing, Hua Y. Geng, Yinghua Li, Yi Zhang, Jun Li, Shourui Li, Xiaohui Chen, Junjie Gao, and Qiang Wu. 2023. "Revisiting the High-Pressure Behaviors of Zirconium: Nonhydrostaticity Promoting the Phase Transitions and Absence of the Isostructural Phase Transition in β-Zirconium" Materials 16, no. 14: 5157. https://doi.org/10.3390/ma16145157
APA StyleLiu, L., Jing, Q., Geng, H. Y., Li, Y., Zhang, Y., Li, J., Li, S., Chen, X., Gao, J., & Wu, Q. (2023). Revisiting the High-Pressure Behaviors of Zirconium: Nonhydrostaticity Promoting the Phase Transitions and Absence of the Isostructural Phase Transition in β-Zirconium. Materials, 16(14), 5157. https://doi.org/10.3390/ma16145157







