Sorption Capacity of Polydimethylsiloxane Foams Filled with Thermal-Treated Bentonite—Polydimethylsiloxane Composite Foams for Oil Spill Remediation
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
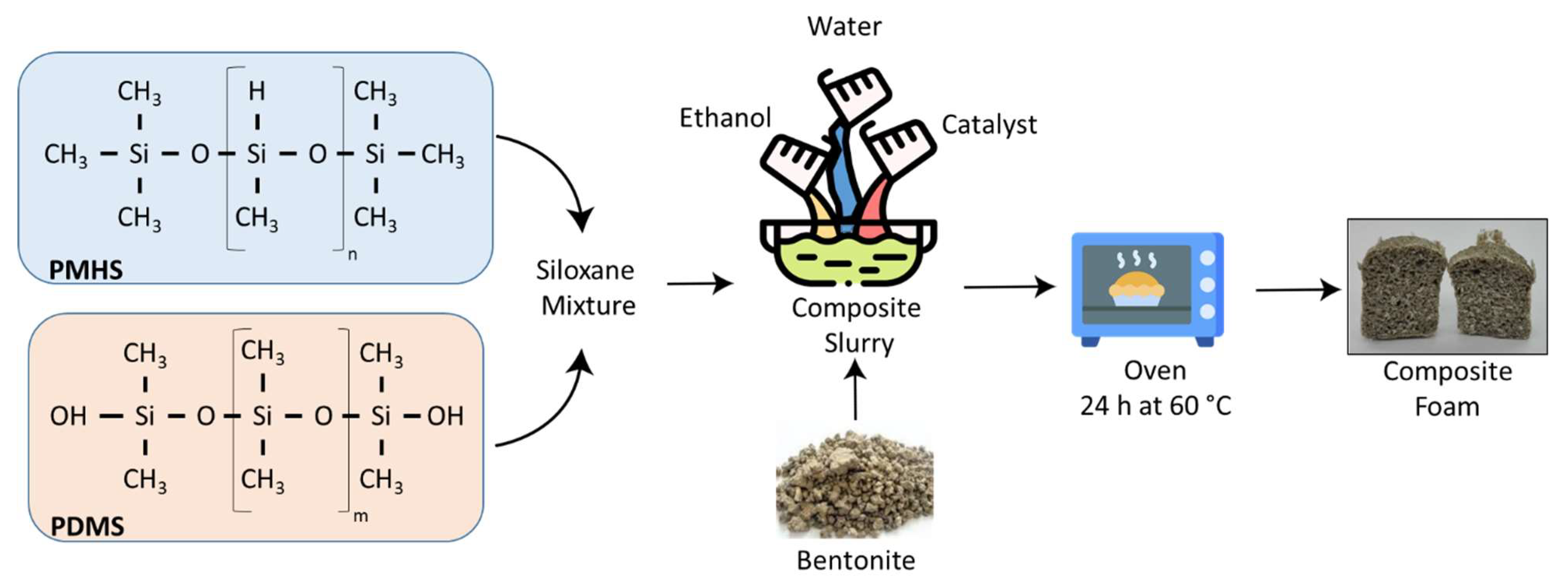
2.2. Synthesis of the Composite Foams
2.3. Sorption Capacity Tests
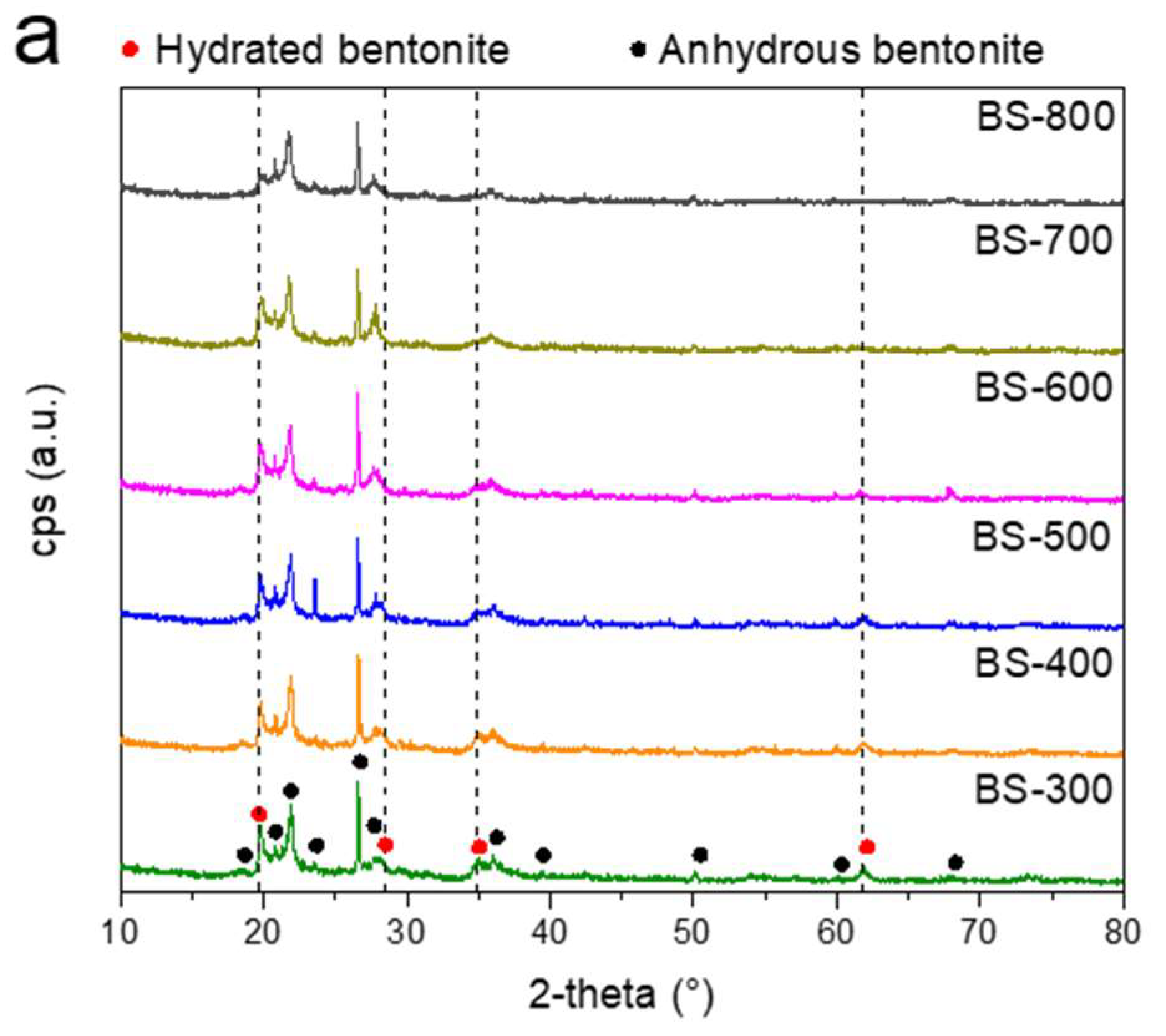
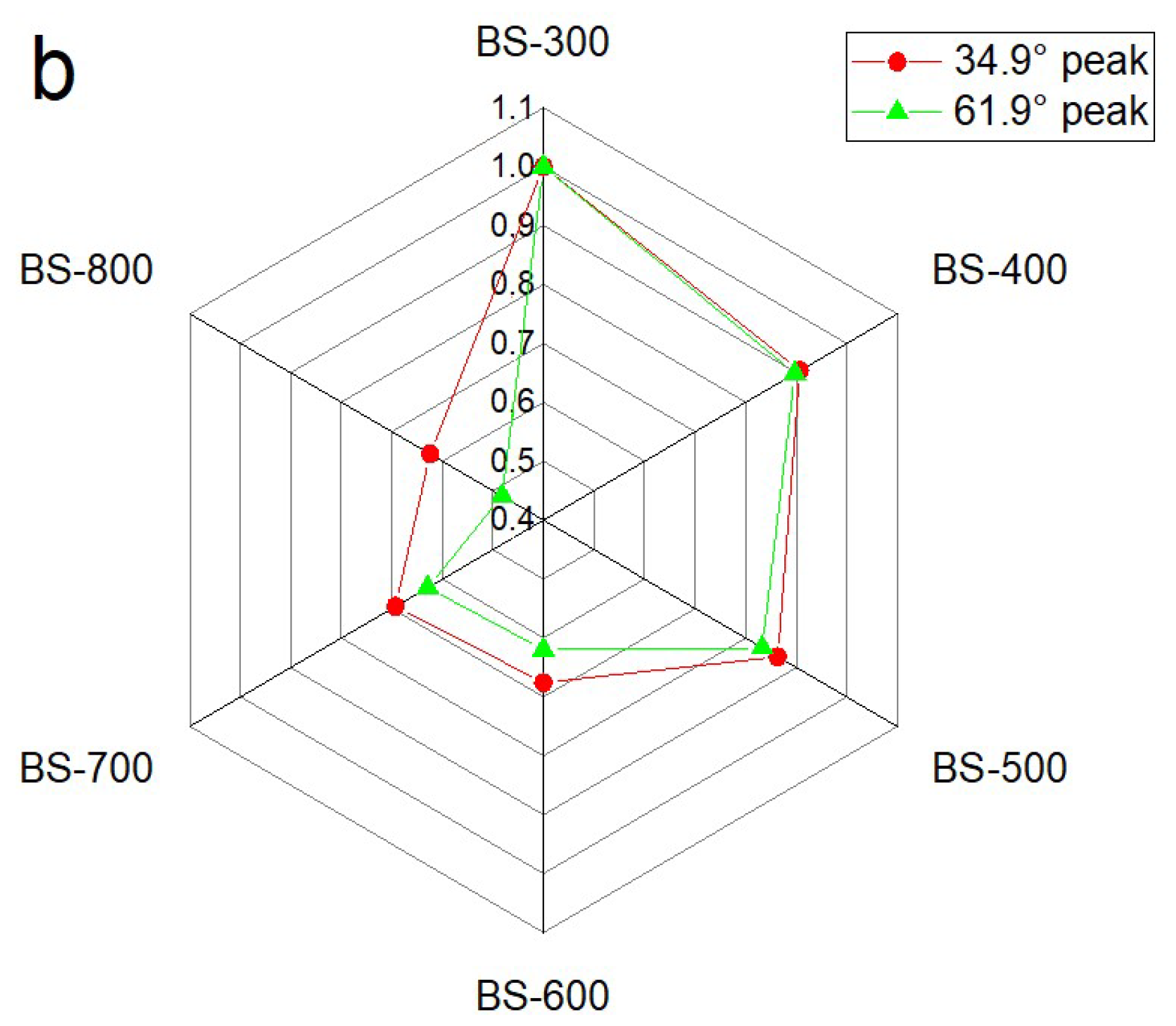
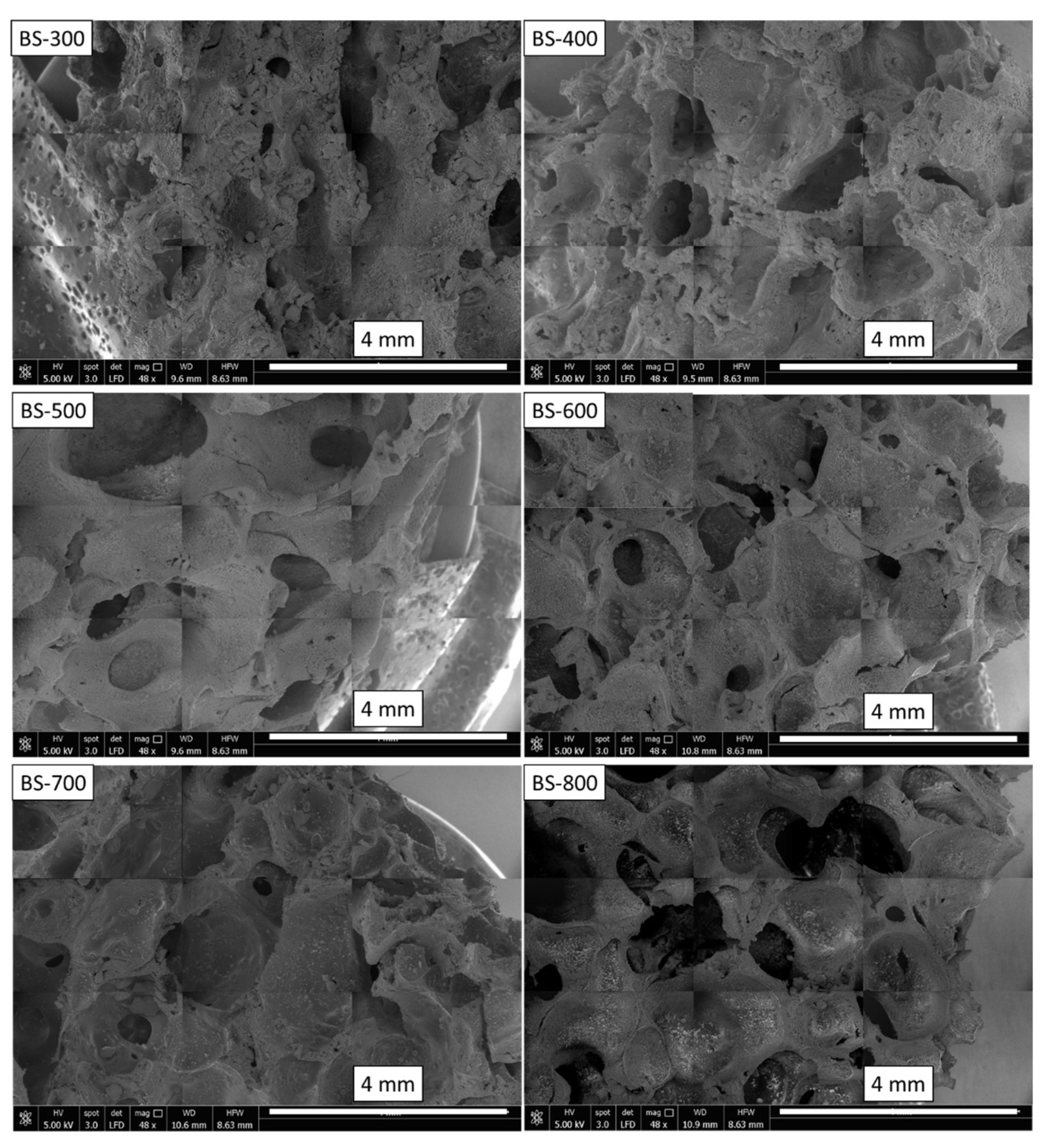
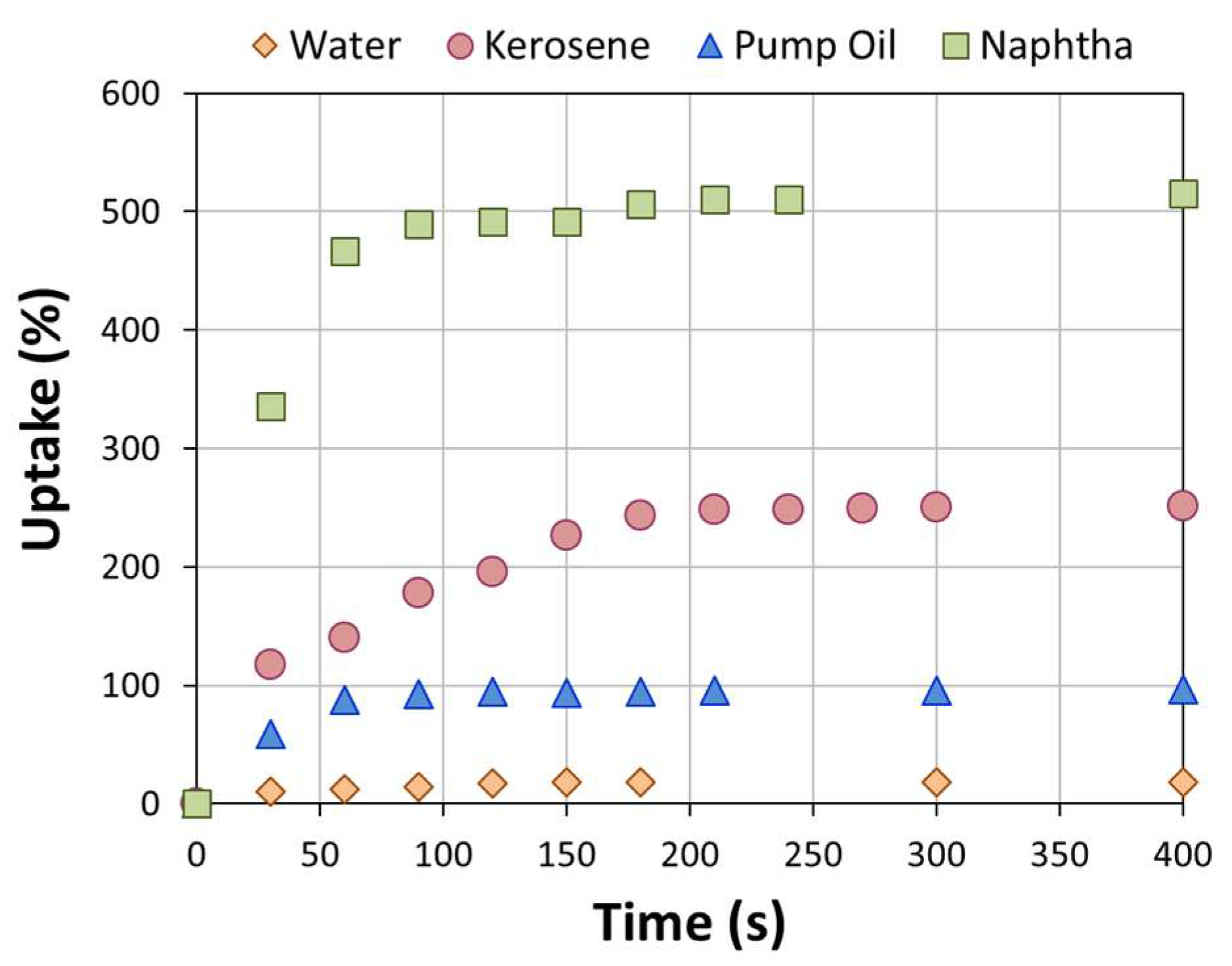
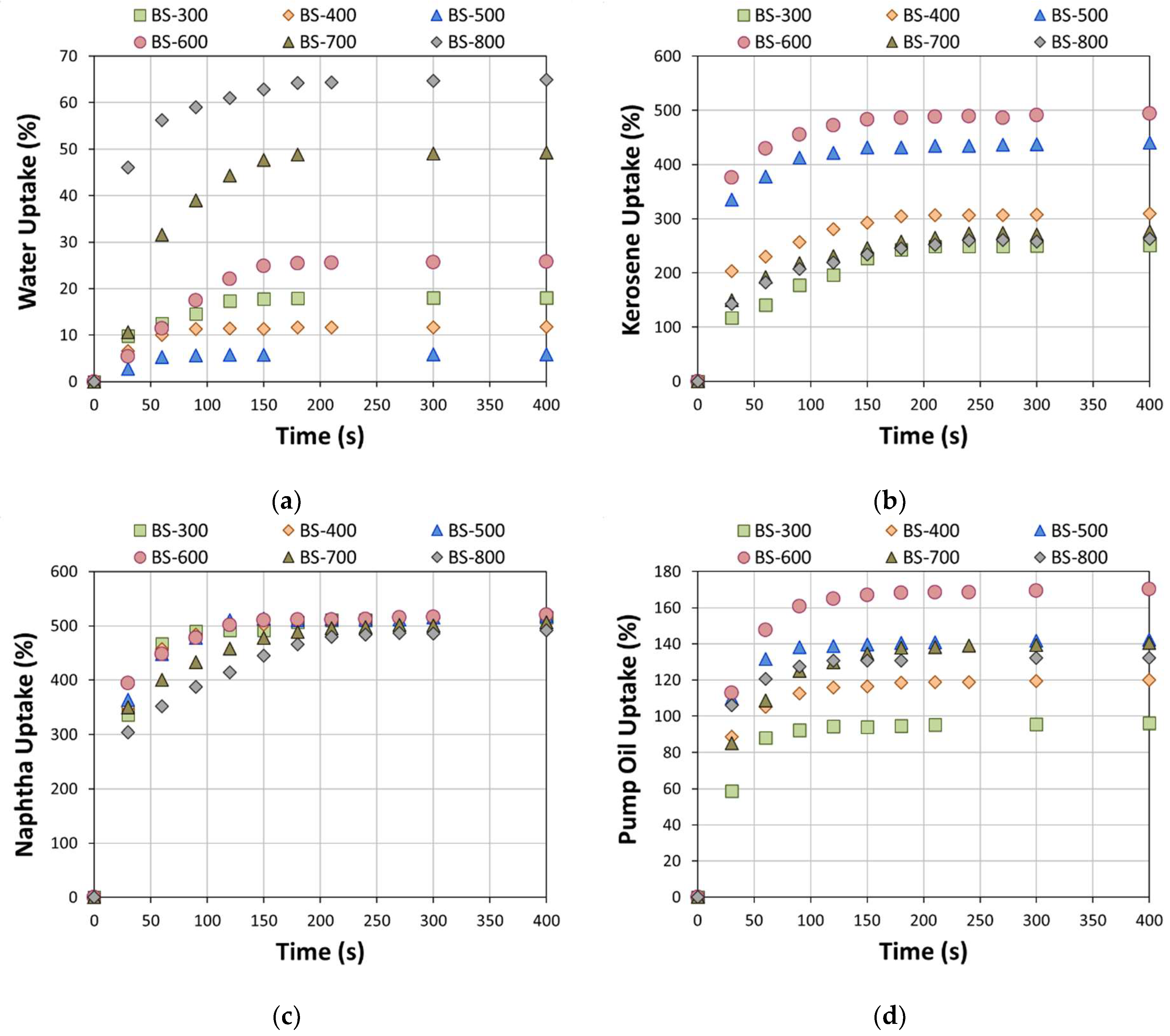
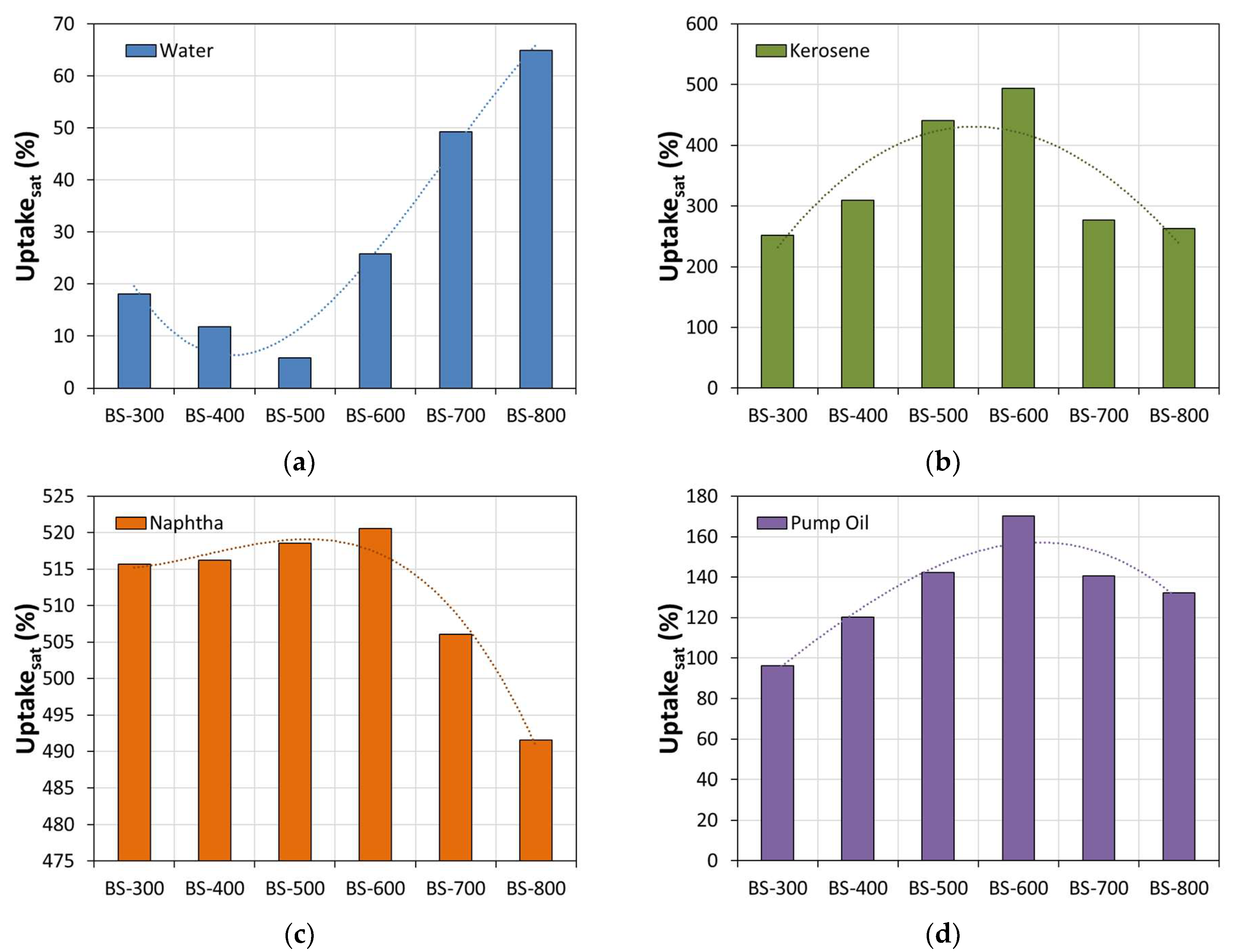
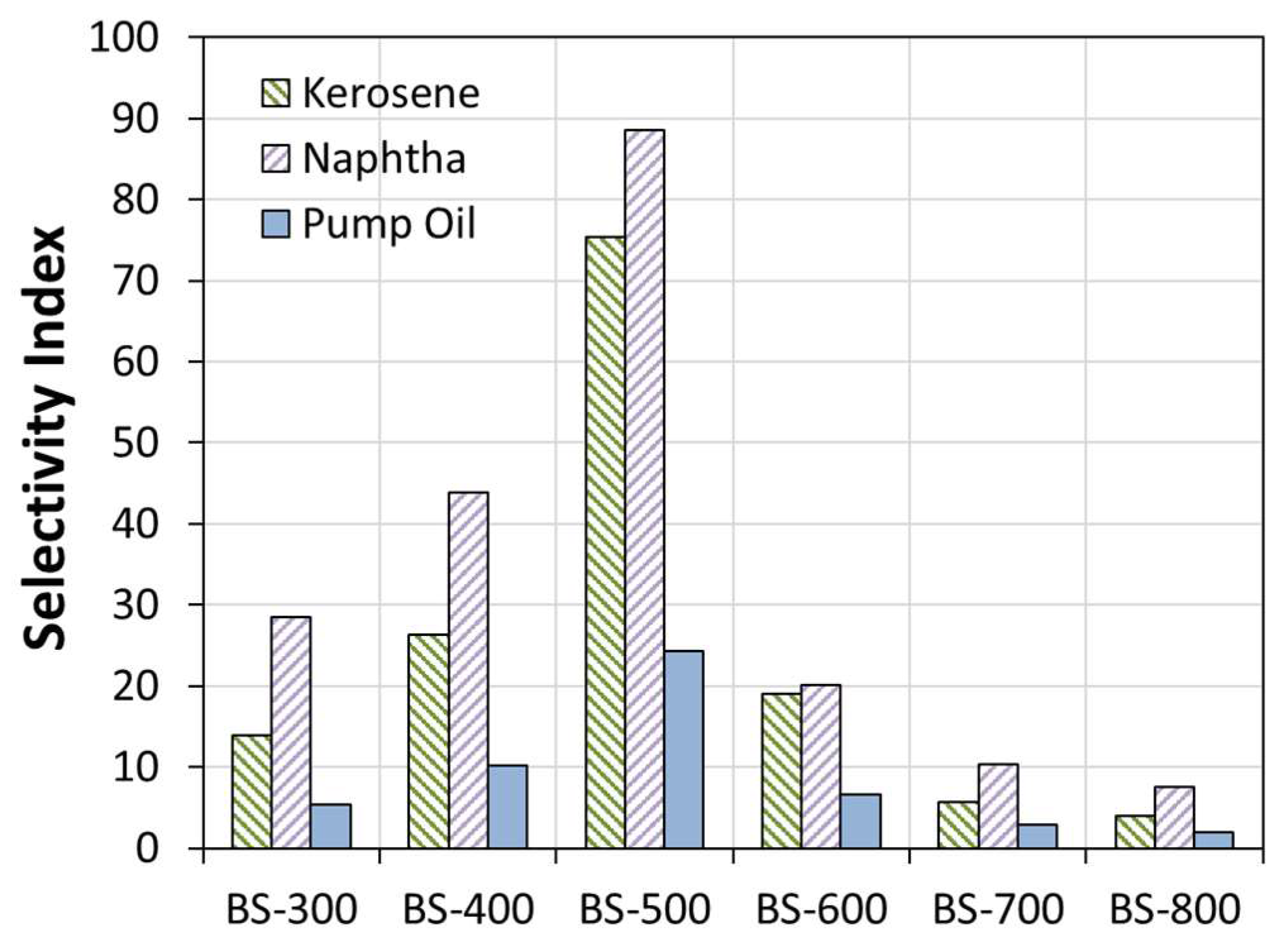
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okeke, E.S.; Okoye, C.O.; Chidike Ezeorba, T.P.; Mao, G.; Chen, Y.; Xu, H.; Song, C.; Feng, W.; Wu, X. Emerging Bio-Dispersant and Bioremediation Technologies as Environmentally Friendly Management Responses toward Marine Oil Spill: A Comprehensive Review. J. Environ. Manag. 2022, 322, 116123. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Bhardwaj, N.; Arya, S.K.; Khatri, M. Environmental Impacts of Oil Spills and Their Remediation by Magnetic Nanomaterials. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100305. [Google Scholar] [CrossRef]
- Cláudio, F.; Barros, D.F.; Constantino, L.; Vasconcellos, G. Removal of Petroleum Spill in Water by Chitin and Chitosan. Orbital Electron. J. Chem. 2014, 6, 2–6. [Google Scholar] [CrossRef]
- Varela, M.; Bode, A.; Lorenzo, J.; Álvarez-Ossorio, M.T.; Miranda, A.; Patrocinio, T.; Anadón, R.; Viesca, L.; Rodríguez, N.; Valdés, L.; et al. The Effect of the “Prestige” Oil Spill on the Plankton of the N–NW Spanish Coast. Mar. Pollut. Bull. 2006, 53, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakakis, E.; Hahladakis, J.; Gidarakos, E. The “Sea Diamond” Shipwreck: Environmental Impact Assessment in the Water Column and Sediments of the Wreck Area. Int. J. Environ. Sci. Technol. 2014, 11, 1421–1432. [Google Scholar] [CrossRef]
- Brody, T.M.; Di Bianca, P.; Krysa, J. Analysis of Inland Crude Oil Spill Threats, Vulnerabilities, and Emergency Response in the Midwest United States. Risk Anal. 2012, 32, 1741–1749. [Google Scholar] [CrossRef]
- Kolokoussis, P.; Karathanassi, V. Oil Spill Detection and Mapping Using Sentinel 2 Imagery. J. Mar. Sci. Eng. 2018, 6, 4. [Google Scholar] [CrossRef]
- Yang, S.-Z.; Jin, H.-J.; Wei, Z.; He, R.-X.; Ji, Y.-J.; Li, X.-M.; Yu, S.-P. Bioremediation of Oil Spills in Cold Environments: A Review. Pedosphere 2009, 19, 371–381. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V.; Elkin, A.A.; Makarov, S.O.; Cunningham, C.J.; Peshkur, T.A.; Atlas, R.M.; Philp, J.C. Oil Spill Problems and Sustainable Response Strategies through New Technologies. Environ. Sci. Process. Impacts 2015, 17, 1201–1219. [Google Scholar] [CrossRef]
- Bidgoli, H.; Mortazavi, Y.; Khodadadi, A.A. A Functionalized Nano-Structured Cellulosic Sorbent Aerogel for Oil Spill Cleanup: Synthesis and Characterization. J. Hazard. Mater. 2019, 366, 229–239. [Google Scholar] [CrossRef]
- Passow, U.; Hetland, R.D. What Happened to All of the Oil? Oceanography 2016, 29, 88–95. [Google Scholar] [CrossRef]
- Warnock, A.M.; Hagen, S.C.; Passeri, D.L. Marine Tar Residues: A Review. Water Air Soil. Pollut. 2015, 226, 68. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.A.; Reddy, G.K. Crude Oil Spill Exposure and Human Health Risks. J. Occup. Environ. Med. 2014, 56, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Fodrie, F.J.; Heck, K.L., Jr. Response of Coastal Fishes to the Gulf of Mexico Oil Disaster. PLoS ONE 2011, 6, e21609. [Google Scholar] [CrossRef] [PubMed]
- Fingas, M. Chapter 12—Physical Spill Countermeasures; Gulf Professional Publishing: Boston, MA, USA, 2011; pp. 303–337. ISBN 978-1-85617-943-0. [Google Scholar]
- Aa, I.; Op, A.; Ujj, I.; Mt, B. A Critical Review of Oil Spills in the Niger Delta Aquatic Environment: Causes, Impacts, and Bioremediation Assessment. Environ. Monit. Assess. 2022, 194, 816. [Google Scholar] [CrossRef] [PubMed]
- de Negreiros, A.C.S.V.; Lins, I.D.; Maior, C.B.S.; Moura, M.J.C. Oil Spills Characteristics, Detection, and Recovery Methods: A Systematic Risk-Based View. J. Loss Prev. Process. Ind. 2022, 80, 104912. [Google Scholar] [CrossRef]
- Mishra, S.; Chauhan, G.; Verma, S.; Singh, U. The Emergence of Nanotechnology in Mitigating Petroleum Oil Spills. Mar. Pollut. Bull. 2022, 178, 113609. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D. Remediation Technologies for Marine Oil Spills: A Critical Review and Comparative Analysis. Am. J. Environ. Sci. 2011, 7, 423–440. [Google Scholar]
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Huynh, T.T. Sorbent-Based Devices for the Removal of Spilled Oil from Water: A Review. Environ. Sci. Pollut. Res. 2021, 28, 28876–28910. [Google Scholar] [CrossRef]
- Wu, Z.; Yue, X.; Cheng, T.; Yu, J.; Yang, H. Effect of Viscosity and Interfacial Tension of Surfactant–Polymer Flooding on Oil Recovery in High-Temperature and High-Salinity Reservoirs. J. Pet. Explor. Prod. Technol. 2014, 4, 9–16. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, G.; Ge, J.; Liao, K.; Pei, H.; Jiang, P.; Li, X. Study on Organic Alkali-Surfactant-Polymer Flooding for Enhanced Ordinary Heavy Oil Recovery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 230–239. [Google Scholar] [CrossRef]
- Joye, B.S.B. Deepwater Horizon, 5 Years on. Science (1979) 2015, 349, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Kizil, S.; Bulbul Sonmez, H. Oil Loving Hydrophobic Gels Made from Glycerol Propoxylate: Efficient and Reusable Sorbents for Oil Spill Clean-Up. J. Environ. Manag. 2017, 196, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of Nanoparticles in Enhanced Oil Recovery: A Critical Review of Recent Progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Choi, S.J.; Kwon, T.H.; Im, H.; Moon, D., II; Baek, D.J.; Seol, M.L.; Duarte, J.P.; Choi, Y.K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Huang, H.-X.; Peng, X.-F.; Turng, L.-S. Superhydrophobic Graphene/Cellulose/Silica Aerogel with Hierarchical Structure as Superabsorbers for High Efficiency Selective Oil Absorption and Recovery. Ind. Eng. Chem. Res. 2018, 57, 1745–1755. [Google Scholar] [CrossRef]
- Wang, N.; Deng, Z. Synthesis of Magnetic, Durable and Superhydrophobic Carbon Sponges for Oil/Water Separation. Mater. Res. Bull. 2019, 115, 19–26. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Khaskhoussi, A.; Proverbio, E.; Milone, C. Thermo-Physical Characterization of Carbon Nanotube Composite Foam for Oil Recovery Applications. Nanomaterials 2020, 10, 86. [Google Scholar] [CrossRef]
- Haridharan, N.; Sundar, D.; Kurrupasamy, L.; Anandan, S.; Liu, C.-H.; Wu, J.J. Oil Spills Adsorption and Cleanup by Polymeric Materials: A Review. Polym. Adv. Technol. 2022, 33, 1353–1384. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Proverbio, E.; Milone, C. Sustainable Reuse of Char Waste for Oil Spill Recovery Foams. Water Air Soil. Pollut. 2020, 231, 293. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Jovanovic, V.S.; Nikolic, J.; Ciric, S.; Milone, C.; Proverbio, E. Bentonite-PDMS Composite Foams for Oil Spill Recovery: Sorption Performance and Kinetics. J. Appl. Polym. Sci. 2022, 139, e53003. [Google Scholar] [CrossRef]
- Stojiljkovic, S.; Miljkovic, V.; Nikolic, G.; Kostic, D.; Arsic, B.; Barber, J.; Savic, I.; Savic, I. The Influence of the Addition of Polymers on the Physico-Chemical Properties of Bentonite Suspensions. Sci. Sinter. 2014, 46, 65–73. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Abdul Rahim, S.H.; Proverbio, E.; Milone, C. Assessment of Sorption Kinetics of Carbon Nanotube-Based Composite Foams for Oil Recovery Application. J. Appl. Polym. Sci. 2019, 136, 47347. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Proverbio, E.; Milone, C. Synthesis of Reusable Silicone Foam Containing Carbon Nanotubes for Oil Spill Remediation. J. Appl. Polym. Sci. 2018, 135, 46067. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Synthesis of SAPO-34 Zeolite Filled Macrocellular Foams for Adsorption Heat Pump Applications: A Preliminary Study. Appl. Therm. Eng. 2017, 124, 1312–1318. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Silicone Composite Foams for Adsorption Heat Pump Applications. Sustain. Mater. Technol. 2017, 12, 27–34. [Google Scholar] [CrossRef]
- Brancato, V.; Calabrese, L.; Palomba, V.; Frazzica, A.; Fullana-Puig, M.; Solé, A.; Cabeza, L.F. MgSO4·7H2O Filled Macro Cellular Foams: An Innovative Composite Sorbent for Thermo-Chemical Energy Storage Applications for Solar Buildings. Sol. Energy 2018, 173, 1278–1286. [Google Scholar] [CrossRef]
- Calabrese, L.; Piperopoulos, E.; Jovanovic, V.S.; Mitic, V.; Mitic, M.; Milone, C.; Proverbio, E. Oil Spill Remediation: Selectivity, Sorption, and Squeezing Capacity of Silicone Composite Foams Filled with Clinoptilolite. J. Appl. Polym. Sci. 2022, 139, e52637. [Google Scholar] [CrossRef]
- Khandelwal, S.; Rhee, K.Y. Evaluation of Pozzolanic Activity, Heterogeneous Nucleation, and Microstructure of Cement Composites with Modified Bentonite Clays. Constr. Build. Mater. 2022, 323, 126617. [Google Scholar] [CrossRef]
- Sarikaya, Y.; Önal, M.; Baran, B.; Alemdaroğlu, T. The Effect of Thermal Treatment on Some of the Physicochemical Properties of a Bentonite. Clays Clay Miner. 2000, 48, 557–562. [Google Scholar] [CrossRef]
- Marszałek, A.; Kamińska, G.; Abdel Salam, N.F. Simultaneous Adsorption of Organic and Inorganic Micropollutants from Rainwater by Bentonite and Bentonite-carbon Nanotubes Composites. J. Water Process. Eng. 2022, 46, 102550. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Freni, A.; Proverbio, E. Morphological and Functional Aspects of Zeolite Filled Siloxane Composite Foams. J. Appl. Polym. Sci. 2018, 135, 45683. [Google Scholar] [CrossRef]
- Brancato, V.; Piperopoulos, E.; Mastronardo, E.; Calabrese, L.; Milone, C.; Proverbio, E. Synthesis and Characterization of Graphite Composite Foams for Oil Spill Recovery Application. J. Compos. Sci. 2020, 4, 154. [Google Scholar] [CrossRef]
- Tong, L.; Liang, T.; Tian, Y.; Zhang, Q.; Pan, Y. Research Progress on Treatment of Mine Wastewater by Bentonite Composite. Arab. J. Geosci. 2022, 15, 681. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of Organic Pollutants by Natural and Modified Clays: A Comprehensive Review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Tadesse, S.H. Application of Ethiopian Bentonite for Water Treatment Containing Zinc. Emerg. Contam. 2022, 8, 113–122. [Google Scholar] [CrossRef]
- Kavil, J.; Anjana, P.M.; Roshni, C.P.; Periyat, P.; Raj, K.G.; Rakhi, R.B. Multifunctional Nanohybrid Material from Discarded Razor Blades as Cost-Effective Supercapacitor Electrodes and Oil-Spill Cleaners. Appl. Surf. Sci. 2019, 487, 109–115. [Google Scholar] [CrossRef]
- Songsaeng, S.; Thamyongkit, P.; Poompradub, S. Natural Rubber/Reduced-Graphene Oxide Composite Materials: Morphological and Oil Adsorption Properties for Treatment of Oil Spills. J. Adv. Res. 2019, 20, 79–89. [Google Scholar] [CrossRef]
- Gan, F.; Zhou, J.; Wang, H.; Du, C.; Chen, X. Removal of Phosphate from Aqueous Solution by Thermally Treated Natural Palygorskite. Water Res. 2009, 43, 2907–2915. [Google Scholar] [CrossRef]
- Yin, H.; Yan, X.; Gu, X. Evaluation of Thermally-Modified Calcium-Rich Attapulgite as a Low-Cost Substrate for Rapid Phosphorus Removal in Constructed Wetlands. Water Res. 2017, 115, 329–338. [Google Scholar] [CrossRef]
- Chen, X.; Wu, L.; Liu, F.; Luo, P.; Zhuang, X.; Wu, J.; Zhu, Z.; Xu, S.; Xie, G. Performance and Mechanisms of Thermally Treated Bentonite for Enhanced Phosphate Removal from Wastewater. Environ. Sci. Pollut. Res. 2018, 25, 15980–15989. [Google Scholar] [CrossRef] [PubMed]
- Martinez Stagnaro, S.Y.; Volzone, C.; Rueda, M.L. Influence of Thermal Treatment on Bentonite Used as Adsorbent for Cd, Pb, Zn Retention from Mono-Solute and Poly-Solute Aqueous Solutions. Mater. Res. 2012, 15, 549–553. [Google Scholar] [CrossRef]
- Önal, M.; Sarıkaya, Y. Thermal Behavior of a Bentonite. J. Therm. Anal. Calorim. 2007, 90, 167–172. [Google Scholar] [CrossRef]
- Sarı Yılmaz, M.; Kalpaklı, Y.; Pişkin, S. Thermal Behavior and Dehydroxylation Kinetics of Naturally Occurring Sepiolite and Bentonite. J. Therm. Anal. Calorim. 2013, 114, 1191–1199. [Google Scholar] [CrossRef]
| Compound | Type | wt.% |
|---|---|---|
| PDMS | Siloxane | 30.8% |
| PMHS | Siloxane | 15.4% |
| Water | Solvent | 6.2% |
| Ethanol | Solvent | 9.2% |
| Sn(II) | Catalyst | 7.6% |
| Bentonite | Filler | 30.8% |
| Bentonite vs. foam [wt.%] | 40.0% | |
| APS [mm] | ρa [kg/m3] | |
|---|---|---|
| BS-300 | 1.02 | 660.31 |
| BS-400 | 1.36 | 539.52 |
| BS-500 | 1.37 | 589.57 |
| BS-600 | 1.40 | 565.35 |
| BS-700 | 1.83 | 414.01 |
| BS-800 | 2.18 | 361.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, L.; Piperopoulos, E.; Stankov Jovanović, V.; Nikolić, J.; Ćirić, S.; Milone, C.; Proverbio, E. Sorption Capacity of Polydimethylsiloxane Foams Filled with Thermal-Treated Bentonite—Polydimethylsiloxane Composite Foams for Oil Spill Remediation. Materials 2023, 16, 4818. https://doi.org/10.3390/ma16134818
Calabrese L, Piperopoulos E, Stankov Jovanović V, Nikolić J, Ćirić S, Milone C, Proverbio E. Sorption Capacity of Polydimethylsiloxane Foams Filled with Thermal-Treated Bentonite—Polydimethylsiloxane Composite Foams for Oil Spill Remediation. Materials. 2023; 16(13):4818. https://doi.org/10.3390/ma16134818
Chicago/Turabian StyleCalabrese, Luigi, Elpida Piperopoulos, Vesna Stankov Jovanović, Jelena Nikolić, Slobodan Ćirić, Candida Milone, and Edoardo Proverbio. 2023. "Sorption Capacity of Polydimethylsiloxane Foams Filled with Thermal-Treated Bentonite—Polydimethylsiloxane Composite Foams for Oil Spill Remediation" Materials 16, no. 13: 4818. https://doi.org/10.3390/ma16134818
APA StyleCalabrese, L., Piperopoulos, E., Stankov Jovanović, V., Nikolić, J., Ćirić, S., Milone, C., & Proverbio, E. (2023). Sorption Capacity of Polydimethylsiloxane Foams Filled with Thermal-Treated Bentonite—Polydimethylsiloxane Composite Foams for Oil Spill Remediation. Materials, 16(13), 4818. https://doi.org/10.3390/ma16134818










