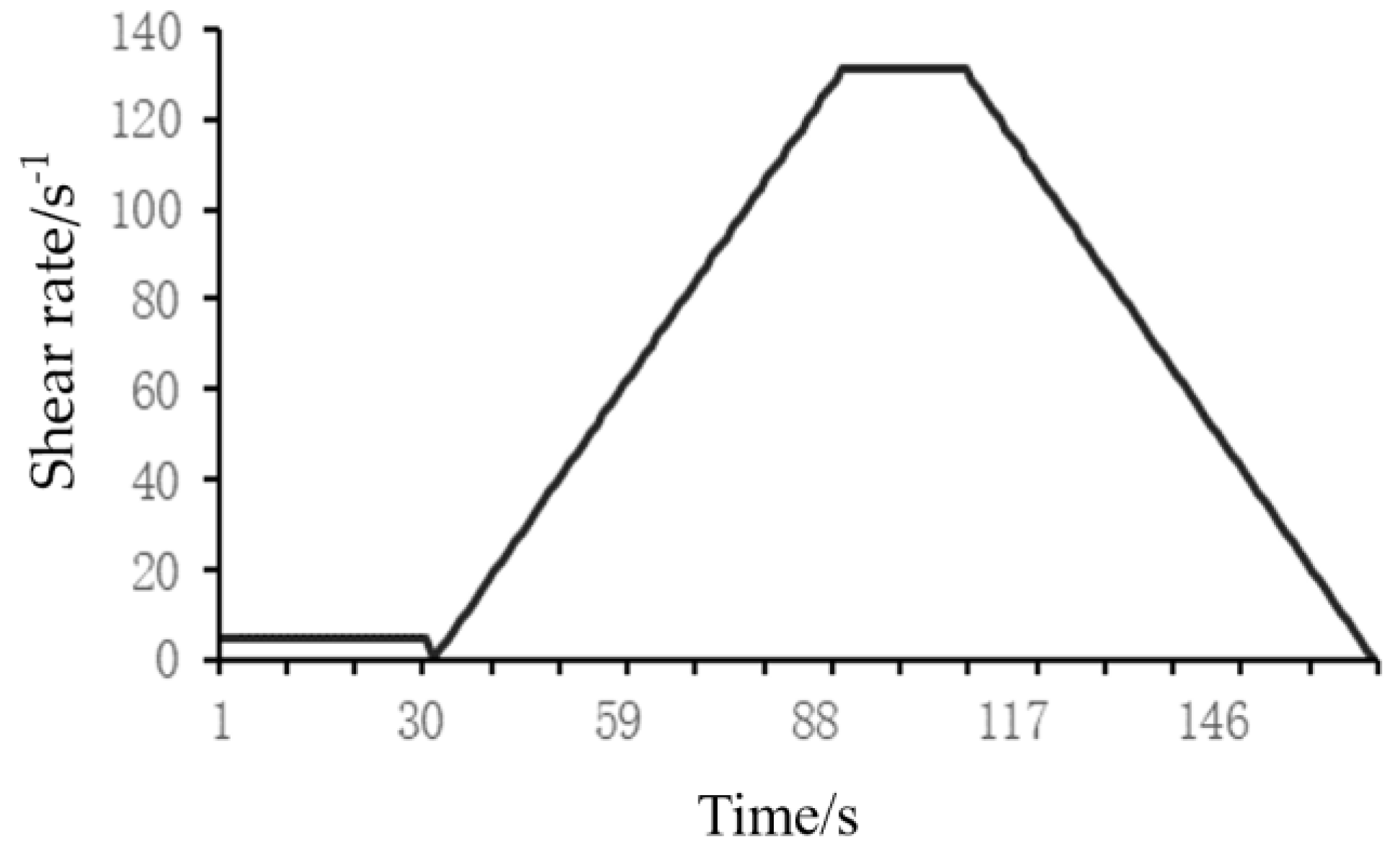
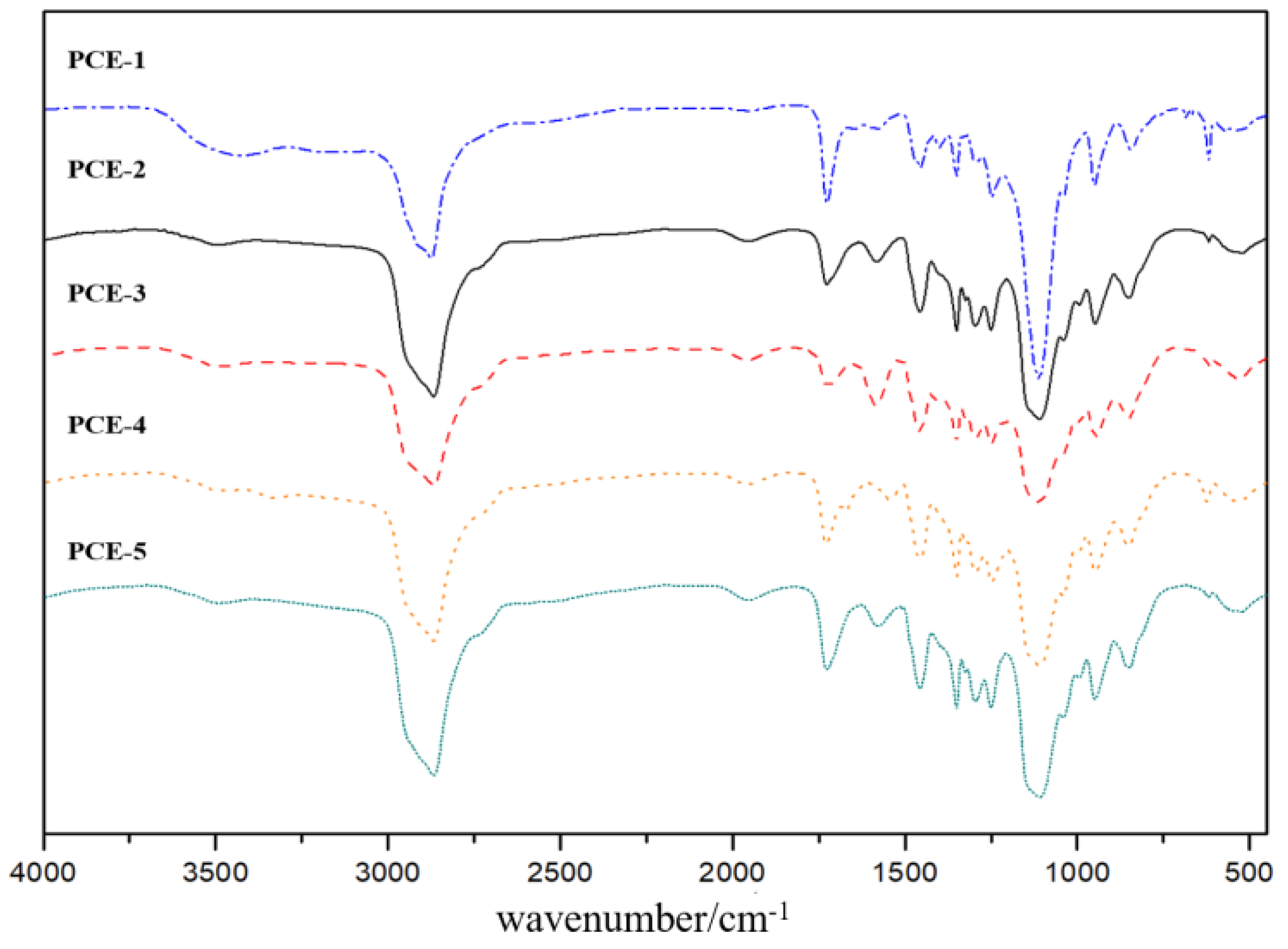
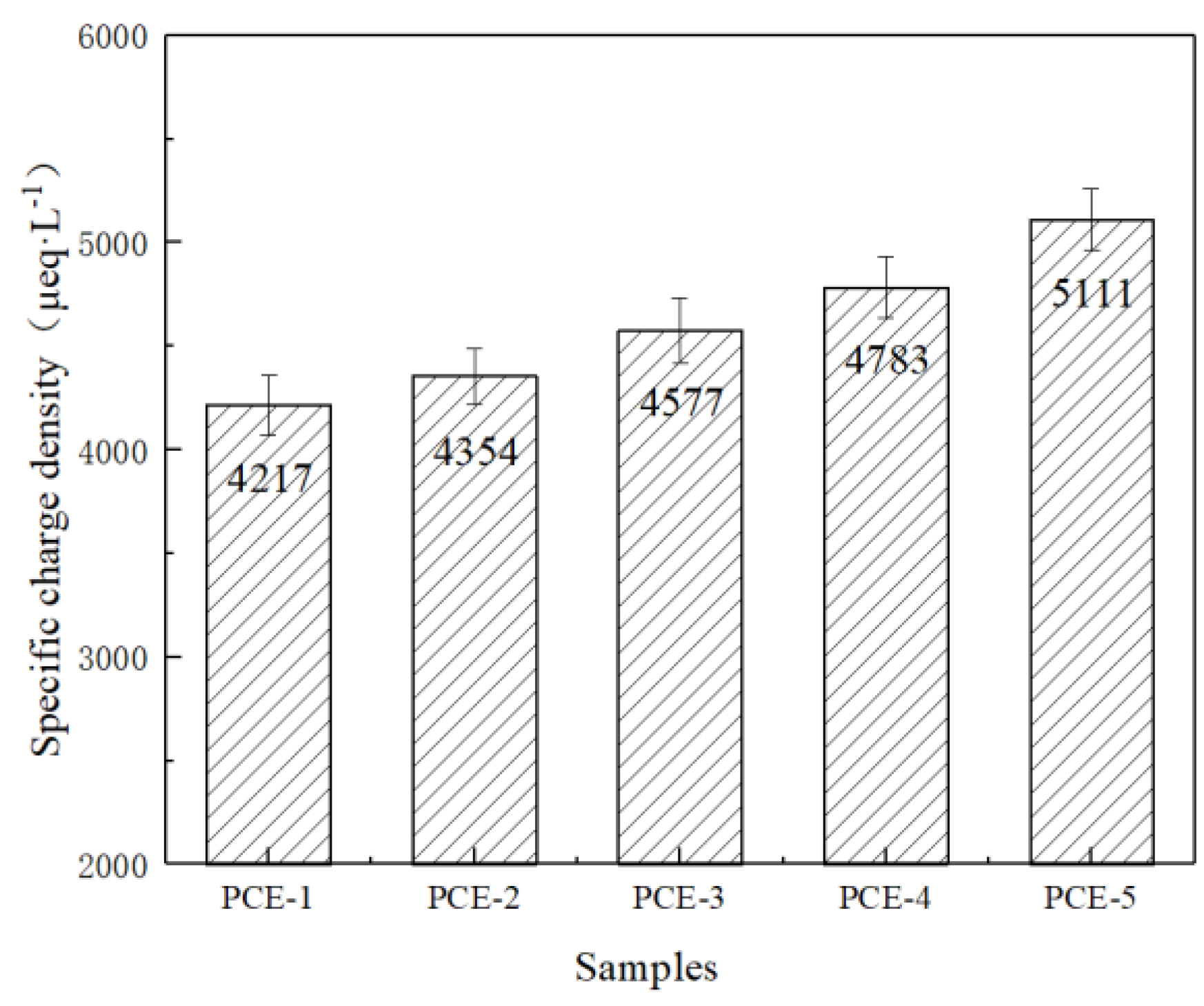
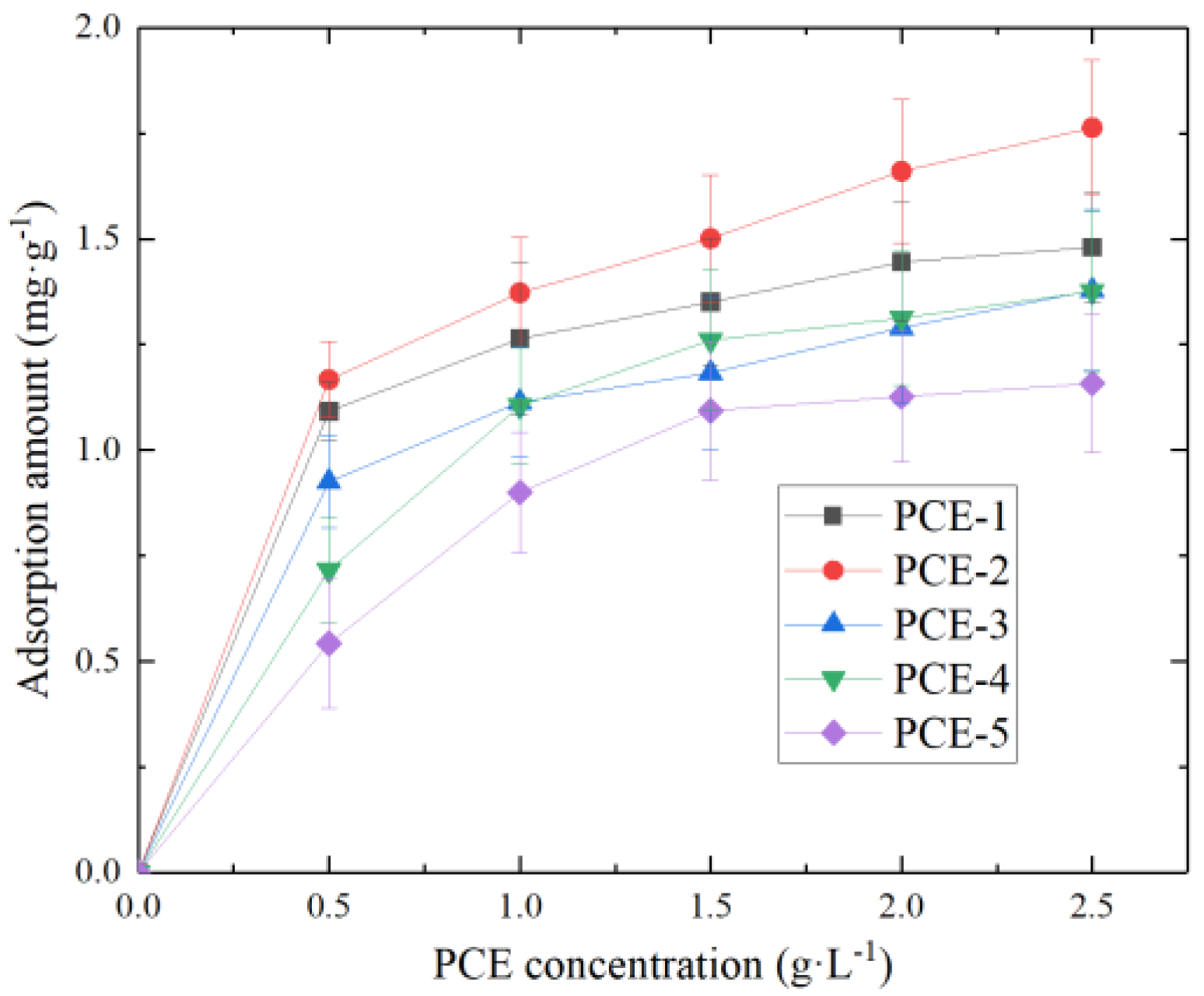
1. Introduction
Concrete admixtures are a relatively effective and convenient technical approach to reducing cement consumption and improving the durability and strength of concrete. They are essential components of modern concrete and key new materials for the development of high-strength, high-performance, and high-durability concrete [
1,
2]. Polycarboxylate ethers (PCEs) have great potential for high performance due to their strong molecular structure adjustability and the ability to produce products with different properties using different monomer compositions and polymerization processes. They have become the most effective, economical, and convenient technical approach to achieving high durability and performance improvement in concrete, gradually replacing lignosulfonate-based superplasticizers, naphthalene-based superplasticizers, etc., and becoming the mainstream variety of concrete admixtures in the market [
3].
The main chain molecular structure of PCE is generally composed of unsaturated acids and their derivatives, with sulfonic acid groups (-SO
3H) and carboxylic acid groups (-COOH) grafted onto the main chain. The side chains are composed of long polyethylene oxide groups [
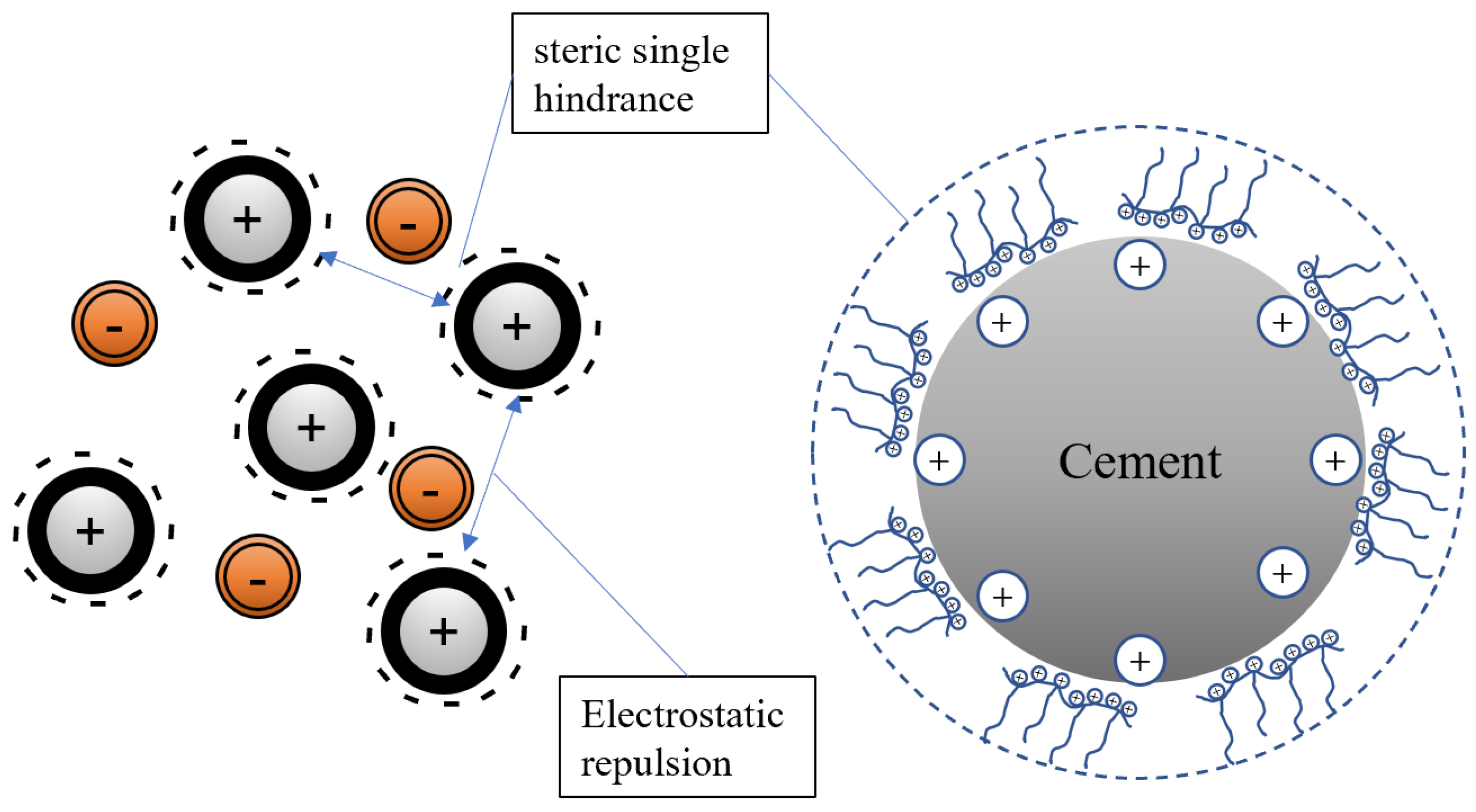
4], which can ionize carboxylic acid groups, sulfonic acid groups, and other functional groups under alkaline conditions in the cement slurry, resulting in a negative charge on the main chain. PCE adsorbs on the surface of positively charged cement particles and hydration products through electrostatic forces and releases free water in the cement particles through the effects of steric hindrance and electrostatic repulsion, greatly increasing the fluidity of the slurry [
5]. After PCEs are adsorbed onto cement particles, the interparticle forces are primarily electrostatic repulsion. However, in actual cement slurries, various high-concentration ions (such as Ca
2+ and OH
−) reduce the electrostatic repulsion, resulting in differences in the origin of repulsive forces between different cement particles.
The molecular structure of PCE can usually be modified. There are many studies on the molecular structure modification and working mechanism of PCE [
6,
7]. Different spatial hindrance effects can be produced by changing the length of the polyethylene oxide side chain or inserting various functional groups into the PCE skeleton.
Different functional groups and structures in PCE molecules can result in varying degrees of affinity to the surface for cement particles and cement hydration products, which in turn leads to significant adsorption. The adsorption process belongs to solid-liquid adsorption and is a dynamic equilibrium process in which adsorption and desorption occur simultaneously [
8]. The large difference in size between solid and liquid phase molecules leads to significant differences in adsorption, which can be studied through adsorption kinetics and thermodynamics. Isothermal adsorption and adsorption kinetics are commonly used to simulate adsorption between solid and liquid phases, and commonly used isothermal and kinetic models are listed in
Table 1 [
9].
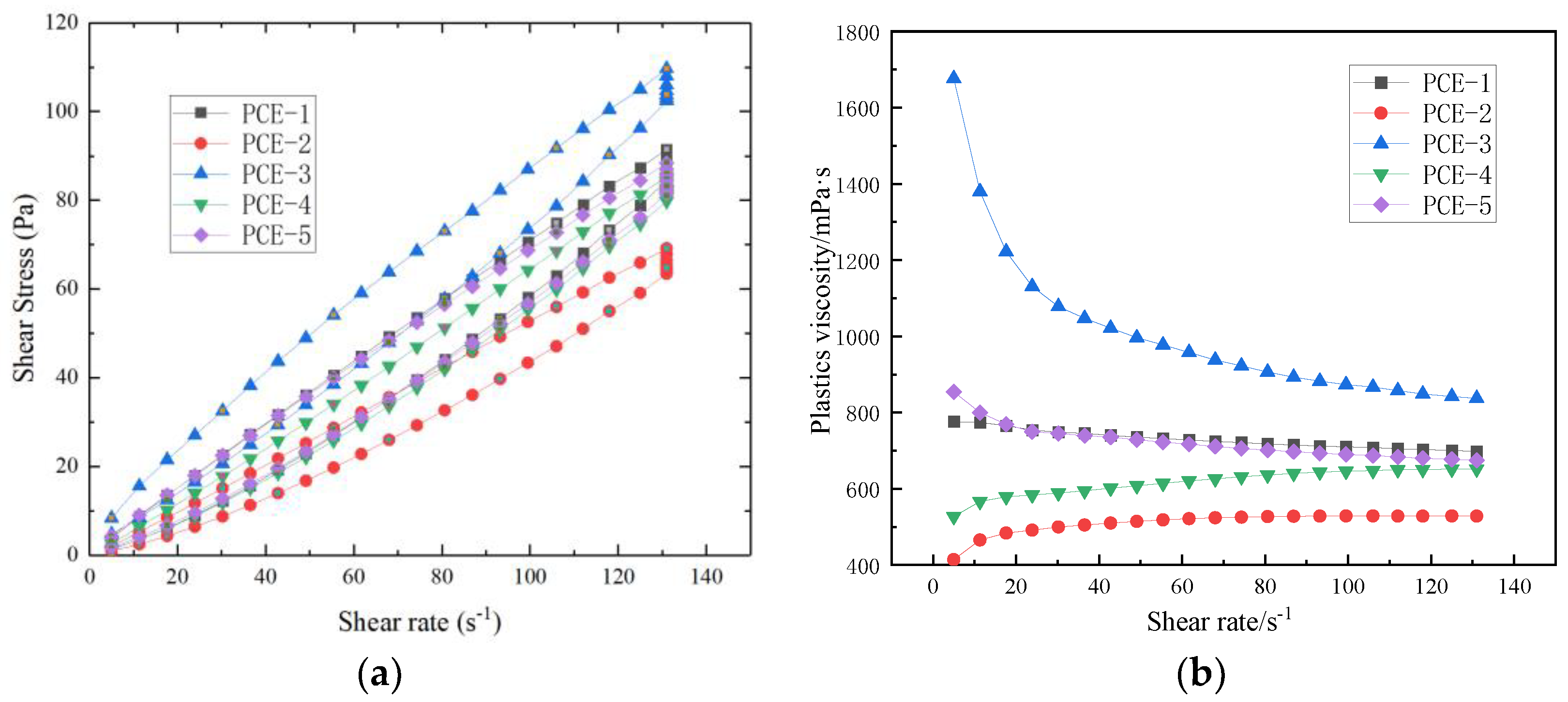
After different molecular structures of PCE are adsorbed onto the surface of cement particles or hydration products, they can have different effects on the rheological properties of fresh cement paste, such as monomer molar ratio, side chain length, and anionic anchoring groups. Yamada K et al. [
4] analyzed and studied the effect of PCE side chain length, main chain polymerization degree, carboxyl and sulfonic acid group composition on dispersibility, and found that PCE with longer side chains, a lower main chain polymerization degree, and higher sulfonic acid group content had better dispersibility. HE Yan [
10] found that the carboxyl density of PCE has a significant effect on its dispersibility, and the dispersibility of PCE increases with increasing carboxyl density, while the viscosity of cement paste is the lowest. Hui Feng [
11] pointed out that PCE with short side chains and high carboxylate density has higher adsorption capacity in mortar, and PCE with sulfonic acid groups has higher adsorption on the surface of cement, resulting in better rheological properties.
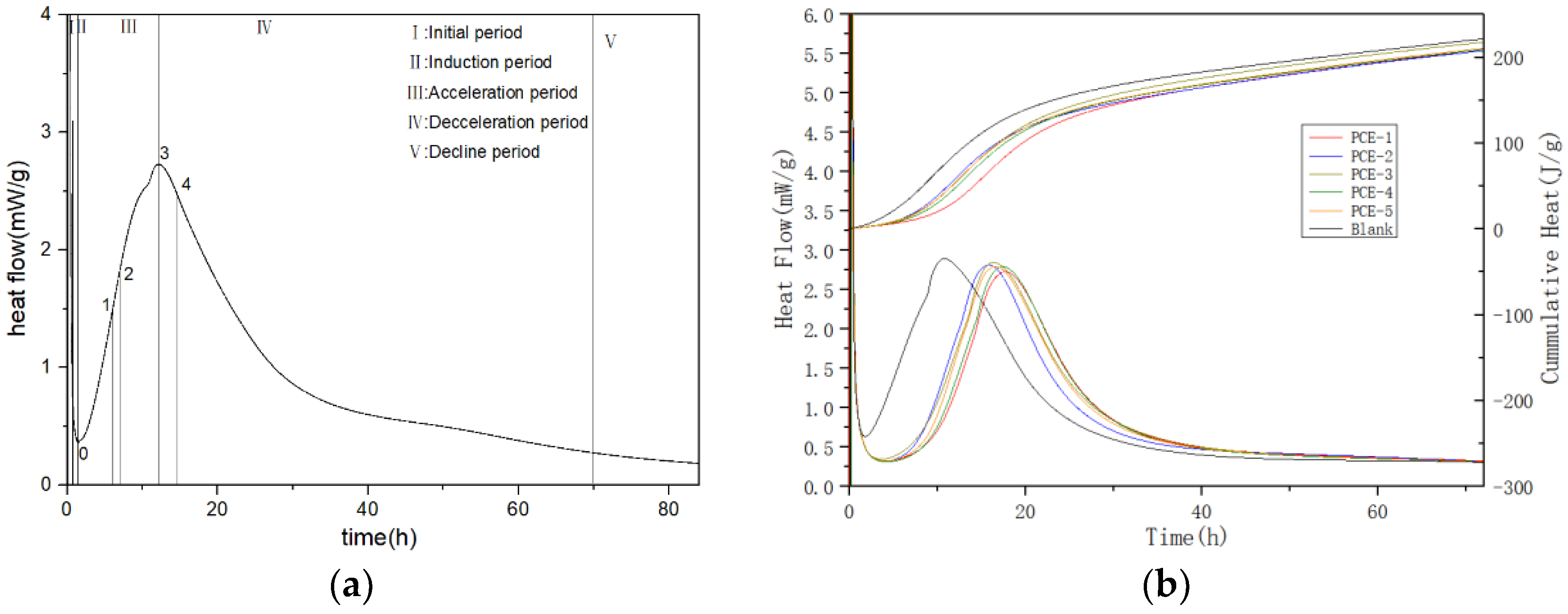
PCE also has a huge impact on cement hydration through the free water released by the dispersed cement particles and the morphology and thickness of the PCE wrapping layer on the surface of the cement particles. In the different stages of hydration, the main sources of heat release are mineral dissolution, nucleation, and the growth of hydration products. Different microstructures of PCE and their interaction with cement particles in the early stage lead to different rates of mineral dissolution, pore solution concentration, nucleation, and growth rate of hydration products. Generally, the new surface film layer caused by adsorption may be thicker than the original one, and the presence of a thick film layer can hinder the exchange of substances and energy between the surface of cement particles and the liquid phase, and also hinder the penetration of external ions into the internal ions, thereby affecting the hydration process of cement. Hongwei Tian [
12] studied its effect on the flowability and early hydration of Portland cement and sulphoaluminate cement systems and showed that PCE can significantly inhibit the early formation of ettringite (AFt) crystals. Karen L. Scrivener [
13] studied the fundamental mechanisms of hydration during different stages and showed that the growth of the main hydration product, C-S-H, is the main factor controlling the main heat evolution peak, with geochemical dissolution theory as an explanation for the induction period.
The hydration heat release curve can be divided into five stages, including (I) Initial period, (II) Induction period, (III) Acceleration period, (IV) Deceleration period, and (V) Decline period. In the induction period, after water is added to cement, mineral phases, such as gypsum and C3A dissolve rapidly and generate AFt. At the same time, the concentration of Ca
2+, OH
−, SO
42−, and other ions in the cement solution continues to increase. When the ion concentration reaches a certain value, the nucleation and crystallization processes occur at the solid-liquid interface, resulting in the production of calcium hydroxide (CH) and AFt crystals. Meanwhile, tricalcium silicate (C3S) is gradually hydrated to form a small amount of short and fibrous hydrated calcium silicate gel (C-S-H). Therefore, it is difficult to observe a smooth induction period in ordinary Portland cement paste without the addition of PCE, and the hydration heat curve shows a sharp “valley” shape. The decrease in the rate and amount of heat released during the induction period can be explained by the double electric layer theory. This theory suggests that a calcium-deficient, silicon-rich layer is formed on the surface of cement particles, which can adsorb calcium ions from the solution and form a double electric layer. The appearance of this double electric layer can prevent further contact between cement particles and water, thereby delaying the hydration of cement and causing it to enter the induction period [
14]. According to the crystal dissolution theory mentioned by Juilland, the duration of this initial reaction is short, and it is difficult to capture the heat release pattern of this stage [
15,
16].
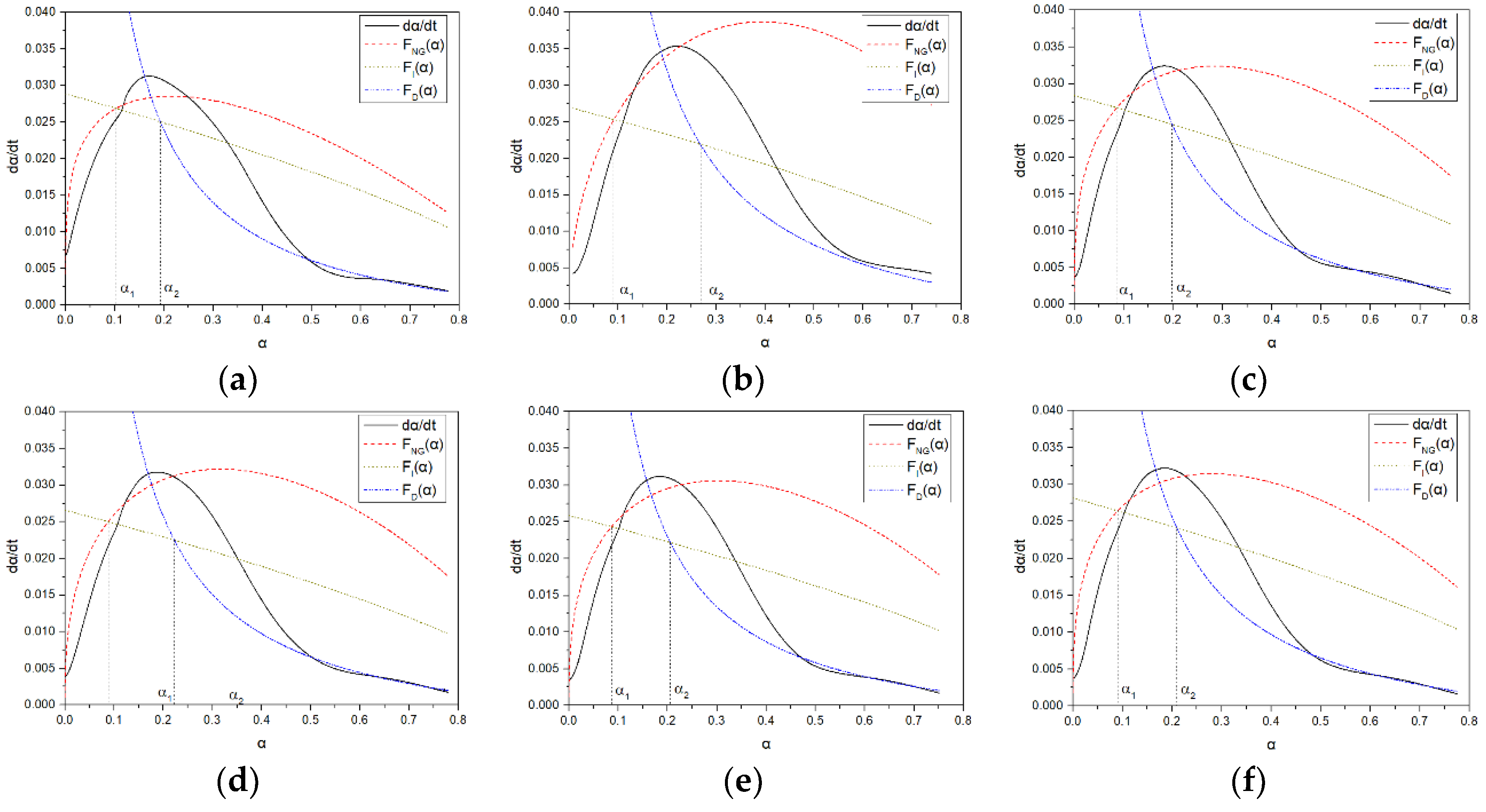
Hydration calorimetry aims to study the exothermic behavior and final state of different hydration processes but lacks the main factors causing exothermic behavior at different stages. Hydration kinetics can characterize the states and reaction processes dominated by different reaction mechanisms during hydration. The Krstulovic–Dabic model for hydration kinetics is currently widely recognized and extensively used in the study of composite cementitious systems [
17,
18,
19]. It considers three stages of the hydration process in cementitious materials: nucleation and crystal growth (NG), interfacial reaction (I), and diffusion (D). It is generally believed that during the NG and I stages, the hydration products continue to grow and are in an accelerated stage. Krstulovic and Dabic characterized these three hydration processes using the JMAK model [
14], the Brown model, and the Jander model, respectively. The kinetic equations governing these three processes are as follow:
Hydration kinetics models have been applied in many aspects, such as studying the variation of n value and apparent activation energy during crystal growth in multiphase systems and investigating the dominant reactions in the hydration process of multiphase systems at different temperatures [
16]. In blended cements with slag content exceeding 50%, interfacial reactions are the main reactions. Typically, hydration kinetics examine reaction parameters at different stages. The main parameters for the NG process are
KNG and n, where
KNG represents the rate constants for nucleation and crystal growth in the NG process, reflecting the speed of the hydration reaction. A larger
KNG indicates easier reaction occurrence, leading to a significant increase in the nucleation rate of calcium silicate hydrate (C-S-H) and calcium hydroxide (CH) crystals. When the concentration of Ca
2+ in the solution increases, C-S-H is more likely to reach a saturated state. Additionally, the NG process is influenced by the reaction order, where the magnitude of the reaction order represents the extent to which concentration affects the reaction rate. Therefore, the fitting curve of the NG process is a parabola.
Existing research on PCE has mainly focused on the effects of side chain structures, such as side chain length and side chain density, on performance, while there is limited research on the influence of main chain structures. In this study, a series of PCEs with different main chain molecular structures were synthesized and prepared. Mathematical models, including the isothermal adsorption model, adsorption kinetics, rheological model, and hydration kinetics were used to investigate the influence of main-chain molecular structure on the performance of PCEs. Furthermore, the correlation between different main chain molecular structures of PCEs and the performance of cement slurries was systematically elucidated, along with the mechanism of “adsorption-dispersion-hydration” interaction.