A Novel One-Step Reactive Extrusion Process for High-Performance Rigid Crosslinked PVC Composite Fabrication Using Triazine Crosslinking Agent@Melamine-Formaldehyde Microcapsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
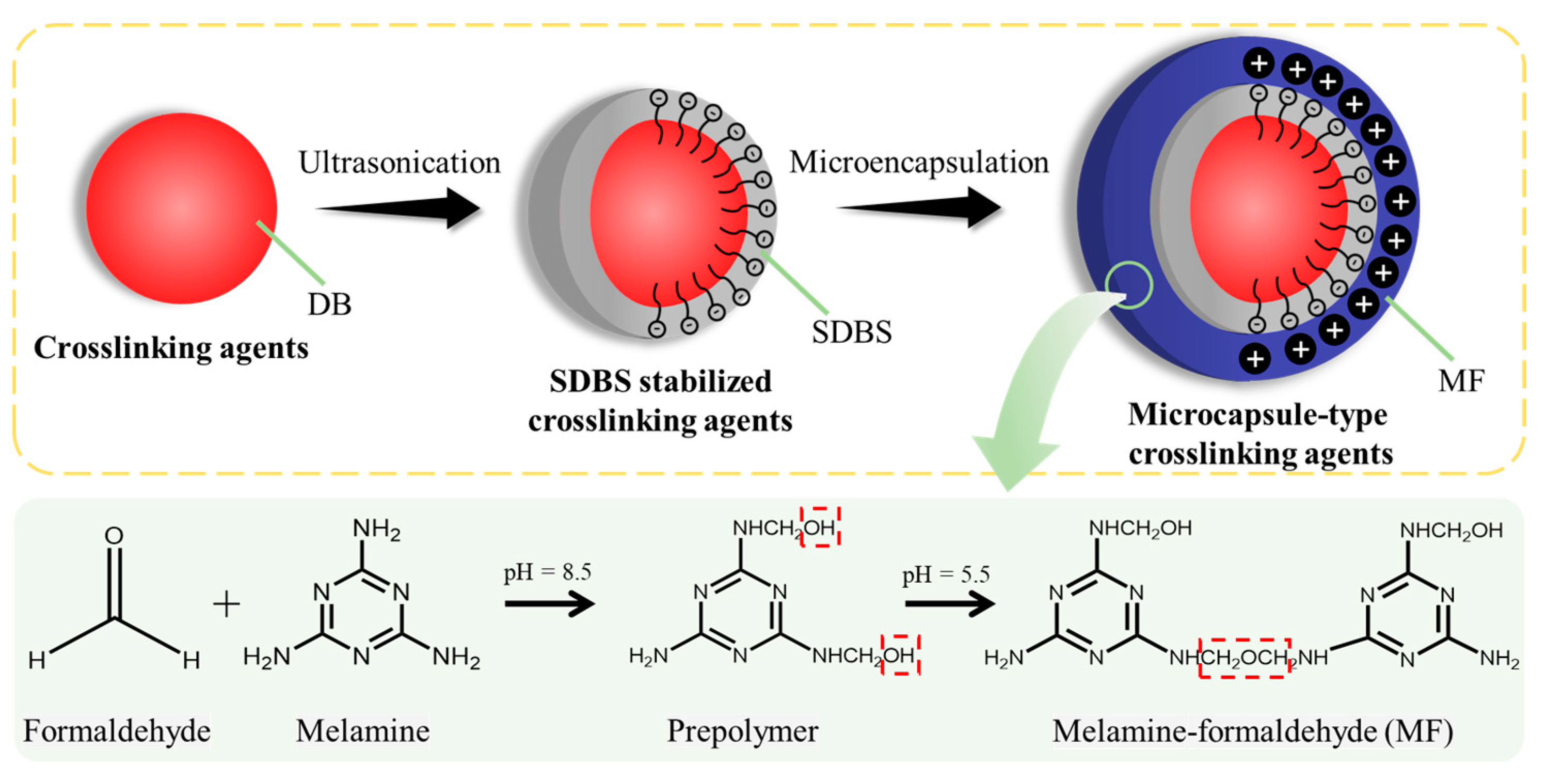
2.2. Preparation of DB@MF and PVC/DB@MF Composites
2.3. Instrumentation
2.4. Core Content of DB@MF
3. Results
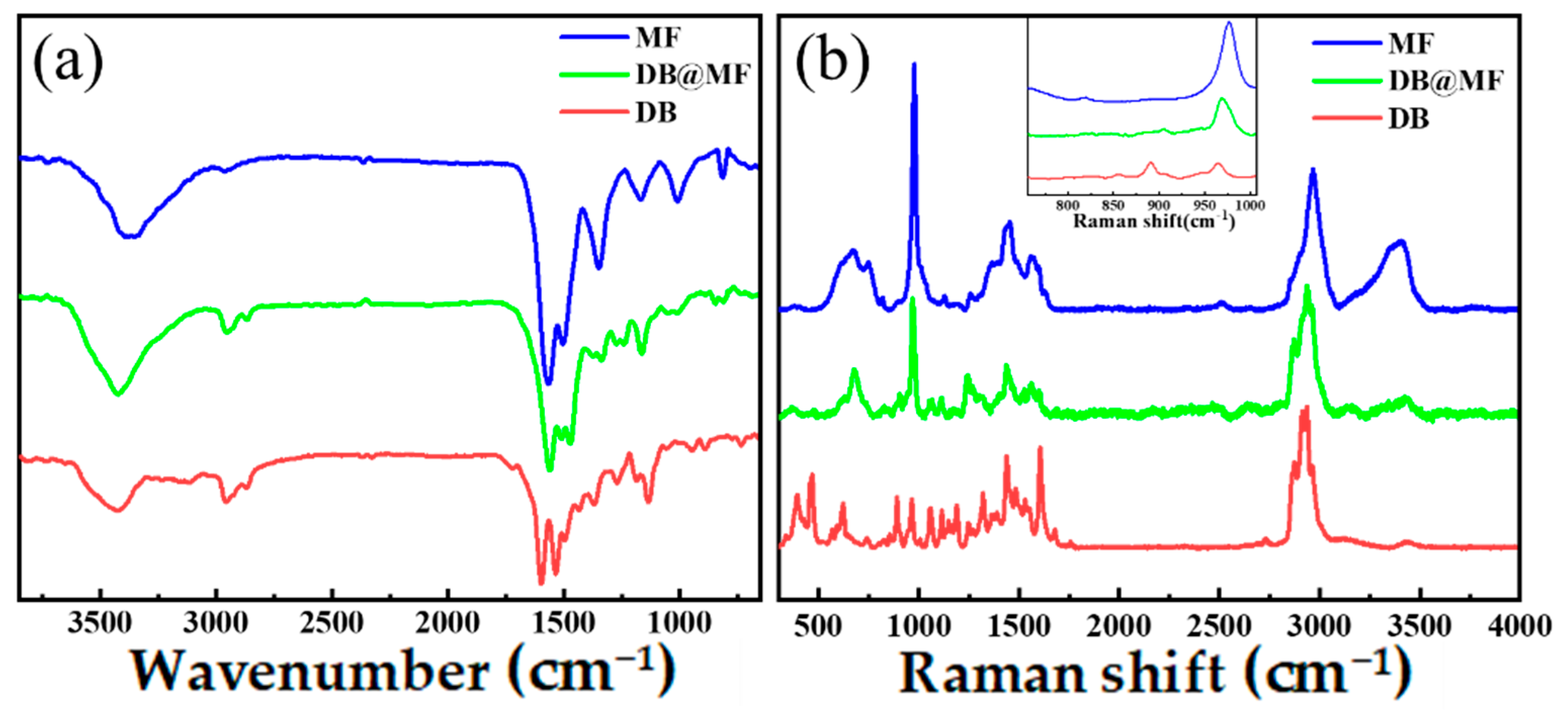
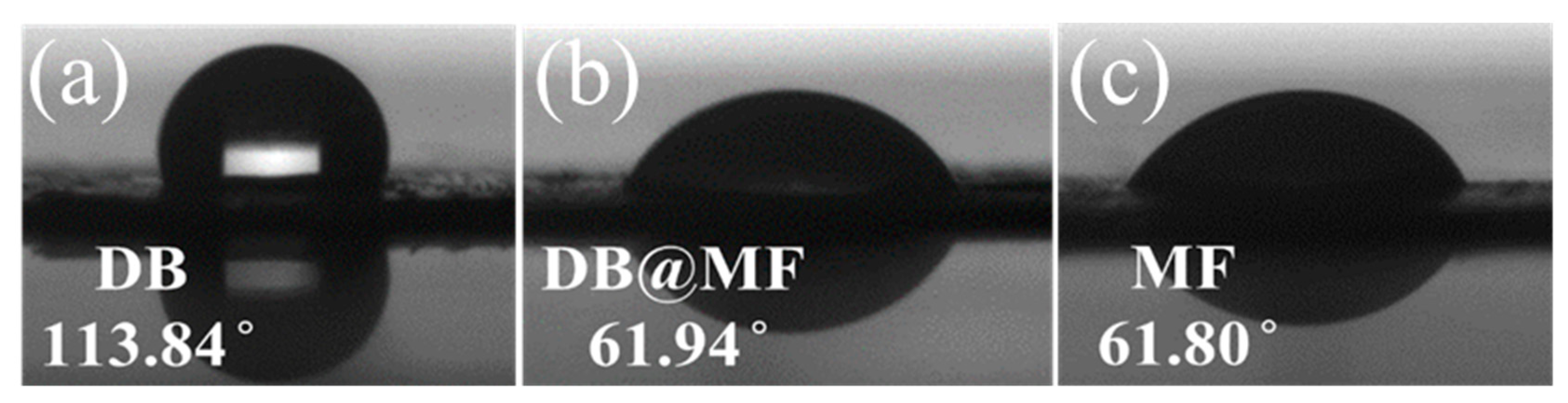
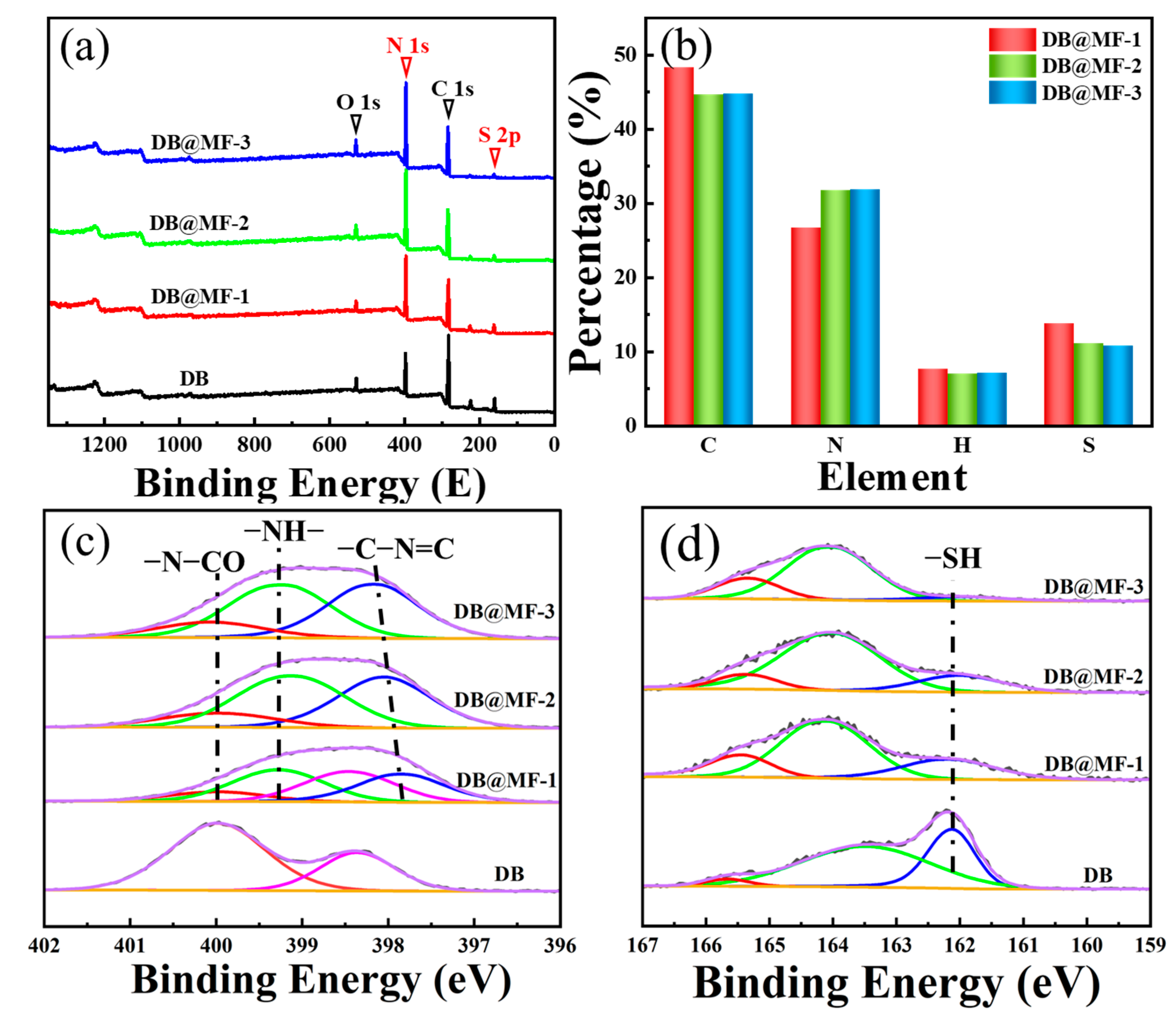
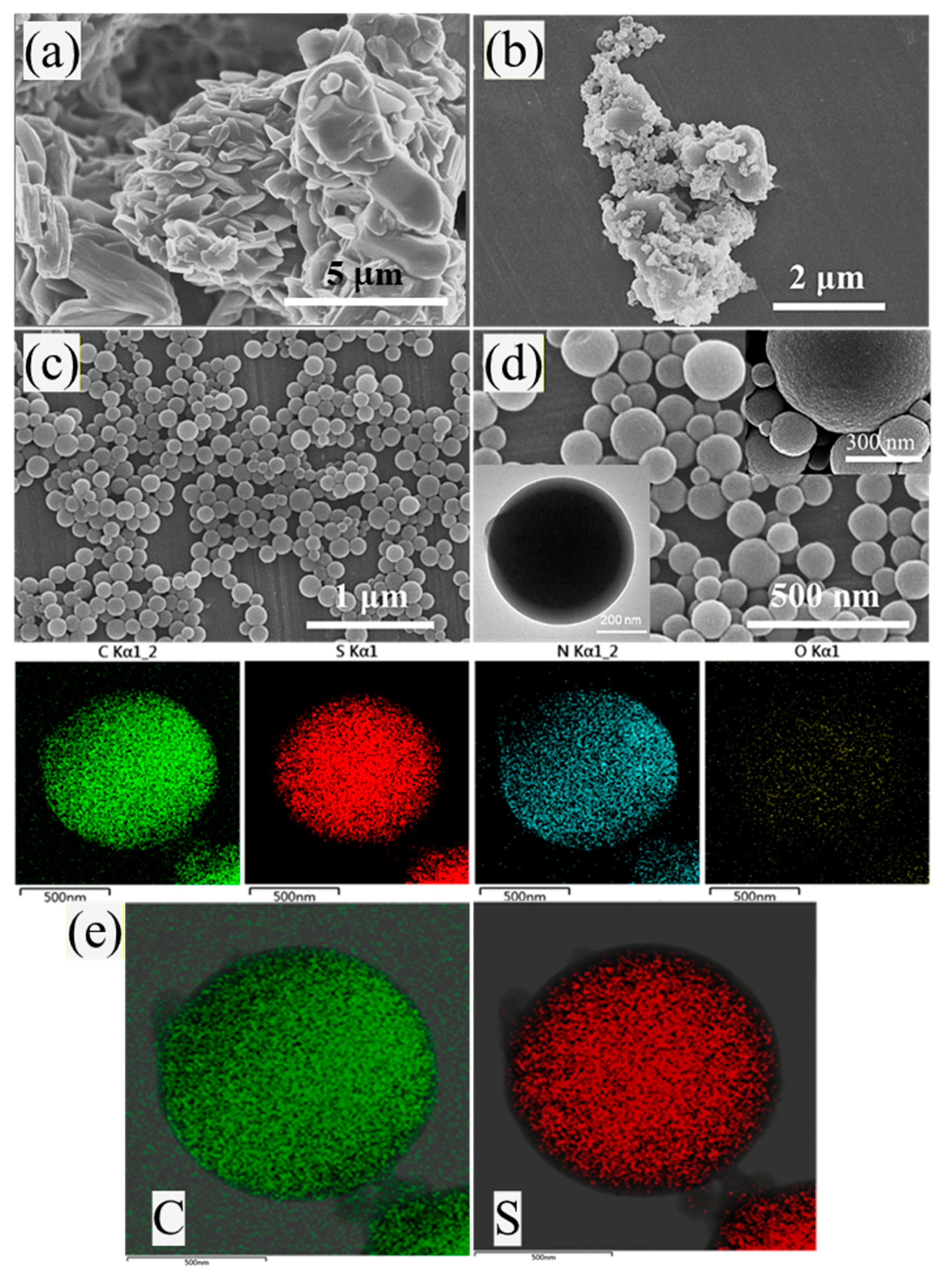
3.1. Characterization of Microcapsules
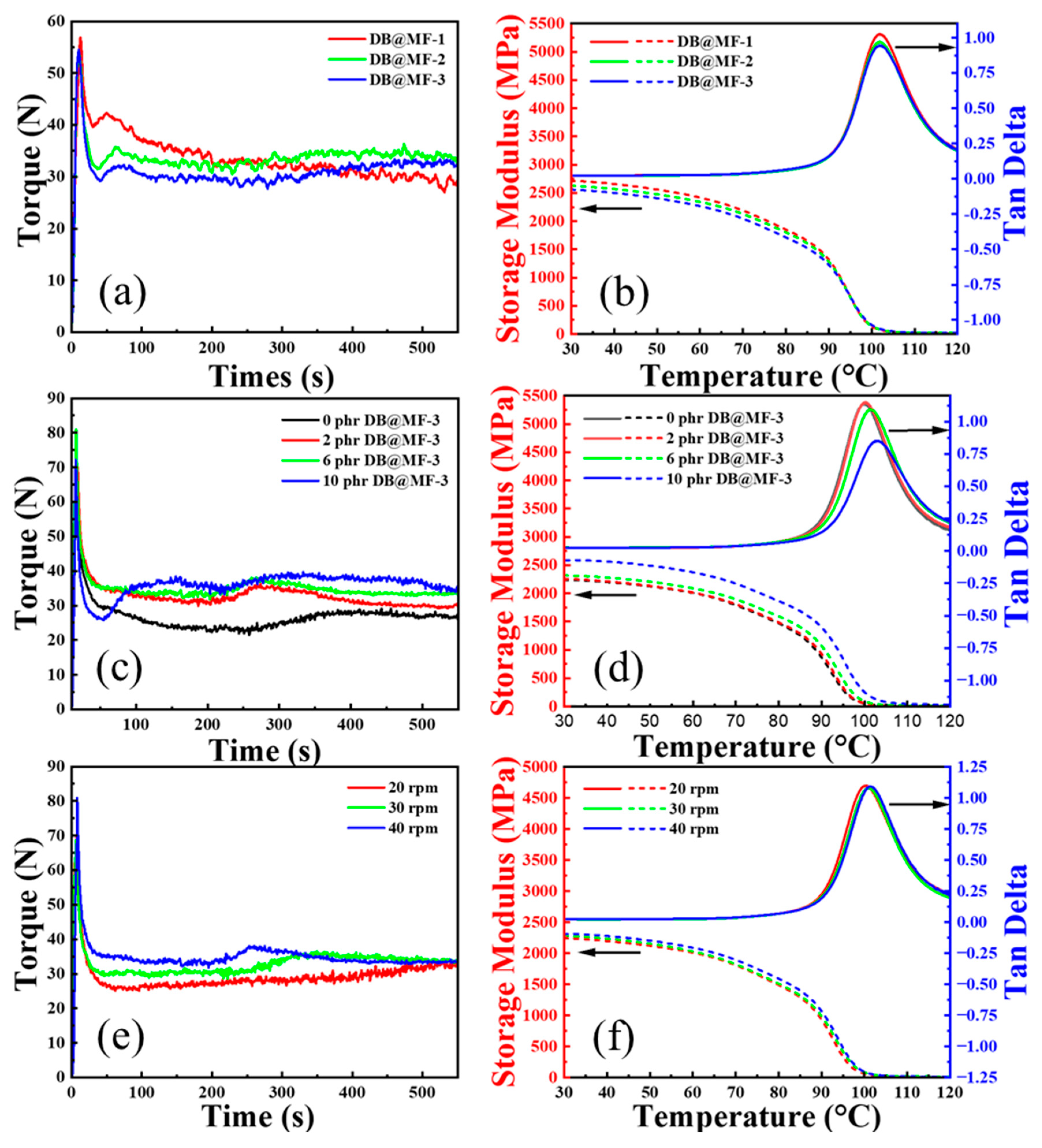
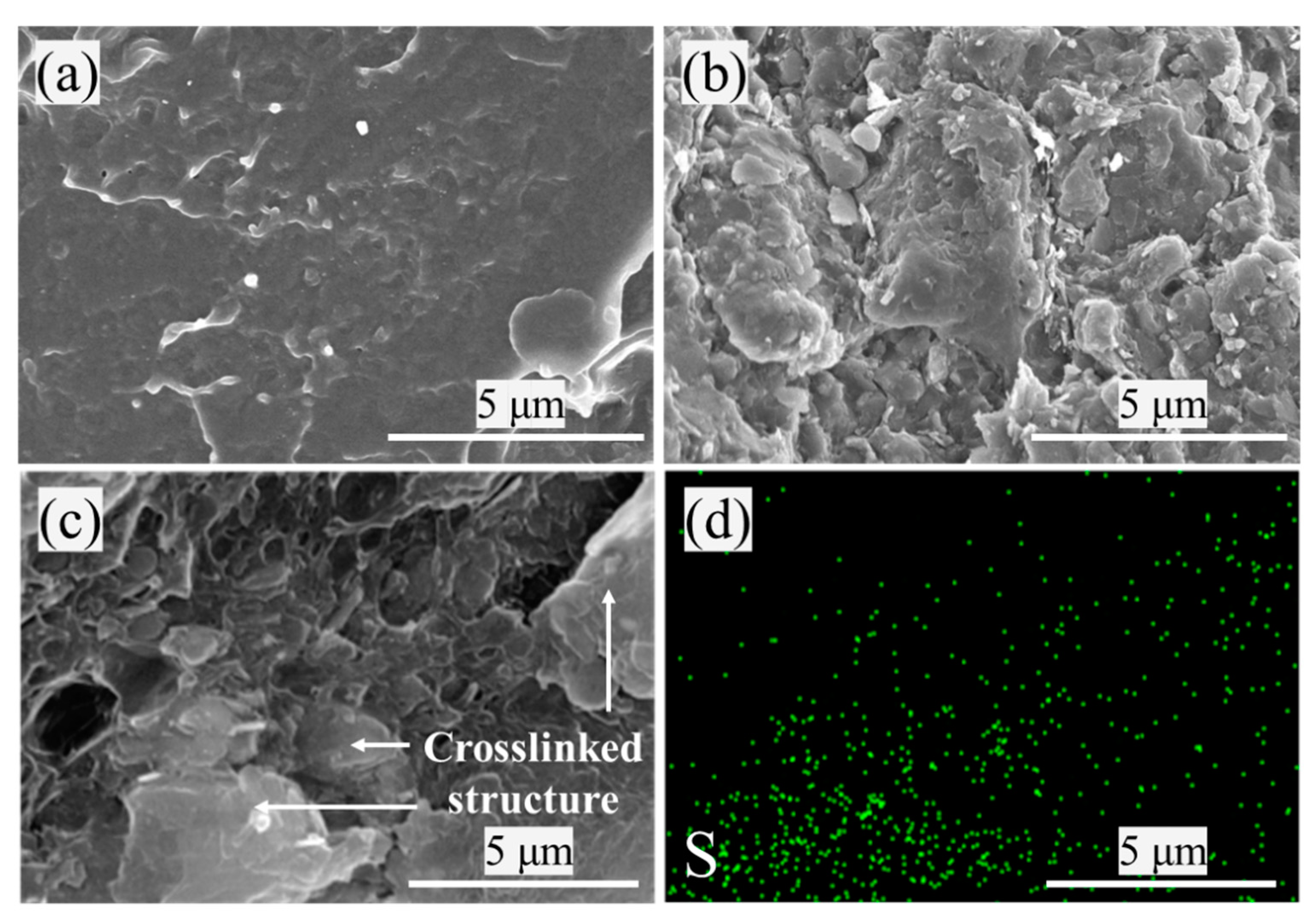
3.2. Characterization of PVC Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.C.; Lv, L.D.; Wang, W.S.; Zhang, J.X.; Lin, J.; Zhou, J.Y.; Dong, M.Y.; Gan, Y.F.; Seok, I.; Guo, Z.H. Effects of chlorinated polyethylene and antimony trioxide on recycled polyvinyl chloride/acryl-butadiene-styrene blends: Flame retardancy and mechanical properties. Polymer 2020, 190, 122198. [Google Scholar] [CrossRef]
- Klapiszewski, L.; Pawlak, F.; Tomaszewska, J.; Jesionowski, T. Preparation and characterization of novel PVC/silica-lignin composites. Polymers 2015, 7, 1767–1788. [Google Scholar] [CrossRef]
- Wang, K.T.; He, Y.; Song, X.L.; Cui, X.M. Effects of the metakaolin-based geopolymer on high-temperature performances of geopolymer/PVC composite materials. Appl. Clay Sci. 2015, 114, 586–592. [Google Scholar] [CrossRef]
- Tillet, G.; Boutevin, B.; Ameduri, B. Chemical reactions of polymer crosslinking and post-crosslinking at room and medium temperature. Prog. Polym. Sci. 2011, 36, 191–217. [Google Scholar] [CrossRef]
- Bednarek, M.; Borska, K.; Kubisa, P. New polylactide-based materials by chemical crosslinking of PLA. Polym. Rev. 2020, 61, 493–519. [Google Scholar] [CrossRef]
- Romero Tendero, P.M.; Jimenez, A.; Greco, A.; Maffezzoli, A. Viscoelastic and thermal characterization of crosslinked PVC. Eur. Polym. J. 2006, 42, 961–969. [Google Scholar] [CrossRef]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable crosslinks in polymeric materials: Resolving the intersection of thermoplastics and thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef]
- Lewandowski, A.; Wilczynski, K. Modeling of twin screw extrusion of polymeric materials. Polymers 2022, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Sauceau, M.; Fages, J.; Common, A.; Nikitine, C.; Rodier, E. New challenges in polymer foaming: A review of extrusion processes assisted by supercritical carbon dioxide. Prog. Polym. Sci. 2011, 36, 749–766. [Google Scholar] [CrossRef]
- Vlachopoulos, J.; Strutt, D. Polymer processing. Mater. Sci. Technol. 2013, 19, 1161–1169. [Google Scholar] [CrossRef]
- Garcia-Quesada, J.C.; Marcilla, A.; Gilbert, M. Thermal degradation of silane crosslinked unplasticized PVC. J. Anal. Appl. Pyrolysis 2001, 60, 159–177. [Google Scholar] [CrossRef]
- Zhang, J.; Coulston, R.J.; Jones, S.T.; Geng, J.; Scherman, O.A.; Abell, C. One-step fabrication of supramolecular microcapsules from microfluidic droplets. Science 2012, 335, 690–694. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Wang, B.; Xie, S.; Li, L.; Lu, H.; Jin, M.; Wang, X.; Zhou, G.; Shui, L. Microfluidic-assisted fabrication of monodisperse core-shell microcapsules for pressure-sensitive adhesive with enhanced performance. Nanomaterials 2020, 10, 274. [Google Scholar] [CrossRef]
- Luo, J.; Gao, Y.; Liu, Y.; Du, J.; Zhang, D.X.; Cao, H.; Jing, T.; Li, B.X.; Liu, F. Using a reactive emulsifier to construct simple and convenient nanocapsules loaded with lambda-cyhalothrin to achieve efficient foliar delivery and insecticidal synergies. Nanoscale 2021, 13, 15647–15658. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Lee, M.W.; Yarin, A.L.; Yoon, S.S. A review on corrosion-protective extrinsic self-healing: Comparison of microcapsule-based systems and those based on core-shell vascular networks. Chem. Eng. J. 2018, 344, 206–220. [Google Scholar] [CrossRef]
- Zhang, W.X.; Abbaspourrad, A.; Chen, D.; Campbell, E.; Zhao, H.; Li, Y.W.; Li, Q.N.; Weitz, D.A. Osmotic pressure triggered rapid release of encapsulated enzymes with enhanced activity. Adv. Funct. Mater. 2017, 27, 1700975. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Adhikari, B.; Wang, M.Q. Microencapsulation of Sichuan pepper essential oil in soybean protein isolate-Sichuan pepper seed soluble dietary fiber complex coacervates. Food Hydrocoll. 2022, 125, 107421. [Google Scholar] [CrossRef]
- Brossault, D.F.F.; McCoy, T.M.; Routh, A.F. Preparation of multicore colloidosomes: Nanoparticle-assembled capsules with adjustable size, internal structure, and functionalities for oil encapsulation. ACS Appl. Mater. Interfaces 2021, 13, 51495–51503. [Google Scholar] [CrossRef]
- Zhao, H.; Fei, X.; Cao, L.; Zhang, B.; Liu, X. Relation between the particle size and release characteristics of aromatic melamine microcapsules in functional textile applications. RSC Adv. 2019, 9, 25225–25231. [Google Scholar] [CrossRef]
- Feng, L.; Dong, S.; Zhou, H.; Yang, L.; Yuan, F.; Yang, Y.; Lei, J.; Bao, L.; Bian, L.; Wang, J. n-Dodecanol nanocapsules with supramolecular lock shell layer for thermal energy storage. Chem. Eng. J. 2020, 389, 124483. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Xue, R.; Fan, J.; Yu, H.; Guan, J.; Wang, H.; Li, M.; Yu, W.; Xie, Z.; et al. All-in-one theranostic platform based on hollow microcapsules for intragastric-targeting antiulcer drug delivery, CT imaging, and synergistically healing gastric ulcer. Small 2022, 18, 2104660. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, W.; Fan, W.; Zhang, X.; Song, L.; Xiong, C.; Gao, X.; Liu, X. Accelerated self-healing performance of magnetic gradient coating. Chem. Eng. J. 2018, 332, 658–670. [Google Scholar] [CrossRef]
- Allahbakhsh, A. PVC/rice straw/SDBS-modified graphene oxide sustainable Nanocomposites: Melt mixing process and electrical insulation characteristics. Compos. Part A Appl. Sci. Manuf. 2020, 134, 105902. [Google Scholar] [CrossRef]
- Barnard, J.L.; Robertson, D.D.; van Reenen, A.J. Tracking lubricants during single screw extrusion of uPVC. Polym. Test. 2020, 87, 106523. [Google Scholar] [CrossRef]
- Herrmann, A. Controlled release of volatiles under mild reaction conditions: From nature to everyday products. Angew. Chem. Int. Ed. 2007, 46, 5836–5863. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Chen, X.; Zhang, Y.; Li, A.; Wang, G. Advanced multifunctional composite phase change materials based on photo-responsive materials. Nano Energy 2021, 80, 105454. [Google Scholar] [CrossRef]
- Pretzl, M.; Neubauer, M.; Tekaat, M.; Kunert, C.; Kuttner, C.; Leon, G.; Berthier, D.; Erni, P.; Ouali, L.; Fery, A. Formation and mechanical characterization of aminoplast core/shell microcapsules. ACS Appl. Mater. Interfaces 2012, 4, 2940–2948. [Google Scholar] [CrossRef]
- Chong, Y.B.; Sun, D.; Zhang, X.; Yue, C.Y.; Yang, J. Robust multifunctional microcapsules with antibacterial and anticorrosion features. Chem. Eng. J. 2019, 372, 496–508. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Wang, K.; Huang, Y.; Chen, Z. Highly efficient photothermal conversion capric acid phase change microcapsule: Silicon carbide modified melamine urea formaldehyde. J. Colloid Interface Sci. 2021, 582, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Bah, M.G.; Bilal, H.M.; Wang, J. Fabrication and application of complex microcapsules: A review. Soft Matter 2020, 16, 570–590. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Rong, M.Z.; Zhang, M.Q. Preparation and characterization of microencapsulated polythiol. Polymer 2008, 49, 2531–2541. [Google Scholar] [CrossRef]
- Nguon, O.; Lagugné-Labarthet, F.; Brandys, F.A.; Li, J.; Gillies, E.R. Microencapsulation by in situ polymerization of amino resins. Polym. Rev. 2017, 58, 326–375. [Google Scholar] [CrossRef]
- Han, S.J.; Chen, Y.P.; Lyu, S.Y.; Chen, Z.L.; Wang, S.Q.; Fu, F. Effects of processing conditions on the properties of paraffin/melamine-urea-formaldehyde microcapsules prepared by in situ polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124046. [Google Scholar] [CrossRef]
- Qin, S.; Yu, X.; Xu, L. Amplified fluorescence detection and adsorption of Au3+ by the fluorescent melamine formaldehyde microspheres incorporated with N and S co-doped carbon dots. J. Hazard. Mater. 2021, 405, 123978. [Google Scholar] [CrossRef]
- Chen, W.L.; Fu, X.W.; Ge, W.B.; Xu, J.J.; Jiang, M.J. Microencapsulation of bisneopentyl glycol dithiopyrophosphate and its flame retardant effect on polyvinyl alcohol. Polym. Degrad. Stab. 2014, 102, 81–87. [Google Scholar] [CrossRef]
- Semenov, A.V.; Pergament, A.L.; Pikalev, A.A. Raman spectroscopy of melamine-formaldehyde resin microparticles exposed to processing in complex plasma. J. Raman Spectrosc. 2016, 47, 1293–1297. [Google Scholar] [CrossRef]
- Dong, W.F.; Ferri, J.K.; Adalsteinsson, T.; Schonhoff, M.; Sukhorukov, G.B.; Mohwald, H. Influence of shell structure on stability, integrity, and mesh size of polyelectrolyte capsules: Mechanism and strategy for improved preparation. Chem. Mater. 2005, 17, 2603–2611. [Google Scholar] [CrossRef]
- Gokce, H.; Ozturk, N.; Ceylan, U.; Alpaslan, Y.B.; Alpaslan, G. Thiol-thione tautomeric analysis, spectroscopic (FT-IR, Laser-Raman, NMR and UV-vis) properties and DFT computations of 5-(3-pyridyl)-4H-1,2,4-triazole-3-thiol molecule. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 163, 170–180. [Google Scholar] [CrossRef]
- Coullerez, G.; Leonard, D.; Lundmark, S.; Mathieu, H.J. XPS and ToF-SIMS study of freeze-dried and thermally cured melamine-formaldehyde resins of different molar ratios. Surf. Interface Anal. 2000, 29, 431–443. [Google Scholar] [CrossRef]
- Laiho, T.; Leiro, J.A.; Lukkari, J. XPS study of irradiation damage and different metal-sulfur bonds in dodecanethiol monolayers on gold and platinum surfaces. Appl. Surf. Sci. 2003, 212–213, 525–529. [Google Scholar] [CrossRef]
- Liu, Y.; Sokolov, I.; Dokukin, M.E.; Xiong, Y.; Peng, P. Can AFM be used to measure absolute values of Young’s modulus of nanocomposite materials down to the nanoscale? Nanoscale 2020, 12, 12432–12443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.H.; Chan, C.M. Transition of phase continuity induced by crosslinking and interfacial reaction during reactive processing of compatibilized PVC/SBR blends. Polymer 1998, 39, 7023–7032. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Liu, H.; Shang, S.; Song, Z.; Song, J. Properties enhancement of room temperature vulcanized silicone rubber by rosin modified aminopropyltriethoxysilane as a cross-linking agent. ACS Sustain. Chem. Eng. 2017, 5, 10002–10010. [Google Scholar] [CrossRef]
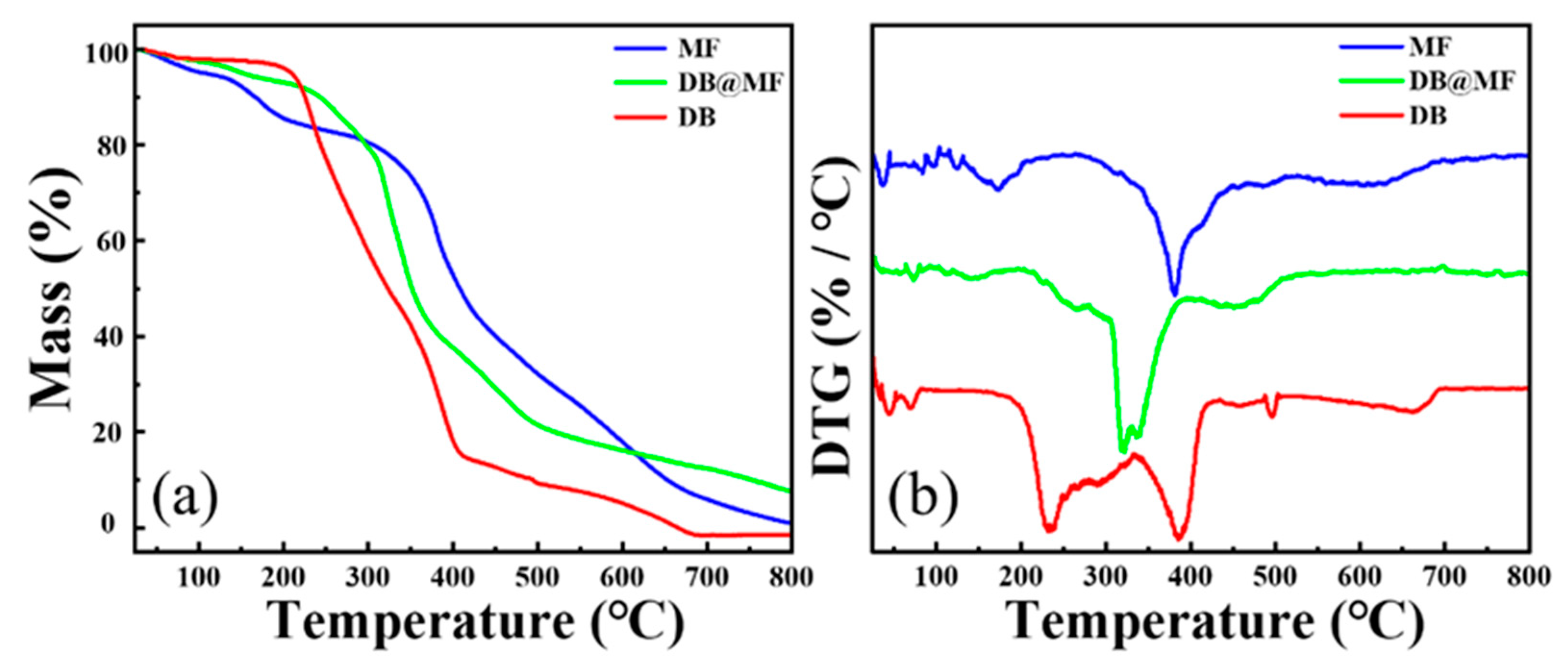
| Sample | T10%/°C | T50%/°C | Tmax/°C |
|---|---|---|---|
| DB | 225 | 323 | 234/388 |
| DB@MF | 243 | 351 | 320 |
| MF | 166 | 408 | 381 |
| Properties | 0 phr | 6 phr | 10 phr | Growth Rate 1 |
|---|---|---|---|---|
| Hardness | 77.87 | 78.75 | 79.00 | 1.44% |
| Tensile strength (MPa) | 31.85 | 39.82 | 44.82 | 40.69% |
| Flexural strength (Mpa) | 51.92 | 54.02 | 66.17 | 27.43% |
| Elongation at break (%) | 57.17 | 13.62 | 11.67 | −79.59% |
| Gel fraction (%) | - | 20.95 | 35.82 | - |
| Vicat softening temperature (°C) | 79.30 | 83.80 | 86.20 | 8.70% |
| Samples | S (%) | Ti (%) | S/Ti Ratio |
|---|---|---|---|
| PVC/DB@MF composites (unprocessed) | 0.13 | 0.09 | 1.44 |
| PVC/DB@MF composites (under torque rheometer) | 0.51 | 0.21 | 2.43 |
| PVC/DB@MF composites (under extrusion process) | 0.44 | 0.18 | 2.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Li, C.; Sui, J.; Jiang, S.; Zhao, W.; Zhang, S.; Wu, R.; Li, J.; Chen, X. A Novel One-Step Reactive Extrusion Process for High-Performance Rigid Crosslinked PVC Composite Fabrication Using Triazine Crosslinking Agent@Melamine-Formaldehyde Microcapsules. Materials 2023, 16, 4600. https://doi.org/10.3390/ma16134600
Zhao J, Li C, Sui J, Jiang S, Zhao W, Zhang S, Wu R, Li J, Chen X. A Novel One-Step Reactive Extrusion Process for High-Performance Rigid Crosslinked PVC Composite Fabrication Using Triazine Crosslinking Agent@Melamine-Formaldehyde Microcapsules. Materials. 2023; 16(13):4600. https://doi.org/10.3390/ma16134600
Chicago/Turabian StyleZhao, Jinshun, Chun Li, Jiayang Sui, Shuai Jiang, Weizhen Zhao, Shihao Zhang, Rong Wu, Jintong Li, and Xuhuang Chen. 2023. "A Novel One-Step Reactive Extrusion Process for High-Performance Rigid Crosslinked PVC Composite Fabrication Using Triazine Crosslinking Agent@Melamine-Formaldehyde Microcapsules" Materials 16, no. 13: 4600. https://doi.org/10.3390/ma16134600
APA StyleZhao, J., Li, C., Sui, J., Jiang, S., Zhao, W., Zhang, S., Wu, R., Li, J., & Chen, X. (2023). A Novel One-Step Reactive Extrusion Process for High-Performance Rigid Crosslinked PVC Composite Fabrication Using Triazine Crosslinking Agent@Melamine-Formaldehyde Microcapsules. Materials, 16(13), 4600. https://doi.org/10.3390/ma16134600







