Unveiling the Potential of Marine Biopolymers: Sources, Classification, and Diverse Food Applications
Abstract
1. Introduction
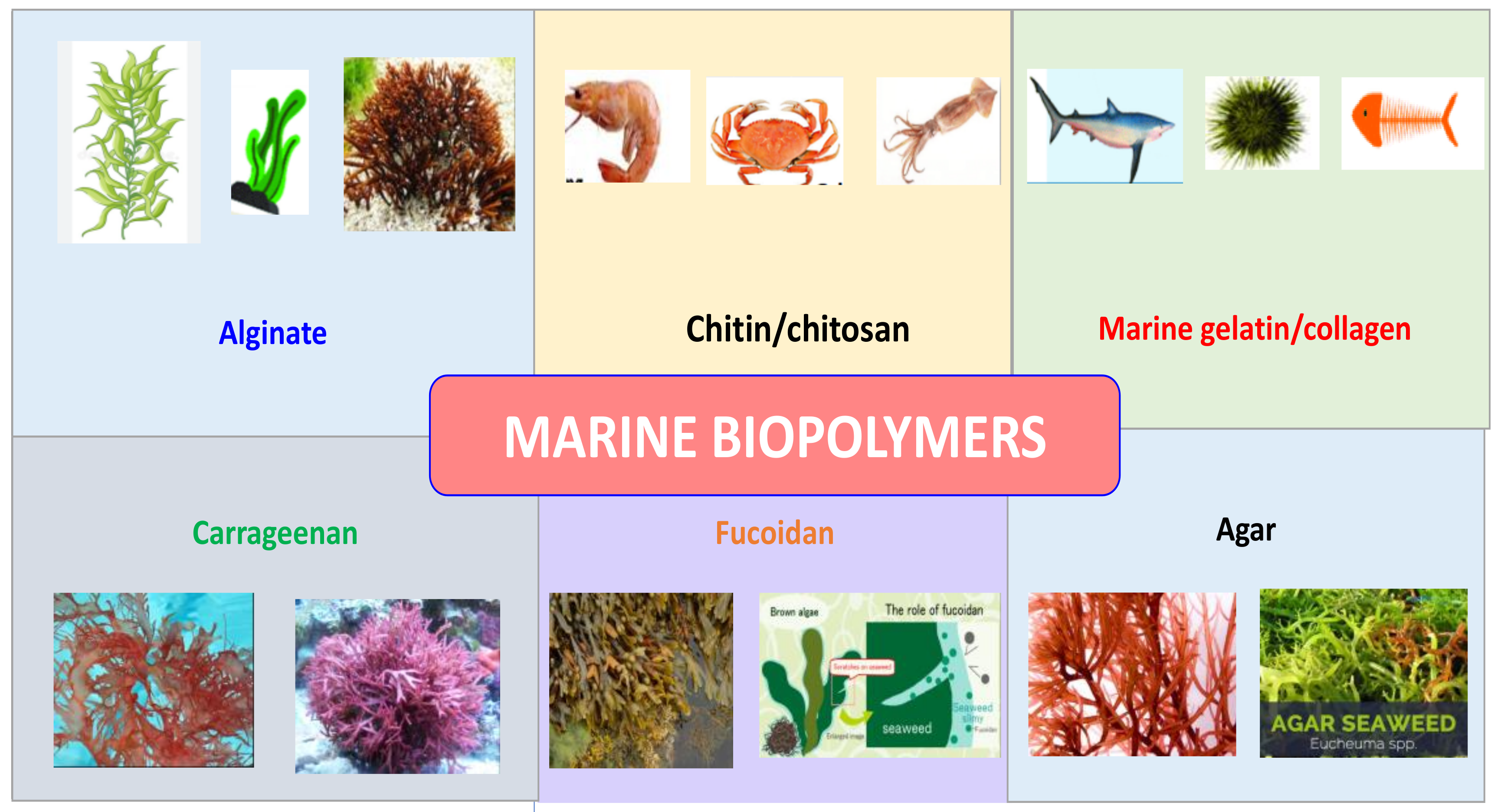
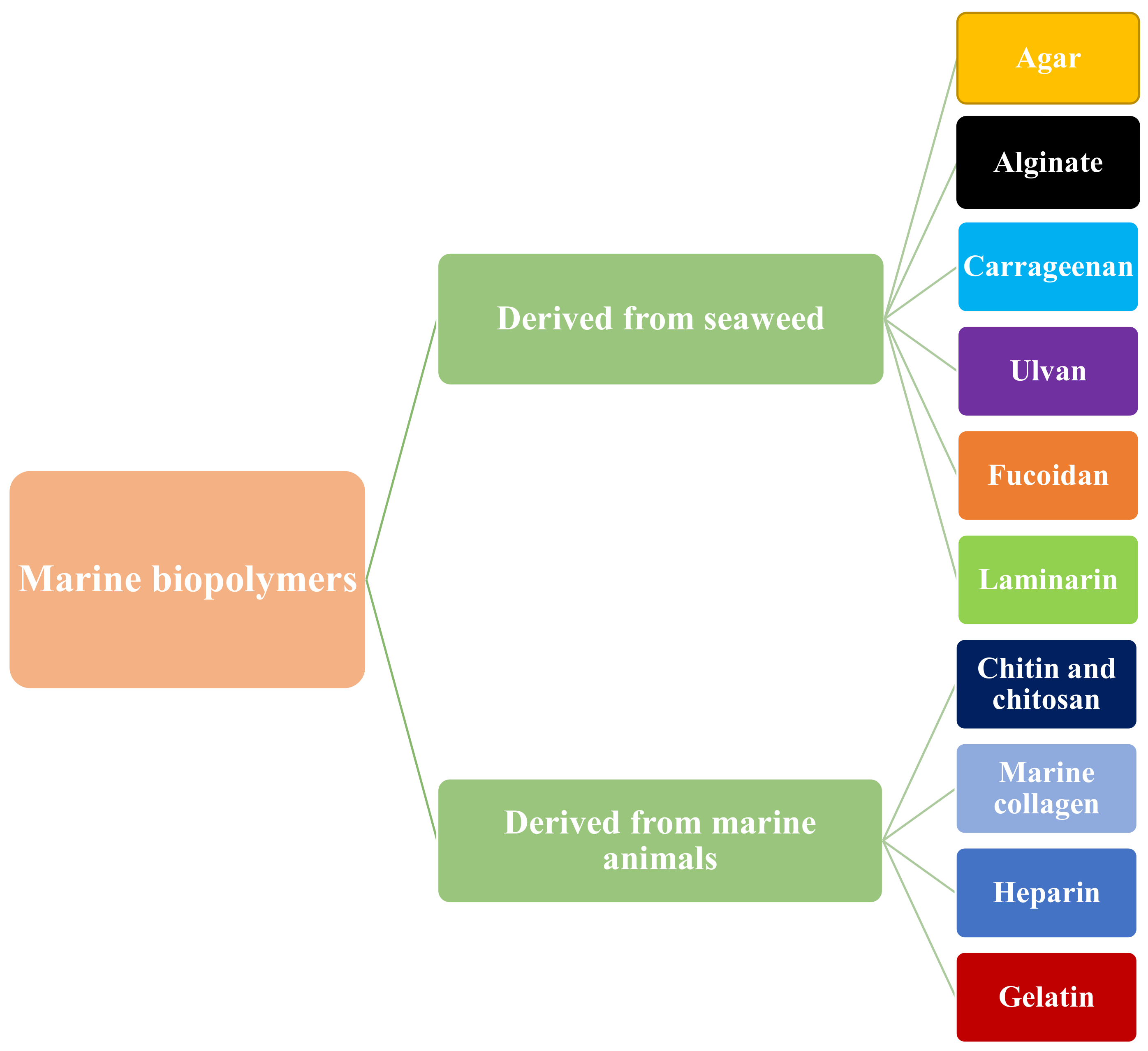
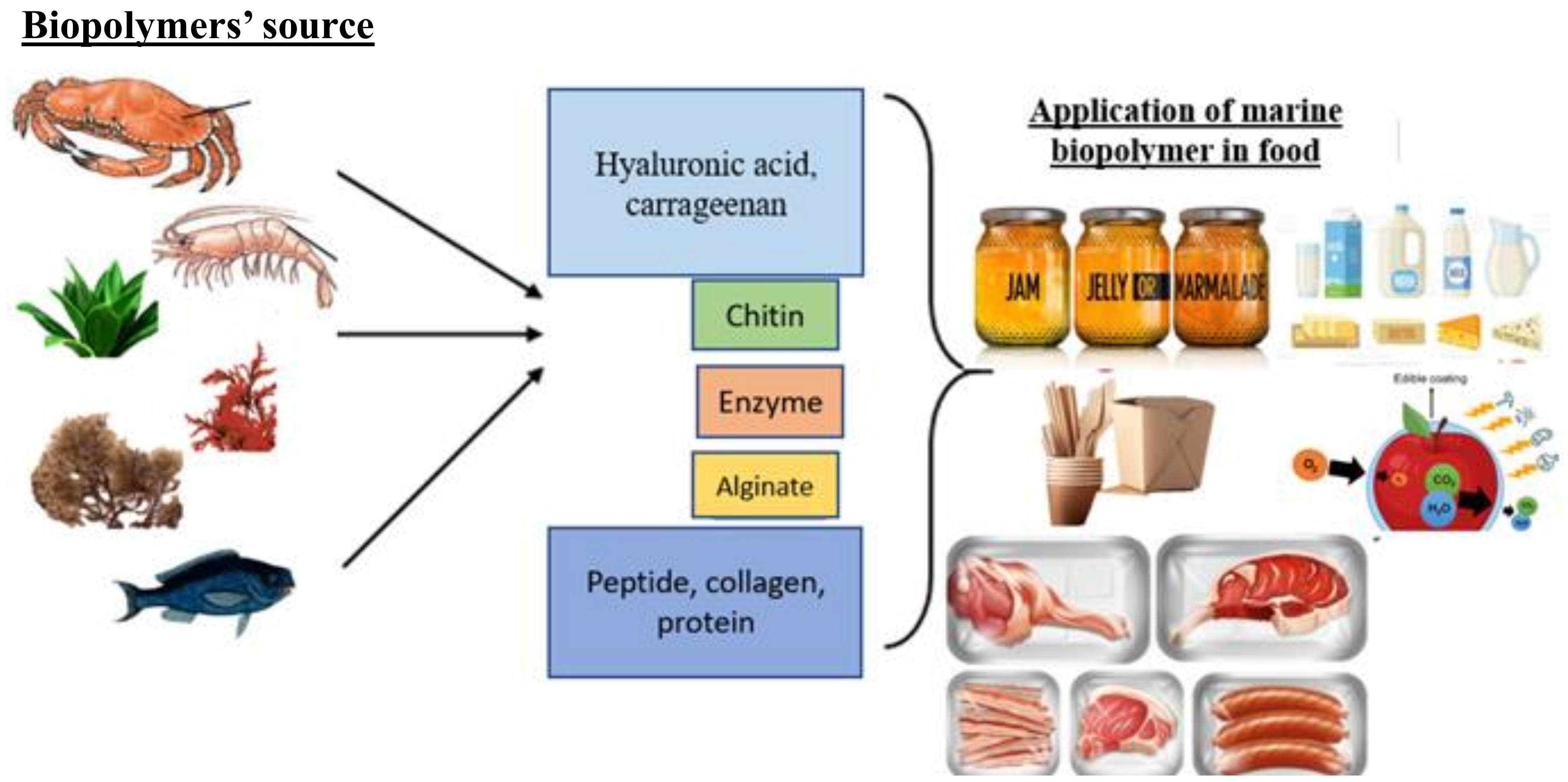
2. Sources of Marine Biopolymers
2.1. Biopolymers Derived from Seaweed
2.1.1. Agar
2.1.2. Alginate
2.1.3. Carrageenan
2.1.4. Ulvan
2.1.5. Fucoidans
2.1.6. Laminarins
2.2. Biopolymers Derived from Marine Animals
2.2.1. Chitin and Chitosan
2.2.2. Marine Collagen
2.2.3. Marine Gelatin
2.2.4. Heparins and Heparan Sulfates
3. Classification of Marine Biopolymers
3.1. Polysaccharide-Based Marine Biopolymers
3.2. Protein-Based Marine Biopolymers
4. Marine Biopolymers in Food Applications
4.1. Fish and Meat Products
4.2. Fruits and Vegetables
4.3. Milk and Milk Products
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tibolla, H.; Pelissari, F.M.; Martins, J.T.; Lanzoni, E.M.; Vicente, A.A.; Menegalli, F.C.; Cunha, R.L. Banana Starch Nanocomposite with Cellulose Nanofibers Isolated from Banana Peel by Enzymatic Treatment: In Vitro Cytotoxicity Assessment. Carbohydr. Polym. 2019, 207, 169–179. [Google Scholar] [CrossRef]
- Rai, P.K.; Choure, K. Agriculture waste to bioplastics: A perfect substitution of plastics. In Value-Addition in Agri-Food Industry Waste Through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 299–314. [Google Scholar]
- Juikar, S.K.; Warkar, S.G. Biopolymers for Packaging Applications: An Overview. Packag. Technol. Sci. 2023, 36, 229–251. [Google Scholar] [CrossRef]
- Oyervides-Muñoz, E.; Oyervides-Muñoz, M.A.; Garcia-Lobato, M.A. Chitin and chitosan nanocomposites: From the synthesis to the application. In Green-Based Nanocomposite Materials and Applications; Springer: Cham, Switzerland, 2023; pp. 101–118. [Google Scholar]
- Grzebieniarz, W.; Biswas, D.; Roy, S.; Jamróz, E. Advances in Biopolymer-based Multi-layer Film Preparations and Food Packaging Applications. Food Packag. Shelf Life 2023, 35, 101033. [Google Scholar] [CrossRef]
- Moradali, M.F.; Rehm, B.H.A. Bacterial Biopolymers: From Pathogenesis to Advanced Materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef]
- Mahmud, N.; Islam, J.; Tahergorabi, R. Marine Biopolymers: Applications in Food Packaging. Processes 2021, 9, 2245. [Google Scholar] [CrossRef]
- Vera, M.; Mella, C.; García, Y.; Jiménez, V.A.; Urbano, B.F. Recent Advances in Tannin-Containing Food Biopackaging. Trends Food Sci. Technol. 2023, 133, 28–36. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the Marine Environment with Pharmacological, Cosmeceutical and Nutraceutical Potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- Uranga, J.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. Biopolymers derived from marine sources for food packaging applications. In Sustainable Food Packaging Technology; Wiley: Hoboken, NJ, USA, 2021; pp. 35–56. [Google Scholar]
- Chakraborty, K. Recent Advances in Marine Biotechnology. In Frontiers in Aquaculture Biotechnology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 187–217. [Google Scholar]
- Roy, S.; Zhang, W.; Biswas, D.; Ramakrishnan, R.; Rhim, J.-W. Grapefruit Seed Extract-added Functional Films and Coating for Active Packaging Applications: A Review. Molecules 2023, 28, 730. [Google Scholar] [CrossRef]
- Nehra, A.; Biswas, D.; Siracusa, V.; Roy, S. Natural Gum-based Functional Bioactive Films and Coatings: A Review. Int. J. Mol. Sci. 2022, 24, 485. [Google Scholar] [CrossRef]
- Roy, S.; Siracusa, V. Multifunctional Application of Biopolymers and Biomaterials. Int. J. Mol. Sci. 2023, 24, 10372. [Google Scholar] [CrossRef]
- Duarte, C.M.; Bruhn, A.; Krause-Jensen, D. A Seaweed Aquaculture Imperative to Meet Global Sustainability Targets. Nat. Sustain. 2022, 5, 185–193. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable Biopolymers for Active Packaging: Demand, Development and Directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Benjamin, T.-A.; Ahmad, I.; Sadiq, M.B. Applications of marine biochemical pathways to develop bioactive and functional products. In Marine Biochemistry; CRC Press: Boca Raton, FL, USA, 2023; pp. 231–259. ISBN 1003303900. [Google Scholar]
- Mistry, P.A.; Konar, M.N.; Latha, S.; Chadha, U.; Bhardwaj, P.; Eticha, T.K. Chitosan Superabsorbent Biopolymers in Sanitary and Hygiene Applications. Int. J. Polym. Sci. 2023, 2023, 4717905. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Alalawy, A.I.; Almutairi, F.M.; Al-Duais, M.A.; Wang, J.; Salama, E.-S. Identification and Characterization of Marine Seaweeds for Biocompounds Production. Environ. Technol. Innov. 2021, 24, 101848. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Kumar, B.R.; Mathimani, T.; Arunkumar, K. A Review on Bioenergy and Bioactive Compounds from Microalgae and Macroalgae-Sustainable Energy Perspective. J. Clean. Prod. 2019, 228, 1320–1333. [Google Scholar] [CrossRef]
- Xue, W.; Zhu, J.; Sun, P.; Yang, F.; Wu, H.; Li, W. Permeability of Biodegradable Film Comprising Biopolymers Derived from Marine Origin for Food Packaging Application: A Review. Trends Food Sci. Technol. 2023, 136, 295–307. [Google Scholar] [CrossRef]
- Bukhari, N.T.M.; Rawi, N.F.M.; Hassan, N.A.A.; Saharudin, N.I.; Kassim, M.H.M. Seaweed Polysaccharide Nanocomposite Films: A Review. Int. J. Biol. Macromol. 2023, 245, 125486. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A Review about Brown Algal Cell Walls and Fucose-Containing Sulfated Polysaccharides: Cell Wall Context, Biomedical Properties and Key Research Challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef]
- Azeem, M.; Batool, F.; Iqbal, N. Chapter 1—Algal-based biopolymers. In Algae Based Polymers, Blends, and Composites; Zia, K.M., Zuber, M., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–31. ISBN 978-0-12-812360-7. [Google Scholar]
- Pandya, Y.H.; Bakshi, M.; Sharma, A.; Pandya, H. Agar-Agar Extraction, Structural Properties and Applications: A Review. Pharma Innov. J. 2022, 11, 1151–1157. [Google Scholar]
- Glicksman, M. Red Seaweed Extracts (Agar, Carrageenans, Furcellaran). In Food Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2019; pp. 73–113. ISBN 0429290373. [Google Scholar]
- Zhang, Y.; Fu, X.; Duan, D.; Xu, J.; Gao, X. Preparation and Characterization of Agar, Agarose, and Agaropectin from the Red Alga Ahnfeltia Plicata. J. Oceanol. Limnol. 2019, 37, 815–824. [Google Scholar] [CrossRef]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1498730507. [Google Scholar]
- Stefanowska, K.; Woźniak, M.; Dobrucka, R.; Ratajczak, I. Chitosan with Natural Additives as a Potential Food Packaging. Materials 2023, 16, 1579. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, F.S.; Zaeim, D. Agar-Based Edible Films for Food Packaging Applications—A Review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Seaweeds as Renewable Sources for Biopolymers and Its Composites: A Review. Curr. Anal. Chem. 2018, 14, 249–267. [Google Scholar] [CrossRef]
- Matheus, J.R.V.; Dalsasso, R.R.; Rebelatto, E.A.; Andrade, K.S.; de Andrade, L.M.; de Andrade, C.J.; Monteiro, A.R.; Fai, A.E.C. Biopolymers as Green-based Food Packaging Materials: A Focus on Modified and Unmodified Starch-based Films. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1148–1183. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Agar-based Antioxidant Composite Films Incorporated with Melanin Nanoparticles. Food Hydrocoll. 2019, 94, 391–398. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Melanin-mediated Synthesis of Copper Oxide Nanoparticles and Preparation of Functional Agar/CuO NP Nanocomposite Films. J. Nanomater. 2019, 2019, 2840517. [Google Scholar] [CrossRef]
- Venugopal, V.; Sasidharan, A. Seafood Industry Effluents: Environmental Hazards, Treatment and Resource Recovery. J. Environ. Chem. Eng. 2021, 9, 104758. [Google Scholar] [CrossRef]
- Gupta, V.; Biswas, D.; Roy, S. A Comprehensive Review of Biodegradable Polymer-Based Films and Coatings and Their Food Packaging Applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Effect of CuS Reinforcement on the Mechanical, Water vapor barrier, UV-light barrier, and Antibacterial Properties of Alginate-based Composite Films. Int. J. Biol. Macromol. 2020, 164, 37–44. [Google Scholar] [CrossRef]
- Rosadiani, D.W.; Purwanti, T.; Purwanto, D.A. Effect of Natrium Alginate Concentration on Physical Characteristics, Viability and Anticancer Activity of Microparticles from a Combination of Probiotics and Tomato Pasta. Res. J. Pharm. Technol. 2018, 11, 2575–2580. [Google Scholar] [CrossRef]
- Kanokpanont, S.; Yamdech, R.; Aramwit, P. Stability Enhancement of Mulberry-Extracted Anthocyanin Using Alginate/Chitosan Microencapsulation for Food Supplement Application. Artif. Cells Nanomed. Biotechnol. 2018, 46, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Dufrane, D.; Goebbels, R.-M.; Gianello, P. Alginate Macroencapsulation of Pig Islets Allows Correction of Streptozotocin-Induced Diabetes in Primates up to 6 Months without Immunosuppression. Transplantation 2010, 90, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Chee, S.-Y.; Wong, P.-K.; Wong, C.-L. Extraction and Characterisation of Alginate from Brown Seaweeds (Fucales, Phaeophyceae) Collected from Port Dickson, Peninsular Malaysia. J. Appl. Phycol. 2011, 23, 191–196. [Google Scholar] [CrossRef]
- Ali, S.W.; Bairagi, S.; Banerjee, S.; Banerjee, S. Plant and marine-based biopolymers for efficient nutrient delivery. In Biopolymers in Nutraceuticals and Functional Foods; Royal Society of Chemistry: London, UK, 2022; pp. 306–328. ISBN 1839168056. [Google Scholar]
- Vasudevan, U.M.; Lee, O.K.; Lee, E.Y. Alginate Derived Functional Oligosaccharides: Recent Developments, Barriers, and Future Outlooks. Carbohydr. Polym. 2021, 267, 118158. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.V.G.; Nagakubo, T.; Toyofuku, M.; Nomura, N.; Utada, A.S. Synergy between Sophorolipid Biosurfactant and SDS Increases the Efficiency of P. Aeruginosa Biofilm Disruption. Langmuir 2020, 36, 6411–6420. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Robal, M.; Brenner, T.; Matsukawa, S.; Ogawa, H.; Truus, K.; Rudolph, B.; Tuvikene, R. Monocationic Salts of Carrageenans: Preparation and Physico-Chemical Properties. Food Hydrocoll. 2017, 63, 656–667. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of Carrageenan-based Functional Nanocomposite Films Incorporated with Melanin Nanoparticles. Colloids Surf. B Biointerfaces 2019, 176, 317–324. [Google Scholar] [CrossRef]
- Dong, M.; Xue, Z.; Liu, J.; Yan, M.; Xia, Y.; Wang, B. Preparation of Carrageenan Fibers with Extraction of Chondrus via Wet Spinning Process. Carbohydr. Polym. 2018, 194, 217–224. [Google Scholar] [CrossRef]
- Nguyen, P.T.M.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of Different Hydrocolloids on Texture, Rheology, Tribology and Sensory Perception of Texture and Mouthfeel of Low-Fat Pot-Set Yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef]
- Qiu, S.-M.; Aweya, J.J.; Liu, X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.-L. Bioactive Polysaccharides from Red Seaweed as Potent Food Supplements: A Systematic Review of Their Extraction, Purification, and Biological Activities. Carbohydr. Polym. 2022, 275, 118696. [Google Scholar] [CrossRef]
- Gupta, I.; Cherwoo, L.; Bhatia, R.; Setia, H. Biopolymers: Implications and Application in the Food Industry. Biocatal. Agric. Biotechnol. 2022, 50, 102534. [Google Scholar] [CrossRef]
- Skryplonek, K.; Henriques, M.; Gomes, D.; Viegas, J.; Fonseca, C.; Pereira, C.; Dmytrów, I.; Mituniewicz-Małek, A. Characteristics of Lactose-Free Frozen Yogurt with κ-Carrageenan and Corn Starch as Stabilizers. J. Dairy Sci. 2019, 102, 7838–7848. [Google Scholar] [CrossRef] [PubMed]
- Martiny, T.R.; Pacheco, B.S.; Pereira, C.M.P.; Mansilla, A.; Astorga–España, M.S.; Dotto, G.L.; Moraes, C.C.; Rosa, G.S. A Novel Biodegradable Film Based on Κ-carrageenan Activated with Olive Leaves Extract. Food Sci. Nutr. 2020, 8, 3147–3156. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A Review on Synthesis, Properties and Applications of Natural Polymer Based Carrageenan Blends and Composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L. Carrageenan: A Review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Gopi, S.; Balakrishnan, P.; Brai, M. Biopolymers in Nutraceuticals and Functional Foods; Royal Society of Chemistry: London, UK, 2022; ISBN 1839168056. [Google Scholar]
- Yu-Qing, T.; Mahmood, K.; Shehzadi, R.; Ashraf, M.F. Ulva Lactuca and Its Polysaccharides: Food and Biomedical Aspects. J. Biol. Agric. Healthc. 2016, 6, 140–151. [Google Scholar]
- Madany, M.A.; Abdel-Kareem, M.S.; Al-Oufy, A.K.; Haroun, M.; Sheweita, S.A. The Biopolymer Ulvan from Ulva Fasciata: Extraction towards Nanofibers Fabrication. Int. J. Biol. Macromol. 2021, 177, 401–412. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A Systematic Review of Extraction, Composition and Function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Sankaranarayanan, S.; Gajaria, T.K.; Li, G.; Kujawski, W.; Kujawa, J.; Navia, R. A Short Review on the Valorization of Green Seaweeds and Ulvan: Feedstock for Chemicals and Biomaterials. Biomolecules 2020, 10, 991. [Google Scholar] [CrossRef]
- Ben Amor, C.; Jmel, M.A.; Chevallier, P.; Mantovani, D.; Smaali, I. Efficient Extraction of a High Molecular Weight Ulvan from Stranded Ulva Sp. Biomass: Application on the Active Biomembrane Synthesis. Biomass Convers. Biorefin. 2021, 13, 3975–3985. [Google Scholar] [CrossRef]
- Zheng, W.; Hao, Y.; Wang, D.; Huang, H.; Guo, F.; Sun, Z.; Shen, P.; Sui, K.; Yuan, C.; Zhou, Q. Preparation of Triamcinolone Acetonide-Loaded Chitosan/Fucoidan Hydrogel and Its Potential Application as an Oral Mucosa Patch. Carbohydr. Polym. 2021, 272, 118493. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and Structure of Cell Wall Ulvans Recovered from Ulva Spp. along the Swedish West Coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Shanmugam, M.; Bhat, R. Producing Novel Edible Films from Semi Refined Carrageenan (SRC) and Ulvan Polysaccharides for Potential Food Applications. Int. J. Biol. Macromol. 2018, 112, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Aleissa, M.S.; Alkahtani, S.; Abd Eldaim, M.A.; Ahmed, A.M.; Bungău, S.G.; Almutairi, B.; Bin-Jumah, M.; AlKahtane, A.A.; Alyousif, M.S.; Abdel-Daim, M.M. Fucoidan Ameliorates Oxidative Stress, Inflammation, DNA Damage, and Hepatorenal Injuries in Diabetic Rats Intoxicated with Aflatoxin B1. Oxid. Med. Cell. Longev. 2020, 2020, 9316751. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Latip, J.; Badri, K.H.; Hassan, O.; Yamin, B.M. Characterisation of Fucoidan Extracted from Malaysian Sargassum Binderi. Food Chem. 2016, 209, 267–273. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, J.; Xu, X. Bioactivity of Fucoidan Extracted from Laminaria Japonica Using a Novel Procedure with High Yield. Food Chem. 2018, 245, 911–918. [Google Scholar] [CrossRef]
- Shen, P.; Yin, Z.; Qu, G.; Wang, C. Bioactive Seaweeds for Food Applications; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A Review about the Development of Fucoidan in Antitumor Activity: Progress and Challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, M.; Zhang, P.; Fan, S.; Huang, J.; Yu, S.; Zhang, C.; Li, H. Fucoidan and Galactooligosaccharides Ameliorate High-Fat Diet–Induced Dyslipidemia in Rats by Modulating the Gut Microbiota and Bile Acid Metabolism. Nutrition 2019, 65, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.-Y. Fucoidan as a Marine Anticancer Agent in Preclinical Development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, Structure and Biofunctional Activities of Laminarin from Brown Algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Verdugo, P.; Orellana, M.V.; Chin, W.-C.; Petersen, T.W.; van den Eng, G.; Benner, R.; Hedges, J.I. Marine Biopolymer Self-Assembly: Implications for Carbon Cycling in the Ocean. Faraday Discuss. 2008, 139, 393–398. [Google Scholar] [CrossRef]
- Mitsuya, D.; Yamamoto, M.; Okai, M.; Inoue, A.; Suzuki, T.; Ojima, T.; Urano, N. Continuous Saccharification of Laminarin by Immobilized Laminarinase Ulam111 Followed by Ethanol Fermentation with a Marine-Derived Yeast. Adv. Microbiol. 2017, 7, 387–403. [Google Scholar] [CrossRef]
- Mišurcová, L. Chemical composition of seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Wiley: Hoboken, NJ, USA, 2011; pp. 171–192. [Google Scholar]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef]
- Manivasagan, P.; Oh, J. Marine Polysaccharide-Based Nanomaterials as a Novel Source of Nanobiotechnological Applications. Int. J. Biol. Macromol. 2016, 82, 315–327. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Joni, I.M.; Tjahjono, B. Advances on Biopolymers Derived from Marine and Agricultural Products for Sustainable Food Packaging Applications. Front. Sustain. Food Syst. 2022, 6, 1040144. [Google Scholar] [CrossRef]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and Application of Chitin and Its Derivatives: A Review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and Chitosan: Biopolymers for Wound Management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Sharma, M.; Salama, E.-S.; Ling, Z.; Li, X. Applications of Chitin and Chitosan as Natural Biopolymer: Potential Sources, Pretreatments, and Degradation Pathways. Biomass Convers. Biorefin. 2022, 1–15. [Google Scholar] [CrossRef]
- Rebecca, L.J.; Susithra, G.; Sharmila, S.; Das, M.P. Isolation and Screening of Chitinase Producing Serratia Marcescens from Soil. J. Chem. Pharm. Res. 2013, 5, 192–195. [Google Scholar]
- Claverie, M.; McReynolds, C.; Petitpas, A.; Thomas, M.; Fernandes, S.C.M. Marine-Derived Polymeric Materials and Biomimetics: An Overview. Polymers 2020, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, G.; Guan, F.; Liu, W. Application of Chitin/Chitosan and Their Derivatives in the Papermaking Industry. Polymers 2018, 10, 389. [Google Scholar] [CrossRef]
- Harkin, C.; Mehlmer, N.; Woortman, D.V.; Brück, T.B.; Brück, W.M. Nutritional and Additive Uses of Chitin and Chitosan in the Food Industry. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Springer: Cham, Switzerland, 2019; pp. 1–43. [Google Scholar]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms. Solubility Polysacch. 2017, 3, 20–60. [Google Scholar]
- Merzendorfer, H. The Cellular Basis of Chitin Synthesis in Fungi and Insects: Common Principles and Differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Effect of Chitosan Modified Halloysite on the Physical and Functional Properties of Pullulan/chitosan Biofilm Integrated with Rutin. Appl. Clay Sci. 2021, 211, 106205. [Google Scholar] [CrossRef]
- Joye, J.I.; Julian McClements, D. Biopolymer-Based Delivery Systems: Challenges and Opportunities. Curr. Top. Med. Chem. 2016, 16, 1026–1039. [Google Scholar] [CrossRef]
- Sałek, K.; Gutierrez, T. Surface-Active Biopolymers from Marine Bacteria for Potential Biotechnological Applications. AIMS Microbiol. 2016, 2, 92–107. [Google Scholar] [CrossRef]
- Monteiro, L.P.G.; Borges, J.; Rodrigues, J.M.M.; Mano, J.F. Unveiling the Assembly of Neutral Marine Polysaccharides into Electrostatic-Driven Layer-by-Layer Bioassemblies by Chemical Functionalization. Mar. Drugs 2023, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Yuswan, M.H.; Jalil, N.H.A.; Mohamad, H.; Keso, S.; Mohamad, N.A.; Yusoff, T.S.T.M.; Ismail, N.F.; Manaf, Y.N.A.; Hashim, A.M.; Desa, M.N.M. Hydroxyproline Determination for Initial Detection of Halal-Critical Food Ingredients (Gelatin and Collagen). Food Chem. 2021, 337, 127762. [Google Scholar] [CrossRef] [PubMed]
- Hashim, P.; Ridzwan, M.M.S.; Bakar, J.; Hashim, M.D. Collagen in Food and Beverage Industries. Int. Food Res. J. 2015, 22, 1. [Google Scholar]
- Abd El-Salam, M.H.; El-Shibiny, S. Natural biopolymers as nanocarriers for bioactive ingredients used in food industries. In Encapsulations; Elsevier: Amsterdam, The Netherlands, 2016; pp. 793–829. [Google Scholar]
- Bhagwat, P.K.; Dandge, P.B. Isolation, Characterization and Valorizable Applications of Fish Scale Collagen in Food and Agriculture Industries. Biocatal. Agric. Biotechnol. 2016, 7, 234–240. [Google Scholar] [CrossRef]
- Vilarinho, F.; Sanches Silva, A.; Vaz, M.F.; Farinha, J.P. Nanocellulose in Green Food Packaging. Crit. Rev. Food Sci. Nutr. 2018, 58, 1526–1537. [Google Scholar] [CrossRef]
- Meneghetti, M.C.Z.; Hughes, A.J.; Rudd, T.R.; Nader, H.B.; Powell, A.K.; Yates, E.A.; Lima, M.A. Heparan Sulfate and Heparin Interactions with Proteins. J. R. Soc. Interface 2015, 12, 20150589. [Google Scholar] [CrossRef]
- Nakamoto, M.M.; Assis, M.; de Oliveira Filho, J.G.; Braga, A.R.C. Spirulina Application in Food Packaging: Gaps of Knowledge and Future Trends. Trends Food Sci Technol 2023, 133, 138–147. [Google Scholar] [CrossRef]
- Milovanovic, I.; Hayes, M. Marine Gelatine from Rest Raw Materials. Appl. Sci. 2018, 8, 2407. [Google Scholar] [CrossRef]
- Rathod, N.B.; Bangar, S.P.; Šimat, V.; Ozogul, F. Chitosan and Gelatine Biopolymer-based Active/Biodegradable Packaging for the Preservation of Fish and Fishery Products. Int. J. Food Sci. Technol. 2023, 58, 854–861. [Google Scholar] [CrossRef]
- Mehetre, S.S.; Shankar, R.K.; Ameta, R.K.; Behere, S.S. An introduction to protein-based biopolymers. In Protein-Based Biopolymers; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–40. [Google Scholar]
- Jumaidin, R. Agar based composite as a new alternative biopolymer. In Composites from the Aquatic Environment; Springer: Singapore, 2023; pp. 67–82. [Google Scholar]
- Charoenpol, A.; Crespy, D.; Schulte, A.; Suginta, W. Marine Chitin Upcycling with Immobilized Chitinolytic Enzymes: Current State and Prospects. Green Chem. 2023. [Google Scholar] [CrossRef]
- Beiras, R.; López-Ibáñez, S. A Practical Tool for the Assessment of Polymer Biodegradability in Marine Environments Guides the Development of Truly Biodegradable Plastics. Polymers 2023, 15, 974. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Kwon, Y.-M.; Moon, J.H.; Cho, G.-C.; Kim, Y.U.; Chang, I. Xanthan Gum Biopolymer-Based Soil Treatment (Bpst) as a Construction Material to Mitigate Internal Erosion of Earthen Levee and Embankment Structures: A Field-Scale Study. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4370967 (accessed on 9 June 2023).
- Issa, A.T.; Tahergorabi, R. Barrier, degradation, and cytotoxicity studies for chitin-chitosan bionanocomposites. In Chitin-and Chitosan-Based Biocomposites for Food Packaging Applications; CRC Press: Boca Raton, FL, USA, 2020; pp. 49–58. ISBN 0429299605. [Google Scholar]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate Composites for Bone Tissue Engineering: A Review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Campbell, K.T.; Gharaviram, H.; Madrigal, J.L.; Silva, E.A. Alginate-Chitosan Hydrogels Provide a Sustained Gradient of Sphingosine-1-Phosphate for Therapeutic Angiogenesis. Ann. Biomed. Eng. 2017, 45, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by Vibrational Spectroscopy of Seaweed Polysaccharides with Potential Use in Food, Pharmaceutical, and Cosmetic Industries. Int. J. Carbohydr. Chem. 2013, 2013, 537202. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Saurabh, C.K.; Tye, Y.Y.; Lai, T.K.; Easa, A.M.; Rosamah, E.; Fazita, M.R.N.; Syakir, M.I.; Adnan, A.S.; Fizree, H.M. Seaweed Based Sustainable Films and Composites for Food and Pharmaceutical Applications: A Review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Xiao, Q.; Weng, H.; Ni, H.; Hong, Q.; Lin, K.; Xiao, A. Physicochemical and Gel Properties of Agar Extracted by Enzyme and Enzyme-Assisted Methods. Food Hydrocoll. 2019, 87, 530–540. [Google Scholar] [CrossRef]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.-L. Biosynthesis of Agar in Red Seaweeds: A Review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible Films and Coatings for Food Packaging Applications: A Review. Environ. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C. Recent Applications of Biopolymers Derived from Fish Industry Waste in Food Packaging. Polymers 2021, 13, 2337. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Natural Polymer Based Cling Films for Food Packaging. Int. J. Pharm. Pharm. Sci. 2015, 7, 10–18. [Google Scholar]
- Bourbon, A.I.; Pereira, R.N.; Pastrana, L.M.; Vicente, A.A.; Cerqueira, M.A. Protein-Based Nanostructures for Food Applications. Gels 2019, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Kaewprachu, P.; Osako, K.; Rawdkuen, S. Effects of Plasticizers on the Properties of Fish Myofibrillar Protein Film. J. Food Sci. Technol. 2018, 55, 3046–3055. [Google Scholar] [CrossRef] [PubMed]
- Della Malva, A.; Albenzio, M.; Santillo, A.; Russo, D.; Figliola, L.; Caroprese, M.; Marino, R. Methods for Extraction of Muscle Proteins from Meat and Fish Using Denaturing and Nondenaturing Solutions. J. Food Qual 2018, 2018, 8478471. [Google Scholar]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, G.C. Collagen Extraction Optimization from the Skin of the Small-Spotted Catshark (S. canicula) by Response Surface Methodology. Mar. Drugs 2019, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of Antimicrobial and Antioxidant Gelatin/curcumin Composite Films for Active Food Packaging Application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.-W. Fabrication of Antioxidant and Antimicrobial Pullulan/gelatin Films Integrated with Grape Seed Extract and Sulfur Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 2316–2323. [Google Scholar] [CrossRef]
- Iliou, K.; Kikionis, S.; Ioannou, E.; Roussis, V. Marine Biopolymers as Bioactive Functional Ingredients of Electrospun Nanofibrous Scaffolds for Biomedical Applications. Mar. Drugs 2022, 20, 314. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of Chitosan–Gelatin Composite and Bilayer Coating and Film Effect on the Quality of Refrigerated Rainbow Trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Serrano-León, J.S.; Bergamaschi, K.B.; Yoshida, C.M.P.; Saldaña, E.; Selani, M.M.; Rios-Mera, J.D.; Alencar, S.M.; Contreras-Castillo, C.J. Chitosan Active Films Containing Agro-Industrial Residue Extracts for Shelf Life Extension of Chicken Restructured Product. Food Res. Int. 2018, 108, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Feng, L.; Wang, Y. Effect of Alginate/Nano-Ag Coating on Microbial and Physicochemical Characteristics of Shiitake Mushroom (Lentinus Edodes) during Cold Storage. Food Chem. 2013, 141, 954–960. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid-Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Santos, J.; Campañone, L.A. Application of Osmotic Dehydration and Microwave Drying to Strawberries Coated with Edible Films. Dry. Technol. 2019, 37, 1002–1012. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Rodríguez-Hernández, M.J.; Hernández-Hernández, H.M.; Delgado-Sánchez, L.F.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Effect of an Edible Coating Based on Chitosan and Oxidized Starch on Shelf Life of Carica papaya L., and Its Physicochemical and Antimicrobial Properties. Coatings 2018, 8, 318. [Google Scholar] [CrossRef]
- Rao, P.S.; Sharma, H.; Singh, R.; Meghawal, K.; Pradhan, D. Chitosan and Its Application in Dairy Industry. In Quality Control and Waste Utilization for Agriculture and Dairy Products; Prince Publishing: Delhi, India, 2018. [Google Scholar]
- Roy, S.; Ezati, P.; Khan, A.; Rhim, J.-W. New Opportunities and Advances in Quercetin-added Functional Packaging Films for Sustainable Packaging Applications: A Mini-review. Crit. Rev. Food Sci. Nutr. 2023, in press. [Google Scholar] [CrossRef]
- Babaremu, K.; Oladijo, O.P.; Akinlabi, E. Biopolymers: A Suitable Replacement for Plastics in Product Packaging. Adv. Ind. Eng. Polym. Res. 2023. [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Eyvaz, M.; Al Maskari, T.; Nassani, D.E.; Abu Amr, S.S.; Abujazar, M.S.S.; Bashir, M.J.K. An Overview of Green Bioprocessing of Algae-Derived Biochar and Biopolymers: Synthesis, Preparation, and Potential Applications. Energies 2023, 16, 791. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-Based Nanocomposite Films and Coatings: Recent Advances in Shelf-Life Improvement of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2022, 62, 1912–1935. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. Edible Coatings Enriched with Essential Oils for Extending the Shelf-life of ‘Bravo de Esmolfe’Fresh-cut Apples. Int. J. Food Sci. Technol. 2016, 51, 87–95. [Google Scholar] [CrossRef]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Rhim, J.-W. Gelatin/Agar-Based Multifunctional Film Integrated with Copper-Doped Zinc Oxide Nanoparticles and Clove Essential Oil Pickering Emulsion for Enhancing the Shelf Life of Pork Meat. Food Res. Int. 2022, 160, 111690. [Google Scholar] [CrossRef]
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Johns, M.L.; Azizi, A.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Effectiveness of Gelatine and Chitosan Spray Coating for Extending Shelf Life of Vacuum-packaged Beef. Int. J. Food Sci. Technol. 2021, 56, 4026–4037. [Google Scholar] [CrossRef]
- Sapie, S.R.; Kamari, A.; Jumadi, J. A Brief Review of Propolis as an Additive in Biopolymer Matrix Films for Food Packaging. In Proceedings of the 8th International Conference on Research Implementation and Education of Mathematics and Science (ICRIEMS 2021): Transforming Science Literacy into A New Normal Digital World to Achieve Sustainable Development Goals, Yogyakarta, Indonesia, 27–28 August 2021; AIP Publishing LLC: Melville, NY, USA, 2023; Volume 2556, p. 40002. [Google Scholar]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Recent Advances in Gelatine and Chitosan Complex Material for Practical Food Preservation Application. Int. J. Food Sci. Technol. 2021, 56, 6279–6300. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Biswas, D.; Rhim, J.-W. Shikonin Functionalized Packaging Film for Monitoring the Freshness of Shrimp. Materials 2022, 15, 6615. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, F.; Elabed, N.; Ceylan, Z.; Ocak, E.; Ozogul, Y. Nano-Technological Approaches for Plant and Marine-Based Polysaccharides for Nano-Encapsulations and Their Applications in Food Industry. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 97, pp. 187–236. ISBN 1043-4526. [Google Scholar]
- Zhang, W.; Roy, S.; Rhim, J.-W. Copper-based Nanoparticles for Biopolymer-based Functional Films in Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1933–1952. [Google Scholar] [CrossRef] [PubMed]
- Sudha, P.N.; Sangeetha, K.; Gomathi, T. Introduction to marine biopolymers. In Industrial Applications of Marine Biopolymers; CRC Press: Boca Raton, FL, USA, 2017; pp. 3–18. ISBN 1315313537. [Google Scholar]
- Kim, H.J.; Roy, S.; Rhim, J.-W. Gelatin/agar-based Color-indicator Film Integrated with Clitoria ternatea Flower Anthocyanin and Zinc Oxide Nanoparticles for Monitoring Freshness of Shrimp. Food Hydrocoll. 2022, 124, 107294. [Google Scholar] [CrossRef]
- Da Zavareze, E.R.; el Halal, S.L.M.; Marques e Silva, R.; Dias, A.R.G.; Prentice-Hernández, C. Mechanical, Barrier and Morphological Properties of Biodegradable Films Based on Muscle and Waste Proteins from the W Hitemouth Croaker (M Icropogonias Furnieri). J. Food Process. Preserv. 2014, 38, 1973–1981. [Google Scholar] [CrossRef]
- Vinayak, A.; Sharma, S.; Singh, G.B. Biopolymers from industrial waste. In Biopolymers: Recent Updates, Challenges and Opportunities; Springer: Cham, Switzerland, 2022; pp. 129–149. [Google Scholar]
- Qureshi, D.; Nayak, S.K.; Anis, A.; Ray, S.S.; Kim, D.; Nguyen, T.T.H.; Pal, K. Introduction of Biopolymers: Food and Biomedical Applications. In Biopolymer-Based Formulations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–45. [Google Scholar]
- Araújo, C.S.; Rodrigues, A.M.C.; Joele, M.R.S.P.; Araújo, E.A.F.; Lourenço, L.F.H. Optimizing Process Parameters to Obtain a Bioplastic Using Proteins from Fish Byproducts through the Response Surface Methodology. Food Packag. Shelf Life 2018, 16, 23–30. [Google Scholar] [CrossRef]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; de Ramos, A.L.S.; de Miranda Gomide, L.A.; Ramos, E.M. Selection of a Chitosan Gelatin-Based Edible Coating for Color Preservation of Beef in Retail Display. Meat. Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of Fungal Chitosan Incorporated with Pomegranate Peel Extract as Edible Coating for Microbiological, Chemical and Sensorial Quality Enhancement of Nile Tilapia Fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef]
- Sapper, M.; Chiralt, A. Starch-Based Coatings for Preservation of Fruits and Vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Salem, A.; Nasri, R.; Nasri, M.; Jridi, M. Food Applications of Bioactive Marine Gelatin Films. Curr. Opin. Food Sci. 2022, 43, 206–215. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Kumar, V.; Sharma, R. Chitosan Nanoemulsions as Advanced Edible Coatings for Fruits and Vegetables: Composition, Fabrication and Developments in Last Decade. Int. J. Biol. Macromol. 2020, 152, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of Edible Coating with Essential Oil in Food Preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kulandhaivelu, S.V.; Roy, S. Alginate/carboxymethyl cellulose/starch-based Active Coating with Grapefruit Seed Extract to Extend the Shelf Life of Green Chilli. Ind. Crops Prod. 2023, 199, 116752. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kulandhaivelu, S.V.; Roy, S.; Viswanathan, V.P. Characterisation of Ternary Blend Film of Alginate/carboxymethyl cellulose/starch for Packaging Applications. Ind. Crops Prod. 2023, 193, 116114. [Google Scholar] [CrossRef]
- Di Donato, P.; Poli, A.; Taurisano, V.; Abbamondi, G.R.; Nicolaus, B.; Tommonaro, G. Recent Advances in the Study of Marine Microbial Biofilm: From the Involvement of Quorum Sensing in Its Production up to Biotechnological Application of the Polysaccharide Fractions. J. Mar. Sci. Eng. 2016, 4, 34. [Google Scholar] [CrossRef]
- Kim, S.-K.; Perera, U.; Rajapakse, N.; Kim, S. Seafood Processing By-Products; Springer: New York, NY, USA, 2016; ISBN 1493947591. [Google Scholar]
- Yousefi, M.; Jafari, S.M. Recent Advances in Application of Different Hydrocolloids in Dairy Products to Improve Their Techno-Functional Properties. Trends Food Sci. Technol. 2019, 88, 468–483. [Google Scholar] [CrossRef]
- Ebrahimzadeh, S.; Biswas, D.; Roy, S.; McClements, D.J. Incorporation of Essential Oils in Edible Seaweed-based Films: A Comprehensive Review. Trends Food Sci. Technol. 2023, 135, 43–56. [Google Scholar] [CrossRef]
- Vidanarachchi, J.K.; Kurukulasuriya, M.S.; Samaraweera, A.M.; Silva, K. Applications of Marine Nutraceuticals in Dairy Products. Adv. Food Nutr. Res. 2012, 65, 457–478. [Google Scholar] [PubMed]
- Evdokimov, I.A.; Alieva, L.R.; Varlamov, V.P.; Kharitonov, V.D.; Butkevich, T.V.; Kurchenko, V.P. Usage of Chitosan in Dairy Products Production. Foods Raw Mater. 2015, 3, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Vivek, K. Use of Encapsulated Probiotics in Dairy Based Foods. Int. J. Food Agric. Vet. Sci. 2013, 3, 188–199. [Google Scholar]
- Sharma, H.; Mahajan, G.; Kaur, M.; Gupta, R. Additives in dairy-based food. In Microbes for Natural Food Additives; Springer: Singapore, 2023; pp. 169–203. [Google Scholar]
- Zhao, Y.; Khalesi, H.; He, J.; Fang, Y. Application of Different Hydrocolloids as Fat Replacer in Low-Fat Dairy Products: Ice Cream, Yogurt and Cheese. Food Hydrocoll. 2023, 138, 108493. [Google Scholar] [CrossRef]
| Biopolymer-Based Film/Coating | Food System | Key Outcome | References |
|---|---|---|---|
| Gelatin–chitosan film | Rainbow trout | The shelf life of rainbow trout fillets was extended by the application of a chitosan–gelatin composite film to over 16 days in refrigerated storage conditions, which reduced bacterial contamination. | [128] |
| Chitosan | Chicken | Peanut-skin-extract-added chitosan-based film decreased psychrotrophic microbial growth and improved the oxidative stability of the chicken product. | [129] |
| Alginate | Shiitake mushroom | Inhibition of the growth of mesophilic, psychrophilic pseudomonas and yeasts and molds. | [130] |
| Agar | Hake fillet | A green tea probiotic strain was added to the agar-based biopolymer film, which eventually delayed the growth of microbes and decreased the spoilage indexes. | [131] |
| Alginate | Strawberry | Strawberry coatings were an effective way to sustain water loss while significantly lowering solid gain under the studied osmotic dehydration conditions. | [132] |
| Chitosan and oxidized starch | Papaya | The shelf life of the coated fruit was extended. At room temperature, untreated papayas reached the point of ripening after 5 days, whereas coated papayas reached this point after 15 days, indicating that the coating helped to increase papaya pulp hardness. | [133] |
| Chitosan/lactic acid solution | Cheese | A chitosan/lactic acid solution was added in the starter culture; during refrigeration, it prevented the growth of rotting microorganisms for up to 10 days. | [134] |
| Chitosan modified by antimicrobial monomethyl fumaric acid (MFA) | Beef | Chitosan derivatives reduced the total viable count of lactic-acid bacteria yeast–mold, and psychrotrophic bacteria. Also, application of this derivative increased the shelf life by 8 days. | [135] |
| Chitosan | Fish oil | Free-radical-scavenging activity was increased as compared to the control group, resulting in increased storage life. | [136] |
| Gelatin | Cold-smoked sardines | Gelatin film enriched with oregano and rosemary helped lower the oxidation rate and increased the days of storage. | [137] |
| Chitosan and gelatin | Black grapes | The developed film extended the shelf life of the black grapes to up to 14 days during storage at 37 °C. | [138] |
| Alginate | Fresh-cut apples | The coating conferred increased shelf life by giving apples a good appearance and firmness, inhibiting enzymatic actions of browning, and reducing weight loss. | [139] |
| Agar | Green grapes | The zinc-oxide-added agar-based functional packaging film can be a promising active packaging material. The functional film could significantly improve the shelf life of green grapes. | [140] |
| Gelatin/agar | Pork | The clove essential oils and copper-doped zinc-oxide-included film were effective in reducing the lipid peroxidation and total microbial count in functional-film-wrapped pork. The shelf life of the meat was extended after the application of the packaging. | [141] |
| Gelatin/chitosan | Beef | The application of a gelatin and chitosan spray coating on the vacuum-packed beef enhanced the life span up to three weeks compared to the uncoated counterparts. | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bose, I.; Nousheen; Roy, S.; Yaduvanshi, P.; Sharma, S.; Chandel, V.; Biswas, D. Unveiling the Potential of Marine Biopolymers: Sources, Classification, and Diverse Food Applications. Materials 2023, 16, 4840. https://doi.org/10.3390/ma16134840
Bose I, Nousheen, Roy S, Yaduvanshi P, Sharma S, Chandel V, Biswas D. Unveiling the Potential of Marine Biopolymers: Sources, Classification, and Diverse Food Applications. Materials. 2023; 16(13):4840. https://doi.org/10.3390/ma16134840
Chicago/Turabian StyleBose, Ipsheta, Nousheen, Swarup Roy, Pallvi Yaduvanshi, Somesh Sharma, Vinay Chandel, and Deblina Biswas. 2023. "Unveiling the Potential of Marine Biopolymers: Sources, Classification, and Diverse Food Applications" Materials 16, no. 13: 4840. https://doi.org/10.3390/ma16134840
APA StyleBose, I., Nousheen, Roy, S., Yaduvanshi, P., Sharma, S., Chandel, V., & Biswas, D. (2023). Unveiling the Potential of Marine Biopolymers: Sources, Classification, and Diverse Food Applications. Materials, 16(13), 4840. https://doi.org/10.3390/ma16134840







