Spent Coffee Grounds Derived Carbon Loading C, N Doped TiO2 for Photocatalytic Degradation of Organic Dyes
Abstract
1. Introduction
2. Experiments
2.1. Materials
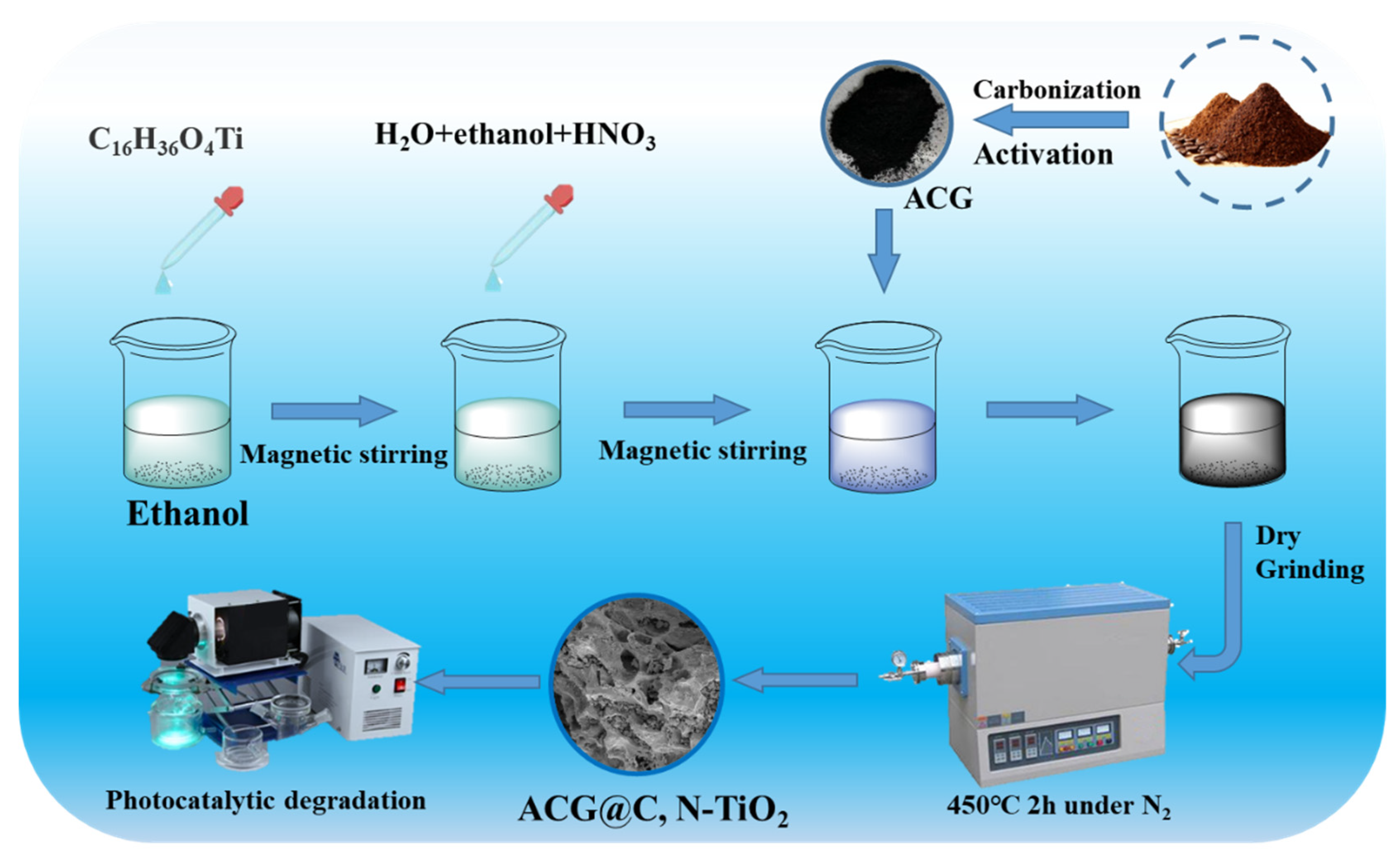
2.2. Preparation of Coffee Grounds-Derived Carbon
2.3. Preparation of C, N–TiO2@ACG
2.4. Materials Characterizations
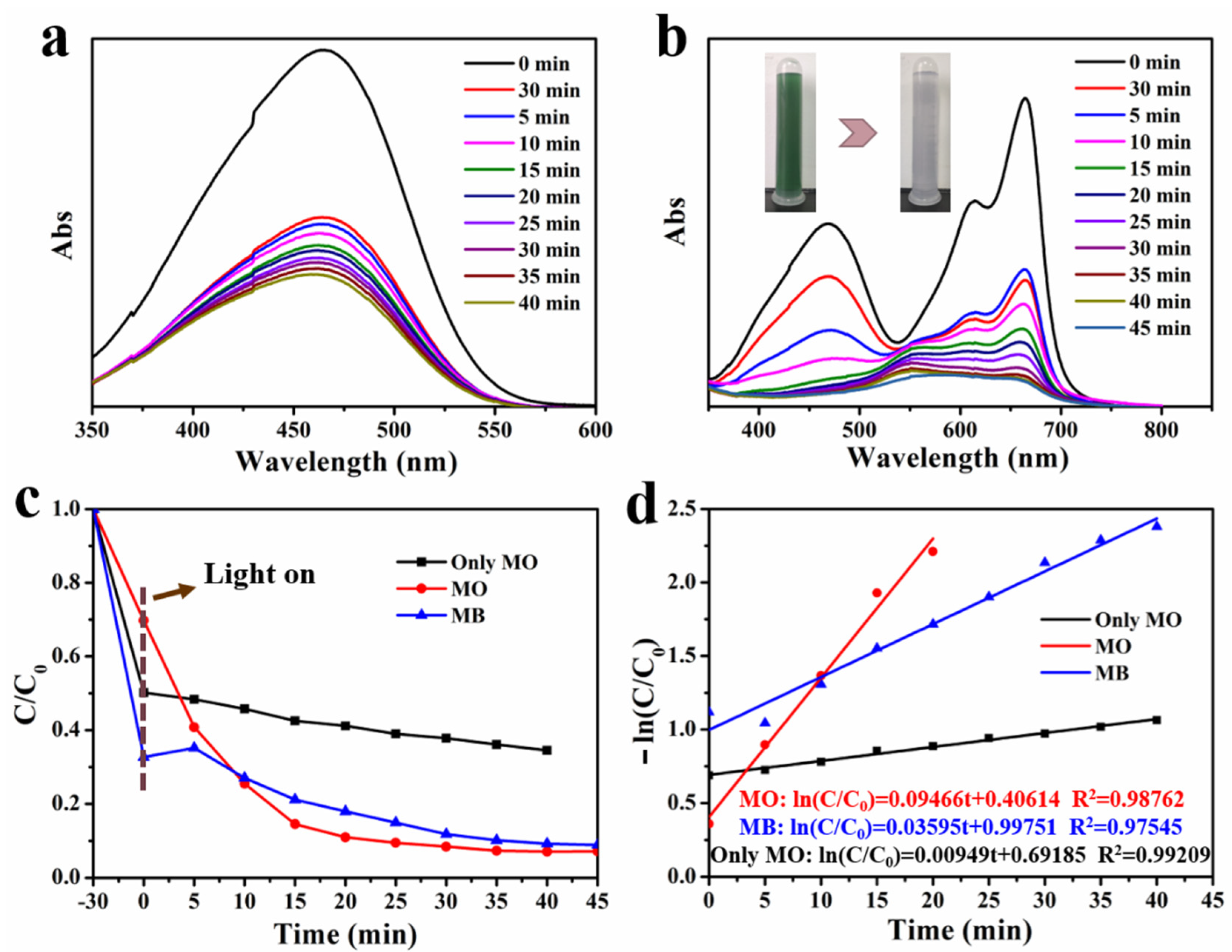
2.5. Photocatalytic Degradation
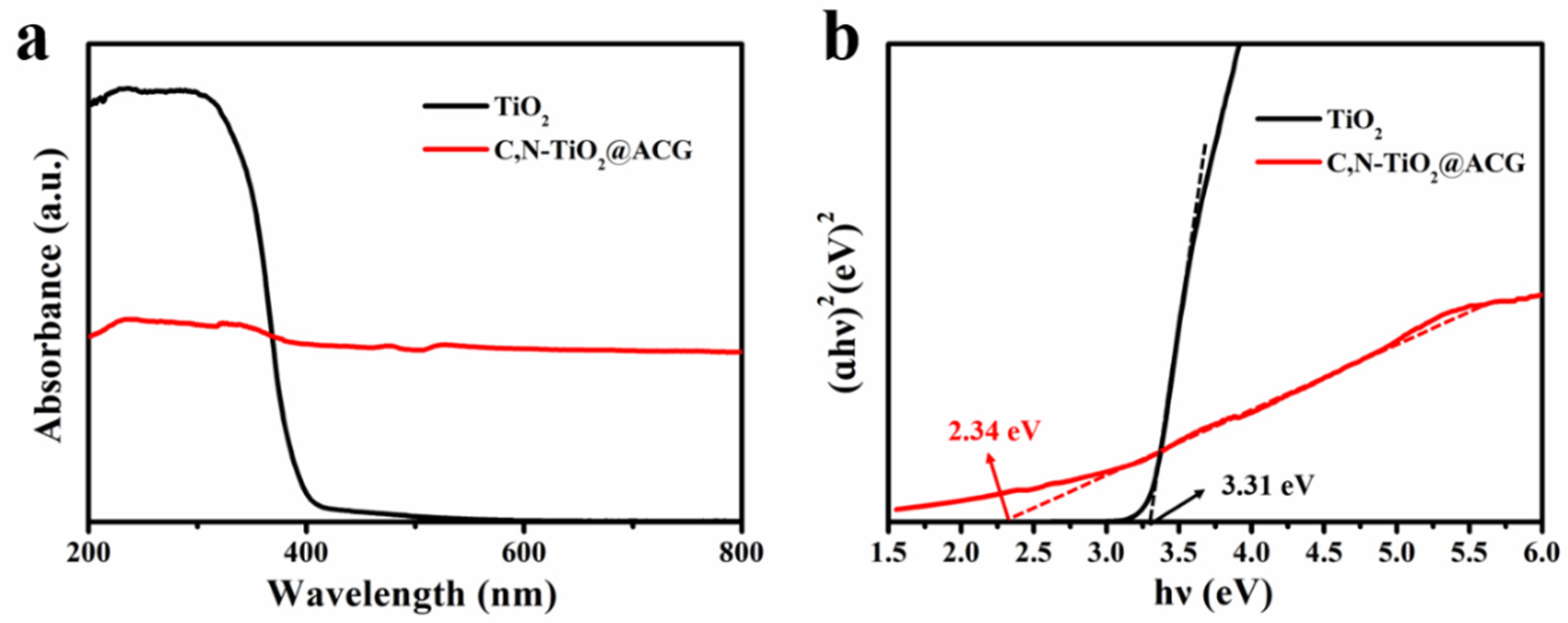
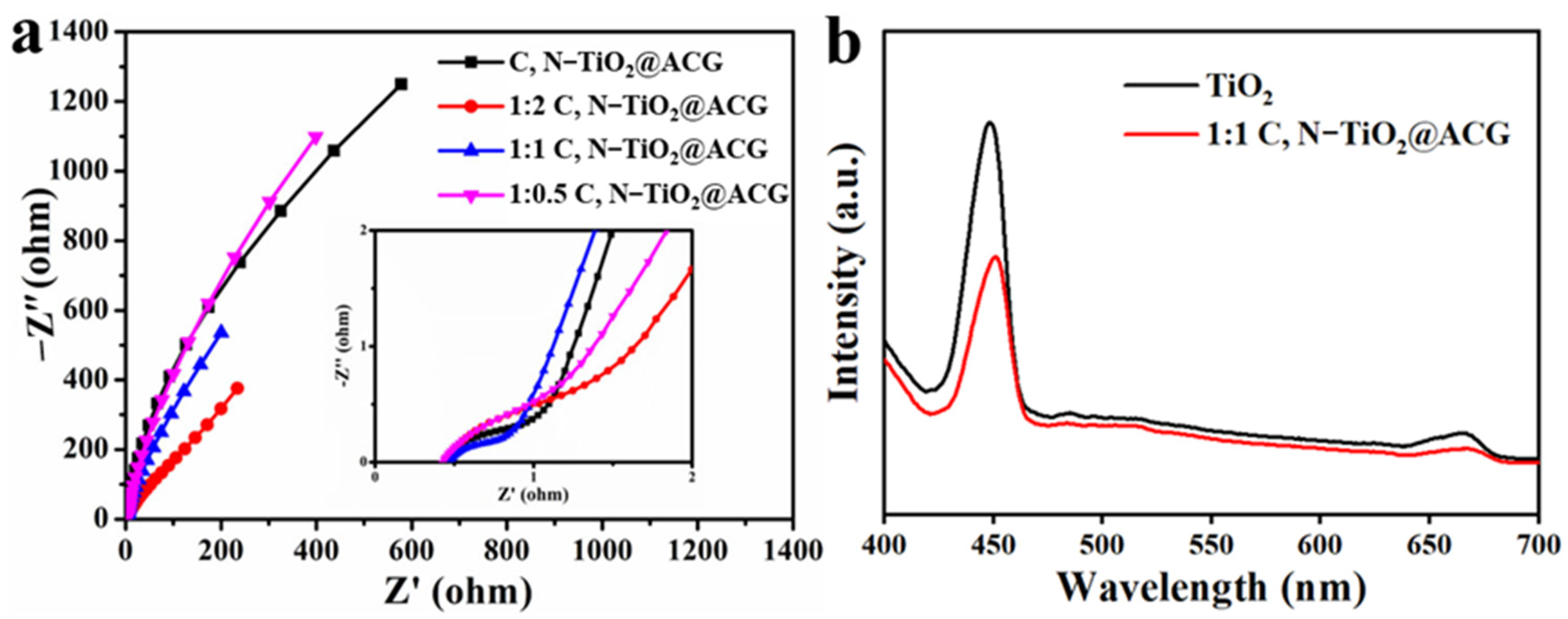
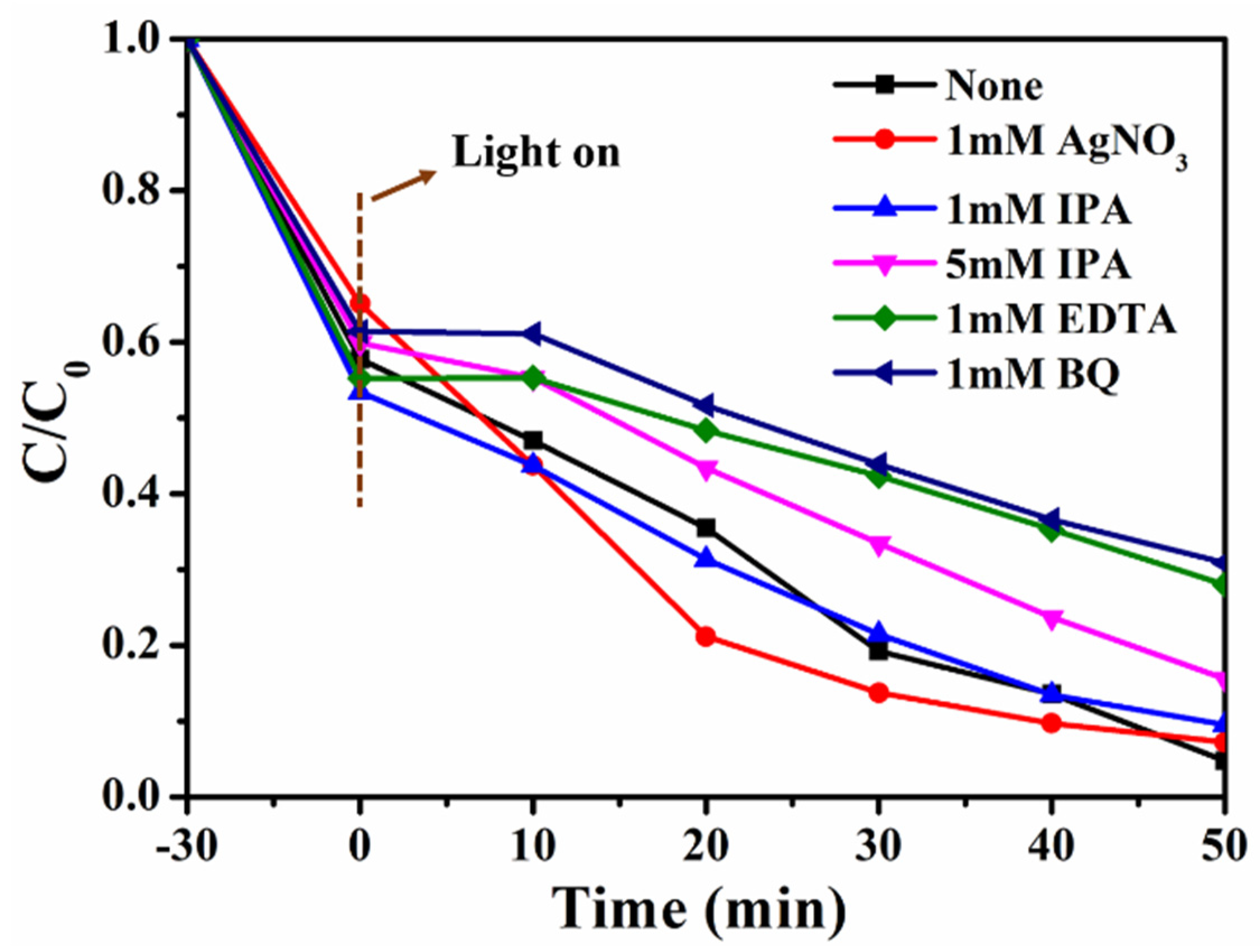
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; Xu, L.; Peng, J.; Han, J.; Guo, S.; Zhang, L.; Han, Z.; Komarneni, S. TiO2@g-C3N4 core/shell spheres with uniform mesoporous structures for high performance visible-light photocatalytic application. Ceram. Int. 2019, 45, 18844–18851. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Deshmukh, S.P.; More, K.V.; Shevale, V.B.; Mullani, S.B.; Dhodamani, A.G.; Delekar, S.D. Sulfated TiO2/WO3 nanocomposite: An efficient photocatalyst for degradation of Congo red and methyl red dyes under visible light irradiation. Mater. Chem. Phys. 2019, 225, 247–255. [Google Scholar] [CrossRef]
- Dai, Z.; Ren, P.-G.; Zhang, H.; Gao, X.; Jin, Y.-L. Nitrogen-doped and hierarchically porous carbon derived from spent coffee ground for efficient adsorption of organic dyes. Carbon Lett. 2021, 31, 1249–1260. [Google Scholar] [CrossRef]
- Huo, M.X.; Jin, Y.L.; Sun, Z.F.; Ren, F.; Pei, L.; Ren, P.G. Facile synthesis of chitosan-based acid-resistant composite films for efficient selective adsorption properties towards anionic dyes. Carbohydr. Polym. 2021, 254, 117473. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, H.N.; Ding, W.; Song, R.Q.; Han, Y.; Yu, H.Y.; Paneth, P. Theoretical Kinetic Isotope Effects in Establishing the Precise Biodegradation Mechanisms of Organic Pollutants. Environ. Sci. Technol. 2023, 57, 4915–4929. [Google Scholar] [CrossRef]
- Rene, E.R.; Kennes, C.; Nghiem, L.D.; Varjani, S. New insights in biodegradation of organic pollutants Preface. Bioresour. Technol. 2022, 347, 126737. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Wei, J.-S.; Song, T.-B.; Zhang, P.; Niu, X.-Q.; Chen, X.-B.; Xiong, H.-M. A new generation of energy storage electrode materials constructed from carbon dots. Mater. Chem. Front. 2020, 4, 729–749. [Google Scholar] [CrossRef]
- Chen, X.; Sun, H.; Zelekew, O.A.; Zhang, J.; Guo, Y.; Zeng, A.; Kuo, D.-H.; Lin, J. Biological renewable hemicellulose-template for synthesis of visible light responsive sulfur-doped TiO2 for photocatalytic oxidation of toxic organic and As(III) pollutants. Appl. Surf. Sci. 2020, 525, 146531. [Google Scholar] [CrossRef]
- Jin, Y.; Tang, W.; Wang, J.; Ren, F.; Chen, Z.; Sun, Z.; Ren, P.-G. Construction of biomass derived carbon quantum dots modified TiO2 photocatalysts with superior photocatalytic activity for methylene blue degradation. J. Alloys Compd. 2023, 932, 167627. [Google Scholar] [CrossRef]
- Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Souza, J.R.B.; Zhang, T.; Pilau, E.J.; Asefa, T.; Almeida, V.C. Sol-gel synthesis of new TiO2/activated carbon photocatalyst and its application for degradation of tetracycline. Ceram. Int. 2017, 43, 4411–4418. [Google Scholar] [CrossRef]
- Mu, R.; Ao, Y.; Wu, T.; Wang, C.; Wang, P. Synergistic effect of molybdenum nitride nanoparticles and nitrogen-doped carbon on enhanced photocatalytic hydrogen evolution performance of CdS nanorods. J. Alloys Compd. 2020, 812, 151990. [Google Scholar] [CrossRef]
- Ye, S.; Yan, M.; Tan, X.; Liang, J.; Zeng, G.; Wu, H.; Song, B.; Zhou, C.; Yang, Y.; Wang, H. Facile assembled biochar-based nanocomposite with improved graphitization for efficient photocatalytic activity driven by visible light. Appl. Catal. B Environ. 2019, 250, 78–88. [Google Scholar] [CrossRef]
- Shirmardi, A.; Teridi, M.A.M.; Azimi, H.R.; Basirun, W.J.; Jamali-Sheini, F.; Yousefi, R. Enhanced photocatalytic performance of ZnSe/PANI nanocomposites for degradation of organic and inorganic pollutants. Appl. Surf. Sci. 2018, 462, 730–738. [Google Scholar] [CrossRef]
- Katsumata, H.; Molla, M.A.I.; Islam, J.B.; Tateishi, I.; Furukawa, M.; Kaneco, S. Dual Z-scheme heterojunction g-C3N4/Ag3PO4/AgBr photocatalyst with enhanced visible-light photocatalytic activity. Ceram. Int. 2022, 48, 21898–21905. [Google Scholar] [CrossRef]
- Feng, X.; Wang, P.; Hou, J.; Qian, J.; Wang, C.; Ao, Y. Oxygen vacancies and phosphorus codoped black titania coated carbon nanotube composite photocatalyst with efficient photocatalytic performance for the degradation of acetaminophen under visible light irradiation. Chem. Eng. J. 2018, 352, 947–956. [Google Scholar] [CrossRef]
- Ouyang, F.; Li, H.; Gong, Z.; Pang, D.; Qiu, L.; Wang, Y.; Dai, F.; Cao, G.; Bharti, B. Photocatalytic degradation of industrial acrylonitrile wastewater by F-S-Bi-TiO2 catalyst of ultrafine nanoparticles dispersed with SiO2 under natural sunlight. Sci. Rep. 2020, 10, 12379. [Google Scholar] [CrossRef]
- Sedaghati, N.; Habibi-Yangjeh, A.; Pirhashemi, M.; Vadivel, S. Boosted visible-light photocatalytic performance of TiO2-x decorated by BiOI and AgBr nanoparticles. J. Photochem. Photobiol. A Chem. 2019, 384, 112066. [Google Scholar] [CrossRef]
- Jin, Y.; Tang, W.; Wang, J.; Chen, Z.; Ren, F.; Sun, Z.; Wang, F.; Ren, P. High photocatalytic activity of spent coffee grounds derived activated carbon-supported Ag/TiO2 catalyst for degradation of organic dyes and antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130316. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Bahnemann, Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahed, M.S.; El-Kalliny, A.S.; Badawy, M.I.; Attia, M.S.; Gad-Allah, T.A. Core double-shell MnFe2O4@rGO@TiO2 superparamagnetic photocatalyst for wastewater treatment under solar light. Chem. Eng. J. 2020, 382, 122936. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Fan, L.; Pakdel, E.; Huang, S.; Wang, X. Immobilization of titanium dioxide on PAN fiber as a recyclable photocatalyst via co-dispersion solvent dip coating. Text. Res. J. 2017, 87, 570–581. [Google Scholar] [CrossRef]
- Wen, C.; Zhu, Y.-J.; Kanbara, T.; Zhu, H.-Z.; Xiao, C.-F. Effects of I and F codoped TiO2 on the photocatalytic degradation of methylene blue. Desalination 2009, 249, 621–625. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhao, D.; Liang, Y.; Wang, H.; Wang, N.; Jiang, W.; Liu, S.; Liu, C.; Ding, W.; et al. Preparation, structural and photocatalytic activity of Sn/Fe co-doped TiO2 nanoparticles by sol-gel method. Ceram. Int. 2021, 48, 8297–8305. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H.; Chen, C.; Li, X.; Niu, M.C.; Gao, X.Y.; Yang, Y.T. Effect of calcination temperature on the structure and visible-light photocatalytic activities of (N, S and C) co-doped TiO2 nano-materials. Appl. Surf. Sci. 2015, 332, 172–180. [Google Scholar] [CrossRef]
- Fahim, S.A.; Zahan, N.; Shathy, R.A.; Quddus, M.S.; Moniruzzaman, M.; Masum, S.M.; Molla, M.A.I. B–Sn/TiO2 nanoparticles for photodegradation of metronidazole antibiotics under different lights. Mater. Chem. Phys. 2023, 305, 127937. [Google Scholar] [CrossRef]
- Qu, G.; Wang, H.; Li, X.; Wang, T.; Zhang, Z.; Liang, D.; Qiang, H. Enhanced removal of acid orange II from aqueous solution by V and N co-doping TiO2-MWCNTs/gamma-Al2O3 composite photocatalyst induced by pulsed discharge plasma. Water Sci. Technol. 2021, 83, 257–270. [Google Scholar] [CrossRef]
- Gong, S.; Fan, J.; Cecen, V.; Huang, C.; Min, Y.; Xu, Q.; Li, H. Noble-metal and cocatalyst free W2N/C/TiO photocatalysts for efficient photocatalytic overall water splitting in visible and near-infrared light regions. Chem. Eng. J. 2021, 405, 126913. [Google Scholar] [CrossRef]
- Tu, B.; Chen, H.; Deng, J.; Xue, S.; Ma, X.; Xu, Y.; Xie, Z.; Tao, H. Preparation of N-I co-doped TiO2 supported on activated carbon photocatalyst for efficient photocatalytic reduction of Cr(Ⅵ) ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126660. [Google Scholar] [CrossRef]
- Delgado-Díaz, D.; Hernández-Ramírez, A.; Guzmán-Mar, J.L.; Villanueva-Rodríguez, M.; Maya-Treviño, L.; Hinojosa-Reyes, L. N-S co-doped TiO2 synthesized by microwave precipitation method: Effective photocatalytic performance for the removal of organoarsenic compounds. J. Environ. Chem. Eng. 2021, 9, 106683. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, C.; Nadagouda, M.N.; Dionysiou, D.D. The fabrication of innovative single crystal N,F-codoped titanium dioxide nanowires with enhanced photocatalytic activity for degradation of atrazine. Appl. Catal. B Environ. 2015, 168–169, 550–558. [Google Scholar] [CrossRef]
- Komatsuda, S.; Asakura, Y.; Vequizo, J.J.M.; Yamakata, A.; Yin, S. Enhanced photocatalytic NO decomposition of visible-light responsive F-TiO2/(N,C)-TiO2 by charge transfer between F-TiO2 and (N,C)-TiO2 through their doping levels. Appl. Catal. B Environ. 2018, 238, 358–364. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Pacchioni, G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Di Valentin, C.; Pacchioni, G. Origin of photoactivity of nitrogen-doped titanium dioxide under visible light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef]
- Mollavali, M.; Falamaki, C.; Rohani, S. Efficient light harvesting by NiS/CdS/ZnS NPs incorporated in C, N-co-doped-TiO2 nanotube arrays as visible-light sensitive multilayer photoanode for solar applications. Int. J. Hydrogen Energy 2018, 43, 9259–9278. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Weng, C.-H.; Lin, Y.-H.; Shiesh, C.-C.; Chen, F.-Y. Effect of C content and calcination temperature on the photocatalytic activity of C-doped TiO2 catalyst. Sep. Purif. Technol. 2013, 116, 114–123. [Google Scholar] [CrossRef]
- Han, X.; An, L.; Hu, Y.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. Ti3C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation. Appl. Catal. B Environ. 2020, 265, 118539. [Google Scholar] [CrossRef]
- Farhadian, N.; Akbarzadeh, R.; Pirsaheb, M.; Jen, T.C.; Fakhri, Y.; Asadi, A. Chitosan modified N, S-doped TiO(2) and N, S-doped ZnO for visible light photocatalytic degradation of tetracycline. Int. J. Biol. Macromol. 2019, 132, 360–373. [Google Scholar] [CrossRef]
- Kholodnaya, G.; Sazonov, R.; Ponomarev, D. TiO2@C nanocomposites—From synthesis to application: A review. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 487–526. [Google Scholar] [CrossRef]
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and antimicrobial activities of chitosan-TiO2 nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Cao, C.; Chen, S.; He, M.; Fang, J.; Chen, J.; Li, X.; Li, D. Investigation of nitrogen doped and carbon species decorated TiO2 with enhanced visible light photocatalytic activity by using chitosan. Appl. Catal. B Environ. 2015, 179, 344–351. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Feizpoor, S.; Seifzadeh, D.; Ghosh, S. Improving visible-light-induced photocatalytic ability of TiO2 through coupling with Bi3O4Cl and carbon dot nanoparticles. Sep. Purif. Technol. 2020, 238, 116404. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A.; Ahadzadeh, I.; Yubuta, K. Oxygen-rich TiO2 decorated with C-Dots: Highly efficient visible-light-responsive photocatalysts in degradations of different contaminants. Adv. Powder Technol. 2019, 30, 1183–1196. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. Acs Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Tokimoto, T.; Kawasaki, N.; Nakamura, T.; Akutagawa, J.; Tanada, S. Removal of lead ions in drinking water by coffee grounds as vegetable biomass. J. Colloid. Interface Sci. 2005, 281, 56–61. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, X.; Li, J.; Sun, H.; Zhang, H.; Wang, W. Electrospinning preparation and dye adsorption capacity of TiO2@Carbon flexible fiber. Ceram. Int. 2019, 45, 11856–11860. [Google Scholar] [CrossRef]
- Qin, J.; Chen, Q.; Sun, M.; Sun, P.; Shen, G. Pyrolysis temperature-induced changes in the catalytic characteristics of rice husk-derived biochar during 1,3-dichloropropene degradation. Chem. Eng. J. 2017, 330, 804–812. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-Derived Advanced Carbon Materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Smith, A.T.; Wang, W.; Sun, L. Versatile Nanostructures from Rice Husk Biomass for Energy Applications. Angew. Chem. Int. Ed. Engl. 2018, 57, 13722–13734. [Google Scholar] [CrossRef]
- Shi, M.; Wei, W.; Jiang, Z.; Han, H.; Gao, J.; Xie, J. Biomass-derived multifunctional TiO2/carbonaceous aerogel composite as a highly efficient photocatalyst. RSC Adv. 2016, 6, 25255–25266. [Google Scholar] [CrossRef]
- Saratale, G.D.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Bhatia, S.K.; Atabani, A.E.; Mulone, V.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, F.M.; Yuan, Q. Organic matter removal from landfill leachate by adsorption using spent coffee grounds activated carbon. Sustain. Mater. Technol. 2020, 23, e00141. [Google Scholar] [CrossRef]
- Pagalan, E., Jr.; Sebron, M.; Gomez, S.; Salva, S.J.; Ampusta, R.; Macarayo, A.J.; Joyno, C.; Ido, A.; Arazo, R. Activated carbon from spent coffee grounds as an adsorbent for treatment of water contaminated by aniline yellow dye. Ind. Crops Prod. 2020, 145, 111953. [Google Scholar] [CrossRef]
- Guo, Z.; Ren, P.; Zhang, Z.; Dai, Z.; Lu, Z.; Jin, Y.; Ren, F. Fabrication of carbonized spent coffee grounds/graphene nanoplates/cyanate ester composites for superior and highly absorbed electromagnetic interference shielding performance. J. Mater. Sci. Technol. 2022, 102, 123–131. [Google Scholar] [CrossRef]
- He, W.; Ren, P.-G.; Dai, Z.; Hou, X.; Ren, F.; Jin, Y.-L. Hierarchical porous carbon composite constructed with 1-D CNT and 2-D GNS anchored on 3-D carbon skeleton from spent coffee grounds for supercapacitor. Appl. Surf. Sci. 2021, 558, 149899. [Google Scholar] [CrossRef]
- Chandrabose, G.; Dey, A.; Gaur, S.S.; Pitchaimuthu, S.; Jagadeesan, H.; Braithwaite, N.S.J.; Selvaraj, V.; Kumar, V.; Krishnamurthy, S. Removal and degradation of mixed dye pollutants by integrated adsorption-photocatalysis technique using 2-D MoS2/TiO2 nanocomposite. Chemosphere 2021, 279, 130467. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Dvoranová, D.; Brezová, V.; Dimotikali, D.; Trapalis, C. Selective removal of organic and inorganic air pollutants by adjusting the g-C3N4/TiO2 ratio. Catal. Today 2021, 361, 37–42. [Google Scholar] [CrossRef]
- Rawal, J.; Kamran, U.; Park, M.; Pant, B.; Park, S.-J. Nitrogen and Sulfur Co-Doped Graphene Quantum Dots Anchored TiO2 Nanocomposites for Enhanced Photocatalytic Activity. Catalysts 2022, 12, 548. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Ibukun, O.; Evans, P.E.; Dowben, P.A.; Jeong, H.K. Titanium dioxide-molybdenum disulfide for photocatalytic degradation of methylene blue. Chem. Phys. 2019, 525, 110419. [Google Scholar] [CrossRef]
- Foura, G.; Chouchou, N.; Soualah, A.; Kouachi, K.; Guidotti, M.; Robert, D. Fe-Doped TiO2 Supported on HY Zeolite for Solar Photocatalytic Treatment of Dye Pollutants. Catalysts 2017, 7, 344. [Google Scholar] [CrossRef]
- Skiba, M.; Vorobyova, V. Synthesis OF AG/TIO2 nanocomposite via plasma liquid interactions and degradation methylene blue. Appl. Nanosci. 2020, 10, 4717–4723. [Google Scholar] [CrossRef]
- Mahanta, U.; Khandelwal, M.; Deshpande, A.S. TiO2@SiO2 nanoparticles for methylene blue removal and photocatalytic degradation under natural sunlight and low-power UV light. Appl. Surf. Sci. 2022, 576, 151745. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.S.; Ficai, A.; Surdu, A.V.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Oprea, O.C.; Vasile, B.S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef] [PubMed]
- Sathiyan, K.; Bar-Ziv, R.; Mendelson, O.; Zidki, T. Controllable synthesis of TiO2 nanoparticles and their photocatalytic activity in dye degradation. Mater. Res. Bull. 2020, 126, 110842. [Google Scholar] [CrossRef]






| Photocatalysts | Irradiation | Degradation Activity | Ref |
|---|---|---|---|
| GQDs–TiO2 | visible light | 99.39%@60 min 98%@40 min | [61] |
| Pd–doped TiO2 | UV | 85.9%@180 min | [62] |
| TiO2–MoS2 | UV | 100%@80 min, 0.040 min−1 | [63] |
| Fe–doped TiO2/zeolite | UV (254 nm) | 98%@60 min | [64] |
| Ag–doped TiO2 | visible light | 96%@50 min | [65] |
| SiO2–TiO2 | sunlight | 98%@120 min | [66] |
| C, N–TiO2@ACG | simulated light | 96.9%@45 min, 0.06348 min−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Wang, J.; Gao, X.; Ren, F.; Chen, Z.; Sun, Z.; Ren, P. Spent Coffee Grounds Derived Carbon Loading C, N Doped TiO2 for Photocatalytic Degradation of Organic Dyes. Materials 2023, 16, 5137. https://doi.org/10.3390/ma16145137
Jin Y, Wang J, Gao X, Ren F, Chen Z, Sun Z, Ren P. Spent Coffee Grounds Derived Carbon Loading C, N Doped TiO2 for Photocatalytic Degradation of Organic Dyes. Materials. 2023; 16(14):5137. https://doi.org/10.3390/ma16145137
Chicago/Turabian StyleJin, Yanling, Jiayi Wang, Xin Gao, Fang Ren, Zhengyan Chen, Zhenfeng Sun, and Penggang Ren. 2023. "Spent Coffee Grounds Derived Carbon Loading C, N Doped TiO2 for Photocatalytic Degradation of Organic Dyes" Materials 16, no. 14: 5137. https://doi.org/10.3390/ma16145137
APA StyleJin, Y., Wang, J., Gao, X., Ren, F., Chen, Z., Sun, Z., & Ren, P. (2023). Spent Coffee Grounds Derived Carbon Loading C, N Doped TiO2 for Photocatalytic Degradation of Organic Dyes. Materials, 16(14), 5137. https://doi.org/10.3390/ma16145137






