Experimental Study on the Physicochemical Properties of Asphalt Modified by Different Anti-Stripping Agents and Their Moisture Susceptibility with Aggregates
Abstract
1. Introduction
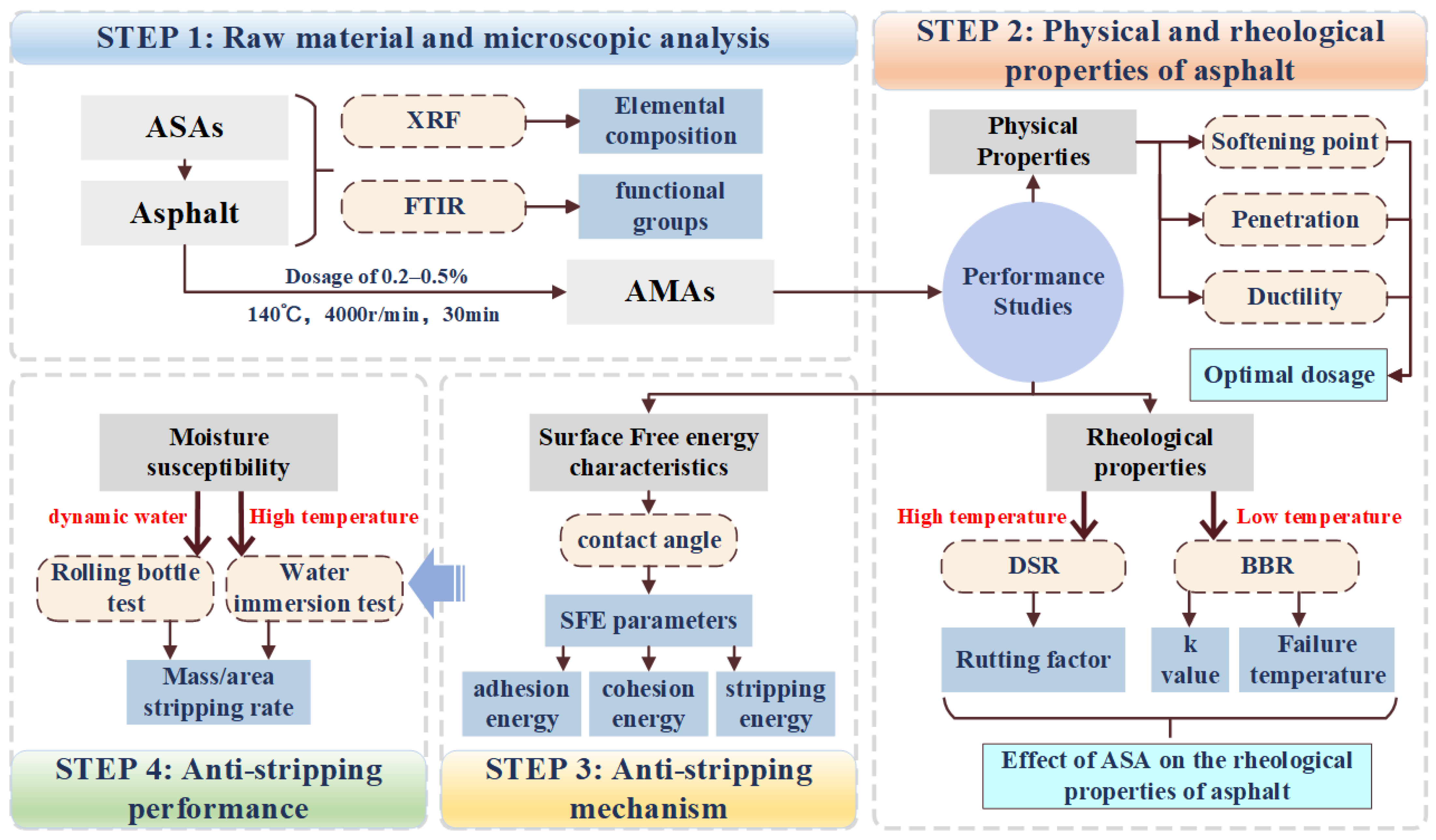
2. Materials and Methods
2.1. Raw Materials
2.1.1. Asphalt Binder and Aggregates
2.1.2. Anti-Stripping Agents
2.2. Experimental Methods
2.2.1. Preparation of AMAs
2.2.2. X-ray Fluorescence Spectroscopy Test
2.2.3. Fourier Transform Infrared Spectrometer Test
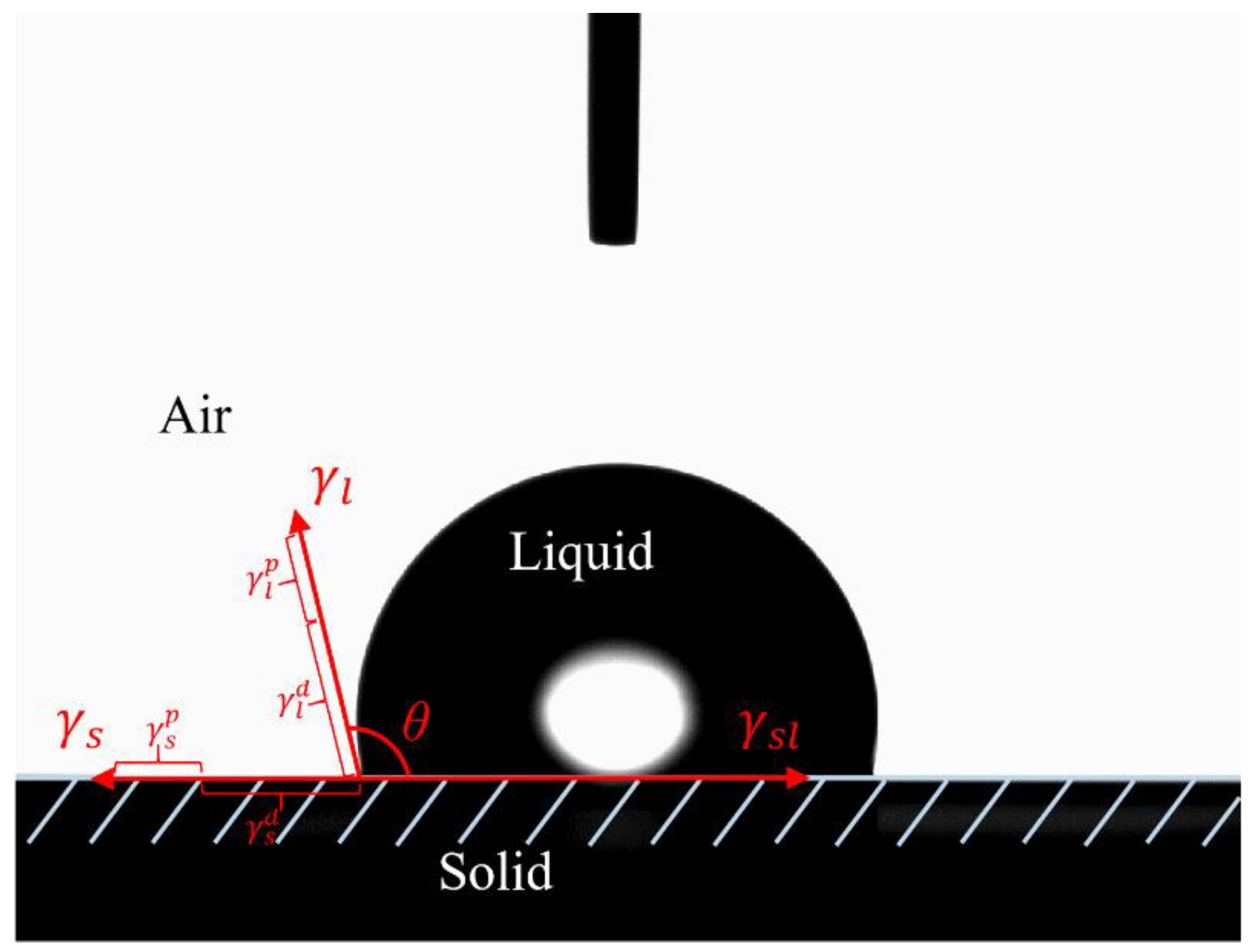
2.2.4. Static Contact Angle Measurement and SFE Calculation
2.2.5. Dynamic Shear Rheometer Test
2.2.6. Bending Beam Rheometer Test
2.2.7. Rolling Bottle Test
2.2.8. High-Temperature Water Immersion Test
2.2.9. Evaluation Method of Anti-Stripping Performance
3. Results and Discussion
3.1. Modification Mechanism of ASAs
3.1.1. Elemental Composition of Aggregates and ASAs
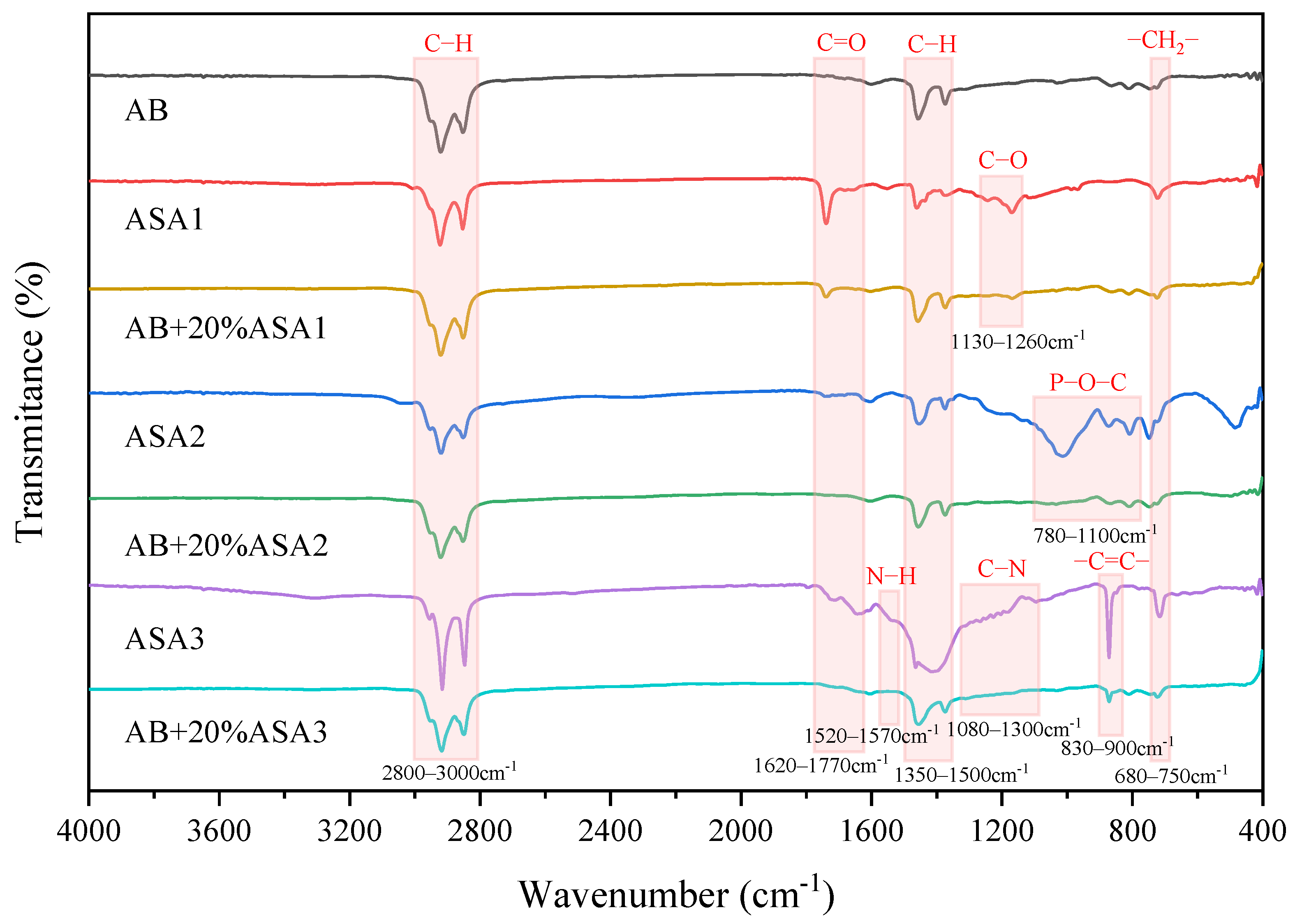
3.1.2. Chemical Components of ASAs
3.2. Effect of ASAs on the Physical Properties of Asphalt
3.3. Effect of ASAs on the Rheological Properties of Asphalt
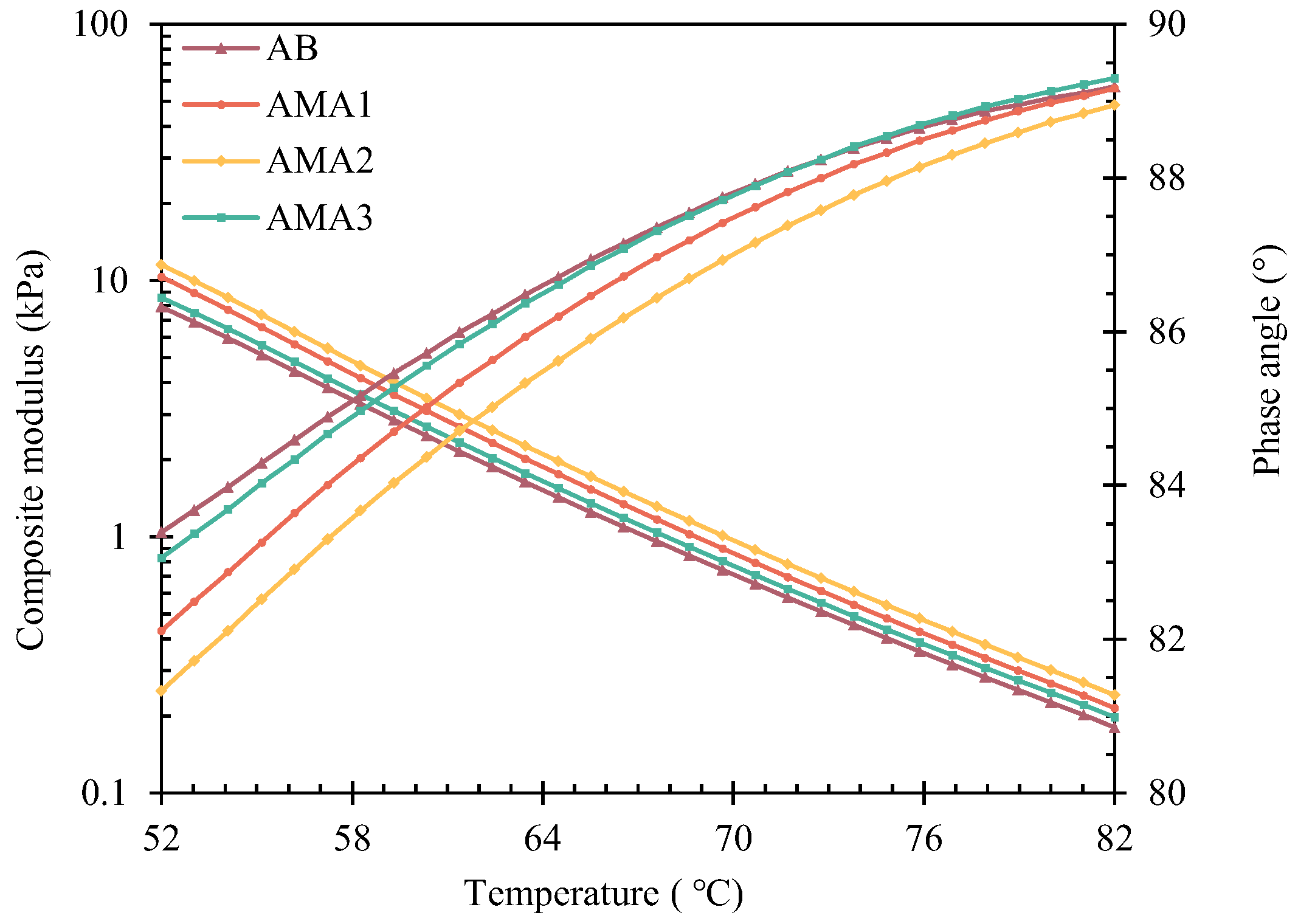
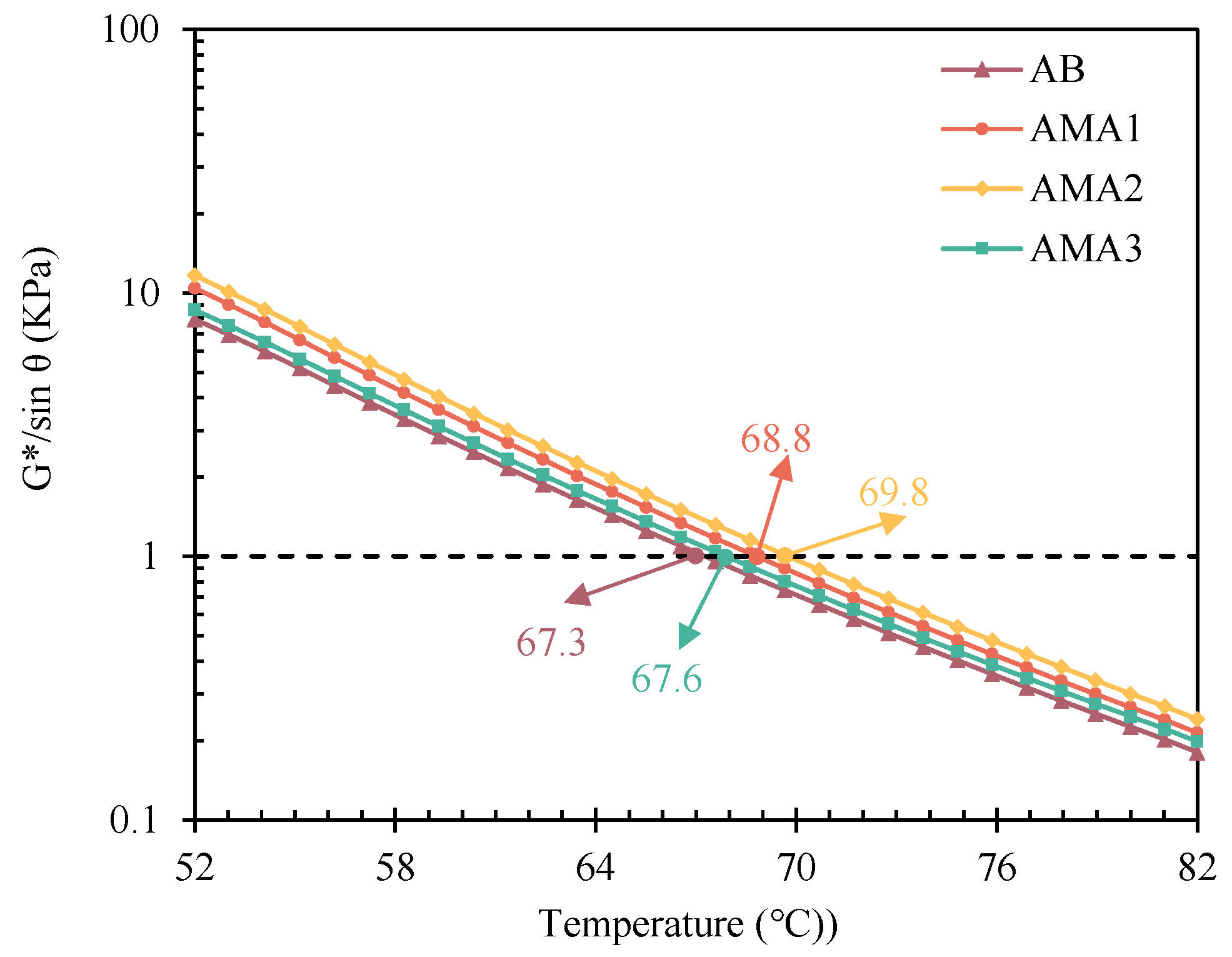
3.3.1. High-Temperature Rheological Properties
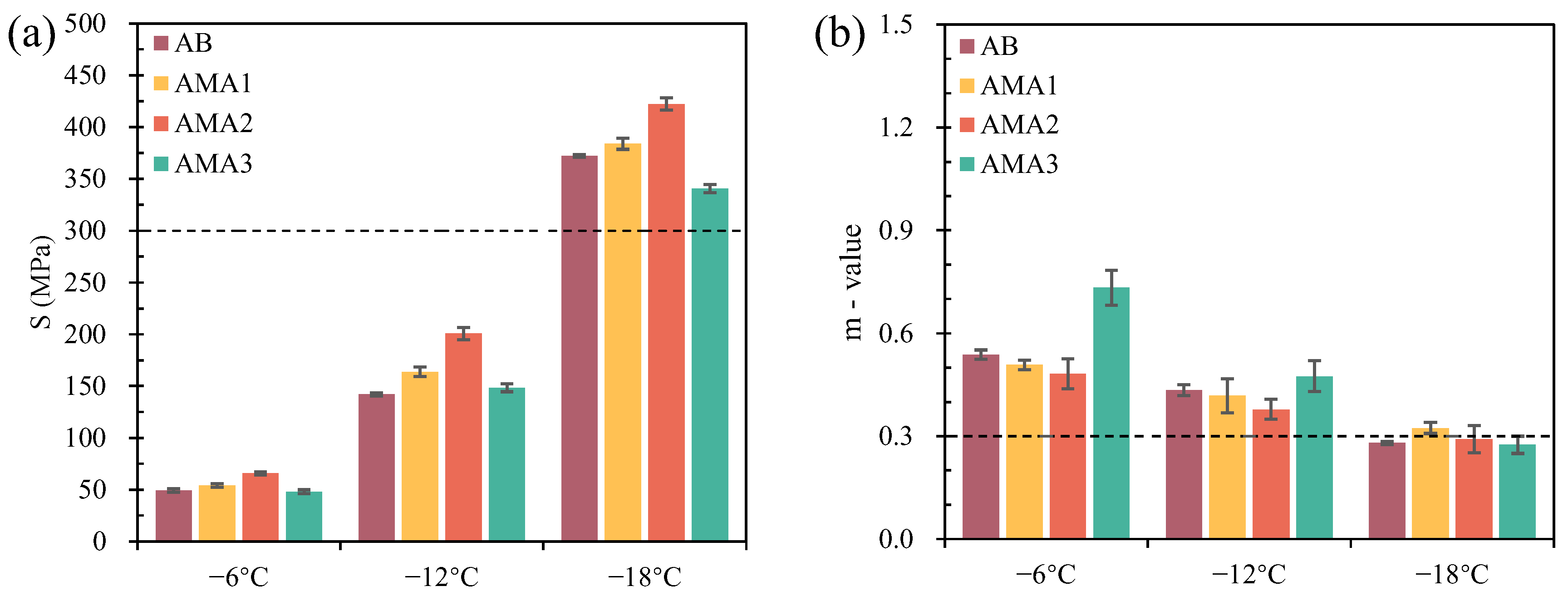
3.3.2. Low-Temperature Rheological Properties
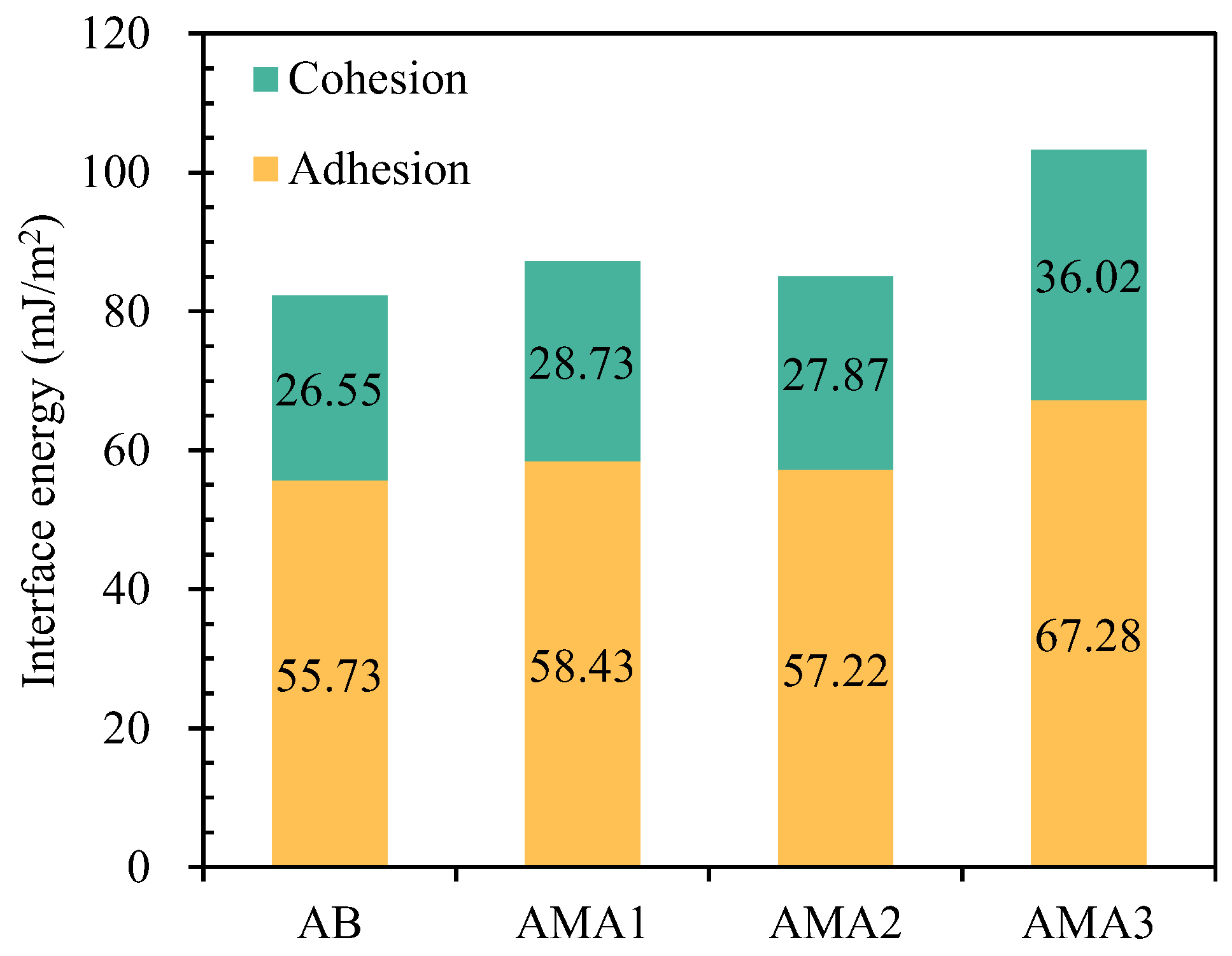
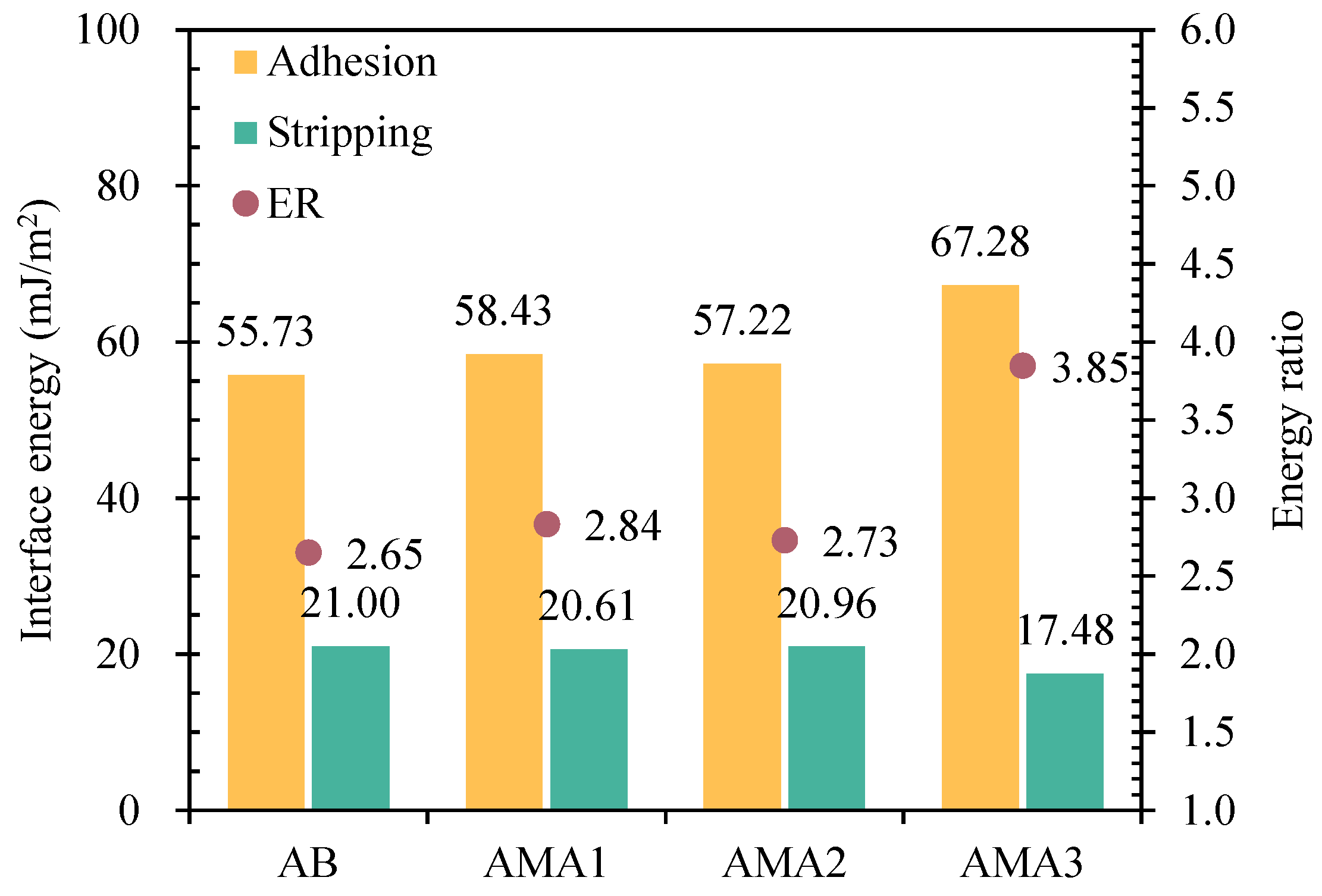
3.4. Surface Free Energy Analysis
3.5. Moisture Susceptibility of AMAs
3.5.1. Effect of Dynamic Water on Anti-Stripping Performance at Room Temperature
3.5.2. Effect of High-Temperature Water Immersion on Anti-Stripping Performance
4. Conclusions
- The main elements of ASA1 are Fe, S and Cl. ASA2 is mainly composed of non-metallic elements such as P and S. ASA3 contains up to 96% Ca. Based on the results of infrared spectra, it is known that there is almost no chemical reaction between the AB and ASA, and the mechanism of the ASA can be classified as a physical modification.
- The optimum dosage of ASAs is 0.3%, as determined by the physical properties of asphalt. ASA1 exhibits the best basic physical properties, and ASA2 shows the best high-temperature deformation resistance, with a 46% increase compared to the base asphalt. ASA3 significantly improves the low-temperature performance of the asphalt, with a 5% improvement.
- ASAs increases the dispersion and polarity components in asphalt. Asphalt adhesion energy is improved and the stripping energy is reduced. ASA3 contains a large amount of metal cations and polar amine groups, which increased the polar component by 2.8 times and the ER value by 45%. The increase in these indexes is significantly higher than that of ASA1 and ASA2.
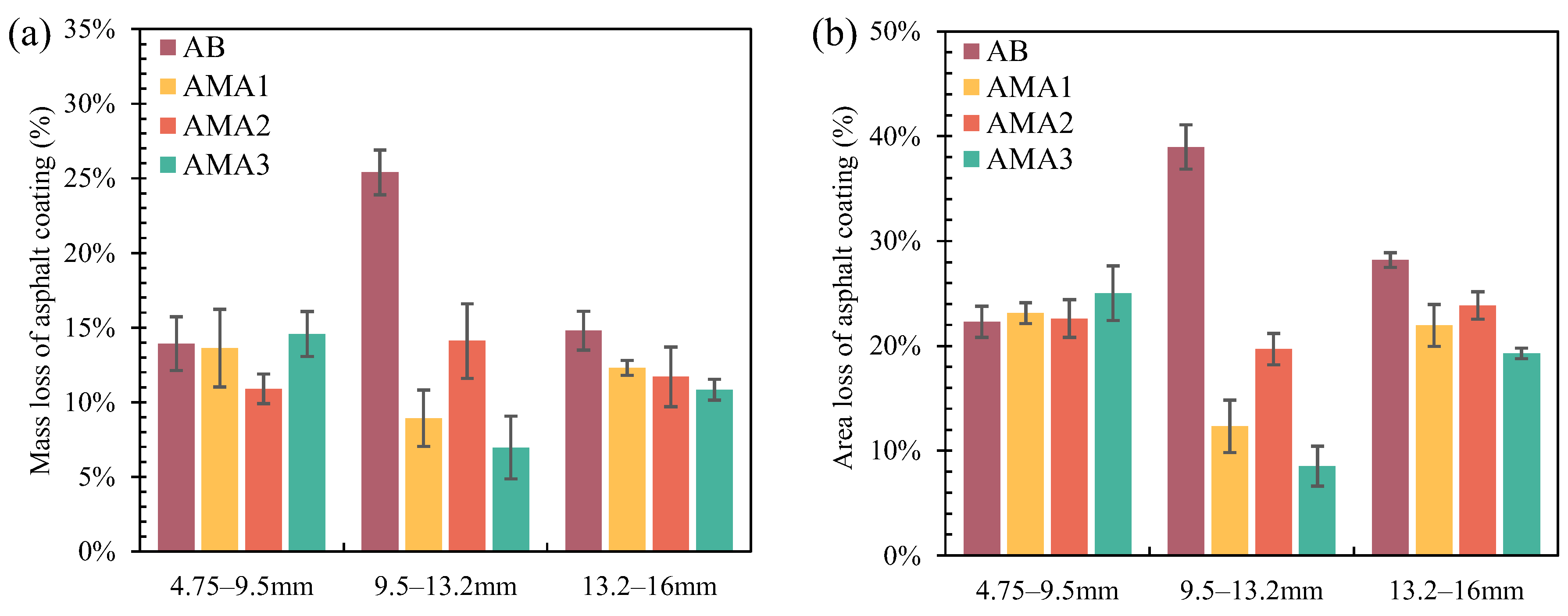
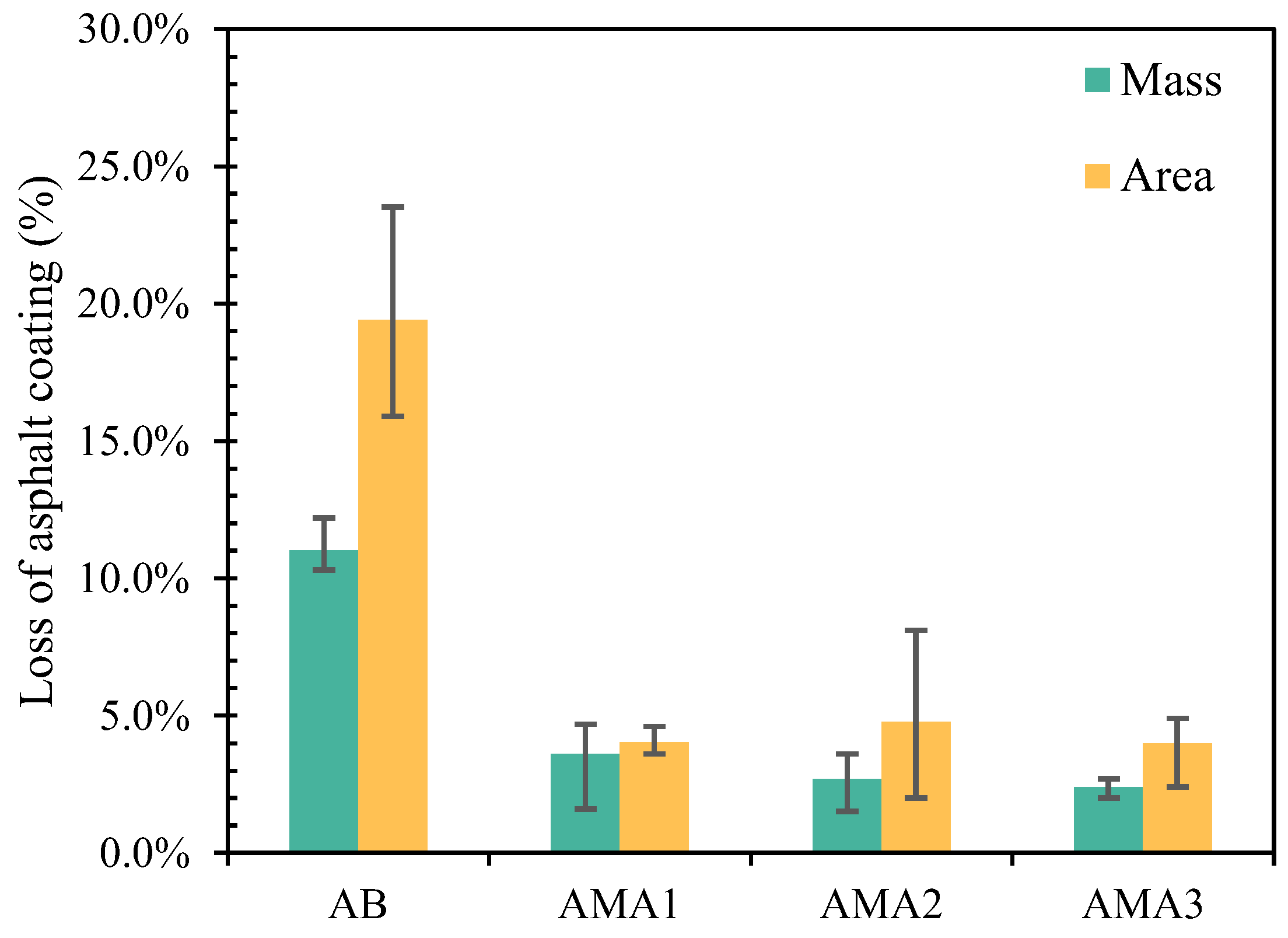
- Compared to other particle sizes of coarse aggregates, ASAs have the best modification effect on the moisture resistance of basalt with a particle size of 9.5–13.2 mm. Under different testing conditions, the adhesion properties of ASA1, ASA2, and ASA3 modifying the asphalt–aggregate interface are enhanced by 65%, 45%, and 70%, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, F.C.; Pei, J.Z.; Zhang, J.P.; Li, R.; Liu, P.F.; Wang, D. Study on Adhesion Property and Moisture Effect between SBS Modified Asphalt Binder and Aggregate Using Molecular Dynamics Simulation. Materials 2022, 15, 6912. [Google Scholar] [CrossRef] [PubMed]
- Eltwati, A.; Jaya, R.P.; Mohamed, A.; Jusli, E.; Al-Saffar, Z.; Hainin, M.R.; Enieb, M. Effect of Warm Mix Asphalt (WMA) Antistripping Agent on Performance of Waste Engine Oil-Rejuvenated Asphalt Binders and Mixtures. Sustainability 2023, 15, 3807. [Google Scholar] [CrossRef]
- Hu, X.Y.; Wang, X.W.; Zheng, N.X.; Li, Q.; Shi, J.Y. Experimental Investigation of Moisture Sensitivity and Damage Evolution of Porous Asphalt Mixtures. Materials 2021, 14, 7151. [Google Scholar] [CrossRef]
- Brondani, C.; Faccin, C.; Specht, L.P.; Nummer, A.V.; Pereira, D.D.; Vestena, P.M.; Baroni, M. Evaluation of Moisture Susceptibility of Asphalt Mixtures: Influence of Aggregates, Visual Analysis, and Mechanical Tests. J. Mater. Civ. Eng. 2023, 35, 04022433. [Google Scholar] [CrossRef]
- Chen, S.Y.; Adhikari, S.; You, Z.P. Relationship of Coefficient of Permeability, Porosity, and Air Voids in Fine-Graded HMA. J. Mater. Civ. Eng. 2019, 31, 04018359. [Google Scholar] [CrossRef]
- Tu, C.Z.; Luo, R.; Huang, T.T. Numerical Simulation of Moisture Diffusion in the Microstructure of Asphalt Mixtures. Materials 2023, 16, 2504. [Google Scholar] [CrossRef]
- Zhang, G.H.; Qiu, J.H.; Zhao, J.Z.; Wei, D.B.; Wang, H. Development of Interfacial Adhesive Property by Novel Anti-Stripping Composite between Acidic Aggregate and Asphalt. Polymers 2020, 12, 473. [Google Scholar] [CrossRef]
- Mei, A.D.; Fusco, R.; Moroni, M.; Fiore, N.; Fontinovo, G.; D’Andrea, A. Affinity between Bitumen and Aggregate in Hot Mix Asphalt by Hyperspectral Imaging and Digital Picture Analysis. Coatings 2021, 11, 648. [Google Scholar] [CrossRef]
- Mahpour, A.; Alipour, S.; Khodadadi, M.; Khodaii, A.; Absi, J. Leaching and mechanical performance of rubberized warm mix asphalt modified through the chemical treatment of hazardous waste materials. Constr. Build. Mater. 2023, 366, 130184. [Google Scholar] [CrossRef]
- Ziari, H.; Nasiri, E.; Amini, A.; Ferdosian, O. The effect of EAF dust and waste PVC on moisture sensitivity, rutting resistance, and fatigue performance of asphalt binders and mixtures. Constr. Build. Mater. 2019, 203, 188–200. [Google Scholar] [CrossRef]
- Lucas, J.L.O.; Da Silva, L.S.V.; Rocha, W.S.; Babadopulos, L.; Soares, J.B. Effects of rheology and adhesiveness of asphalt binders to different substrates on the resistance to moisture conditioning. J. Adhes. 2022, 98, 1937–1956. [Google Scholar] [CrossRef]
- Corrales-Azofeifa, J.; Archilla, A.R.; Miranda-Arguello, F.; Loria-Salazar, L. Effects of moisture damage and anti-stripping agents on hot mix asphalt dynamic modulus. Road Mater. Pavement Des. 2020, 21, 1135–1154. [Google Scholar] [CrossRef]
- Wang, W.Y.; Cheng, H.L.; Sun, L.J.; Sun, Y.R.; Liu, N. Multi-performance evaluation of recycled warm-mix asphalt mixtures with high reclaimed asphalt pavement contents. J. Clean. Prod. 2022, 377, 134209. [Google Scholar] [CrossRef]
- Shu, S.; Zhuang, C.; Zhao, S.; Hao, Y.; Guo, H.; Ye, Y. Study of Anti-Stripping Measures to Improve the Adhesion of Asphalt to Granite Aggregates. Coatings 2022, 12, 1954. [Google Scholar] [CrossRef]
- Ali, S.A.; Zaman, M.; Ghabchi, R.; Rahman, M.A.; Ghos, S.; Rani, S. Effect of additives and aging on moisture-induced damage potential of asphalt mixes using surface free energy and laboratory-based performance tests. Int. J. Pavement Eng. 2022, 23, 285–296. [Google Scholar] [CrossRef]
- Yousefi, A.A.; Haghshenas, H.F.; Underwood, B.S.; Harvey, J.; Blankenship, P. Performance of warm asphalt mixtures containing reclaimed asphalt pavement, an anti-stripping agent, and recycling agents: A study using a balanced mix design approach. Constr. Build. Mater. 2023, 363, 129633. [Google Scholar] [CrossRef]
- Wang, F.H.; Huang, T.; Xin, G.F.; Mu, M.H.; Shen, Q.J. Study on Conventional and Rheological Properties of Corn Stalk Bioasphalt/PPA Composite Modified Asphalt. Adv. Civ. Eng. 2021, 2021, 7928189. [Google Scholar] [CrossRef]
- Xiao, R.; Ding, Y.; Polaczyk, P.; Ma, Y.; Jiang, X.; Huang, B. Moisture damage mechanism and material selection of HMA with amine antistripping agent. Mater. Des. 2022, 220, 110797. [Google Scholar] [CrossRef]
- JTG E20-2011; Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering. China Communications Press: Beijing, China, 2011.
- JTG E42-2005; Test Methods of Aggregate for Highway Engineering. People’s Communications Press: Beijing, China, 2005.
- Hossain, Z.; Alam, M.S.; Baumgardner, G. Evaluation of rheological performance and moisture susceptibility of polyphosphoric acid modified asphalt binders. Road Mater. Pavement Des. 2020, 21, 237–252. [Google Scholar] [CrossRef]
- Rahmad, S.; Yusoff, N.I.M.; Rosyidi, S.A.P.; Badri, K.H.; Widyatmoko, I. Effects of Rediset on the adhesion, stripping, thermal and surface morphologies of PG76 binder. Constr. Build. Mater. 2020, 241, 117923. [Google Scholar] [CrossRef]
- Xu, Y.; Han, B.; Xiao, K.; Yu, J.; Zheng, J.; Liang, S.; Wang, X.; Xu, G.; Huang, X. Revisiting the Surface Energy Parameters of Standard Test Liquids with a Corrected Contact Angle Method over Rough Surfaces. Langmuir 2022, 38, 10760–10767. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.L.; Hao, Y.; Zhuang, C.Y.; Shu, S.Q.; Lv, F.L. Evaluation on Improvement Effect of Different Anti-Stripping Agents on Pavement Performance of Granite-Asphalt Mixture. Materials 2022, 15, 915. [Google Scholar] [CrossRef]
- Zou, Y.X.; Xu, H.Q.; Xu, S.; Chen, A.Q.; Wu, S.P.; Amirkhanian, S.; Wan, P.; Gao, X. Investigation of the moisture damage and the erosion depth on asphalt. Constr. Build. Mater. 2023, 369, 130503. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, M.; Esquena, J. Wetting and interfacial properties of bitumen at room temperature, and the role of naturally present ionic molecules. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125575. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H. Proposed Approach for Determining Consistent Energy Parameters Based on the Surface Free Energy Theory. J. Mater. Civ. Eng. 2018, 30, 04018287. [Google Scholar] [CrossRef]
- Wang, R.; Qi, Z.; Li, R.; Yue, J. Investigation of the effect of aging on the thermodynamic parameters and the intrinsic healing capability of graphene oxide modified asphalt binders. Constr. Build. Mater. 2020, 230, 116984. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Wang, Z.; Jing, H.; Yang, B. Investigations on Adhesion Characteristics between High-Content Rubberized Asphalt and Aggregates. Polymers 2022, 14, 5474. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, W.; Guo, C.; Du, X.; Pan, Y.; Gao, J.; Gao, X. Surface Free Energy Theory for Evaluating Moisture Damage in Expandable Polyurethane Grouting Materials. Adv. Eng. Mater. 2022, 24, 2200409. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, S.; Peng, A. Analysis of moisture susceptibility of foamed warm mix asphalt based on cohesion, adhesion, bond strength, and morphology. J. Clean. Prod. 2020, 277, 123334. [Google Scholar] [CrossRef]
- Peng, C.; Chen, P.; You, Z.; Lv, S.; Zhang, R.; Xu, F.; Zhang, H.; Chen, H. Effect of silane coupling agent on improving the adhesive properties between asphalt binder and aggregates. Constr. Build. Mater. 2018, 169, 591–600. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Xu, G.; Shi, C. Evaluation of aging behaviors of asphalt binders through different rheological indices. Fuel 2018, 221, 78–88. [Google Scholar] [CrossRef]
- Zou, Y.; Pang, L.; Xu, S.; Wu, S.; Yuan, M.; Amirkhanian, S.; Xu, H.; Lv, Y.; Gao, X. Investigation of the Rheological Properties and Chemical Structure of Asphalt under Multiple Aging Conditions of Heat, UV and Aqueous Solution. Materials 2022, 15, 5711. [Google Scholar] [CrossRef] [PubMed]
- Ghabchi, R.; Rani, S.; Zaman, M.; Ali, S.A. Effect of WMA additive on properties of PPA-modified asphalt binders containing anti-stripping agent. Int. J. Pavement Eng. 2021, 22, 418–431. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Wang, H.; Yang, Z.; Zhang, T. Investigating the Rheological Properties of Styrene-Butadiene-Styrene-Based High-Viscosity Modified Asphalt Using Carbon Nanotubes. Sustainability 2023, 15, 71. [Google Scholar] [CrossRef]
- Xue, Z.; Xu, W. A Study on High and Low Temperature Rheological Properties and Oil Corrosion Resistance of Epoxy Resin/SBS Composite Modified Bitumen. Polymers 2023, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- BS EN 12697-11; Test Methods Determination of the Affinity between Aggregate and Bitumen. European Committee for Standardization: Brussels, Belgium, 2020.
- Maia, M.; Dinis-Almeida, M.; Martinho, F.C.G. The Influence of the Affinity between Aggregate and Bitumen on the Mechanical Performance Properties of Asphalt Mixtures. Materials 2021, 14, 6452. [Google Scholar] [CrossRef]
- Jain, S.; Singh, B. Recycled concrete aggregate incorporated cold bituminous emulsion mixture: Mechanical, environmental and economic evaluation. J. Clean. Prod. 2022, 380, 135026. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, M.; Wu, S.; Xie, J.; Kong, D.; Qiao, Z.; Niu, C. Moisture Susceptibility Evaluation of Asphalt Mixtures Containing Steel Slag Powder as Filler. Materials 2019, 12, 3211. [Google Scholar] [CrossRef]
- Chomicz-Kowalska, A. A Study of Adhesion in Foamed WMA Binder-Aggregate Systems Using Boiling Water Stripping Tests. Materials 2021, 14, 6248. [Google Scholar] [CrossRef]
- Li, J.G.; Wang, Z.X.; Jia, M. Comprehensive analysis on influences of aggregate, asphalt and moisture on interfacial adhesion of aggregate-asphalt system. J. Adhes. Sci. Technol. 2021, 35, 641–662. [Google Scholar] [CrossRef]
- Zaidi, S.B.A.; Grenfell, J.; Airey, G.; Ahmad, N.; Ahmed, I.; Fareed, A.; Abed, A. Application of image analysis tools in Matlab to better estimate the degree of binder coverage in rolling bottles test. Road Mater. Pavement Des. 2022, 23, 601–616. [Google Scholar] [CrossRef]
- Asif, S.A.; Ahmad, N. Effect of Different Factors on Bonding Properties of Bitumen-Aggregate Combinations. J. Mater. Civ. Eng. 2022, 34, 04021480. [Google Scholar] [CrossRef]
- Cala, A.; Caro, S.; Lleras, M.; Rojas-Agramonte, Y. Impact of the chemical composition of aggregates on the adhesion quality and durability of asphalt-aggregate systems. Constr. Build. Mater. 2019, 216, 661–672. [Google Scholar] [CrossRef]
- Bai, Y.; Sui, H.; Liu, X.; He, L.; Li, X.; Thormann, E. Effects of the N, O, and S heteroatoms on the adsorption and desorption of asphaltenes on silica surface: A molecular dynamics simulation. Fuel 2019, 240, 252–261. [Google Scholar] [CrossRef]
- Shu, S.Q.; Zhuang, C.Y.; Ren, R.B.; Xing, B.D.; Chen, K.; Li, G. Effect of Different Anti-Stripping Agents on the Rheological Properties of Asphalt. Coatings 2022, 12, 1895. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Z.; Zhang, K.; Li, Z. Rheological Property Evaluation and Microreaction Mechanism of Rubber Asphalt, Desulfurized Rubber Asphalt, and Their Composites. J. Mater. Civ. Eng. 2021, 33, 04021100. [Google Scholar] [CrossRef]
- Zou, Y.; Pang, L.; Chen, S.; Xu, S.; Wu, S.; Amirkhanian, S.; Xu, H.; Zhao, Z. Evaluation of the Physicochemical Properties and Antiaging Properties of Bitumen Mastic Modified by Layered Double Hydroxides. Sustainability 2023, 15, 1546. [Google Scholar] [CrossRef]



| Properties | Unit | Measured Values | Test Methods [19] |
|---|---|---|---|
| Penetration (25 °C, 100 g, 5 s) | 0.1 mm | 68.6 | T0604 |
| Ductility (10 °C) | cm | 39.1 | T0605 |
| Softening point | °C | 49.0 | T0606 |
| Solubility (trichloroethylene) | % | 99.7 | T0607 |
| Properties | Particle Size | Basalt | Technical Indicators | Test Methods [20] |
|---|---|---|---|---|
| Apparent specific gravity (g∙cm−3) | 13.2–16 mm | 2.985 | ≥2.6 | T0304 |
| 9.5–13.2 mm | 2.978 | ≥2.6 | T0304 | |
| 4.75–9.5 mm | 2.976 | ≥2.6 | T0304 | |
| Water absorption (%) | 13.2–16 mm | 0.54 | ≤2.0 | T0308 |
| 9.5–13.2 mm | 0.67 | ≤2.0 | T0308 | |
| 4.75–9.5 mm | 0.72 | ≤2.0 | T0308 | |
| Los Angeles abrasion (%) | 20.9 | ≤28 | T0317 | |
| Crushed value (%) | 16.1 | ≤26 | T0316 |
| Properties | Units | ASA1 | ASA2 | ASA3 |
|---|---|---|---|---|
| Appearance | / | Brown viscous liquid | Dark-brown viscous liquid | Orange solids powder |
| Density (15 °C) | g·cm−3 | 1.05 | 0.95 | 1.00 |
| Melting point | °C | 0 | −5 | 60 |
| Viscosity (100 °C) | cSt | 14 | 19 | / |
| Flash point | °C | >220 | >220 | / |
| Test Liquids | Water | Glycerol | Formamide | Diiodomethane |
|---|---|---|---|---|
| 72.8 | 64.0 | 58.0 | 50.8 | |
| 21.8 | 34.0 | 39.0 | 50.8 | |
| 51.0 | 30.0 | 19.0 | 0.0 |
| Component (%) | Basalt | ASA1 | ASA2 | ASA3 |
|---|---|---|---|---|
| Na2O | 4.98 | 11.900 | 3.888 | 0.286 |
| Al2O3 | 16.11 | 4.753 | 2.656 | 0.473 |
| SiO2 | 38.36 | 7.391 | 1.311 | 0.980 |
| P2O5 | 0.352 | 6.501 | 27.161 | 0.046 |
| SO3 | 0.395 | 19.433 | 57.812 | 0.251 |
| Cl | 0.27 | 15.805 | 5.364 | 0.085 |
| Fe2O3 | 19.34 | 24.487 | 1.808 | 0.439 |
| CaO | 10.39 | / | / | 96.129 |
| MgO | 7.19 | / | / | 1.138 |
| Cr2O3 | 0.085 | 7.947 | / | / |
| Others | 2.528 | 1.783 | / | 0.171 |
| ASA1 | ASA2 | ASA3 | |||
|---|---|---|---|---|---|
| Wavenumber /cm−1 | Functional Group | Wavenumber /cm−1 | Functional Group | Wavenumber /cm−1 | Functional Group |
| 1739 | ν (C=O of carboxylic acid) | 3028 | ν (C−H of benzene ring) | 3310 | ν (N−H of fatty secondary amines) |
| 1660 | ν (C=O of tertiary amides) | 1741 | ν (C=O of aromatic esters) | 1719 | ν (C=O of carboxylic acid) |
| 1551 | ν (C=C of benzene ring) | 1604 | ν (C=C of benzene ring) | 1643 | ν (C=O of amide) |
| 1247 | ν (C−O of aromatic oxides) | 1200 | ν (P=O of phosphate ester) | 1554 | β (N−H of fatty secondary amines) |
| 1170 | νas (C−O−C of fatty ethers) | 1013 | νas (P−O−C of phosphate ester) | 1315–1182 | ν (C−N of aromatic ring) |
| 872 | ν (Meta-substituted of benzene ring) | 1095 | ν (C−N of fatty secondary amines) | ||
| 808 | νas (P−O−C of phosphate ester) | 871 | ν (Para-substituted of benzene ring) | ||
| Testing Samples | AB | AMA1 | AMA2 | AMA3 |
|---|---|---|---|---|
| Fitting temperature of S (°C) | −16.2 | −15.6 | −14.4 | −17.0 |
| Fitting temperature of m-value (°C) | −17.5 | −19.7 | −17.3 | −17.1 |
| Failure temperature (°C) | −16.2 | −15.6 | −14.4 | −17.0 |
| Test Liquids | AB | ASA1 MAB | ASA2 MAB | ASA3 MAB | ||||
|---|---|---|---|---|---|---|---|---|
| Avg. (°) | SD (°) | Avg. (°) | SD (°) | Avg. (°) | SD (°) | Avg. (°) | SD (°) | |
| Water | 104.0 | 0.5 | 105.0 | 0.1 | 104.4 | 0.6 | 93.4 | 0.3 |
| Glycerol | 102.2 | 0.3 | 101.8 | 0.5 | 101.1 | 0.4 | 92.6 | 0.3 |
| Formamide | 88.9 | 0.7 | 85.4 | 0.6 | 88.6 | 0.1 | 79.4 | 0.2 |
| Samples | Basalt | AB | AMA1 | AMA2 | AMA3 |
|---|---|---|---|---|---|
| (mJ/m2) | 38.74 | 11.34 | 11.97 | 11.82 | 12.59 |
| (mJ/m2) | 24.63 | 1.94 | 2.40 | 2.11 | 5.42 |
| (mJ/m2) | 63.38 | 13.28 | 14.37 | 13.93 | 18.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Chen, A.; Wu, S.; Li, Y.; Zou, Y.; Zhu, Y.; Wang, K. Experimental Study on the Physicochemical Properties of Asphalt Modified by Different Anti-Stripping Agents and Their Moisture Susceptibility with Aggregates. Materials 2023, 16, 4545. https://doi.org/10.3390/ma16134545
Lu Z, Chen A, Wu S, Li Y, Zou Y, Zhu Y, Wang K. Experimental Study on the Physicochemical Properties of Asphalt Modified by Different Anti-Stripping Agents and Their Moisture Susceptibility with Aggregates. Materials. 2023; 16(13):4545. https://doi.org/10.3390/ma16134545
Chicago/Turabian StyleLu, Ziyu, Anqi Chen, Shaopeng Wu, Yuanyuan Li, Yingxue Zou, Yunsheng Zhu, and Kaifeng Wang. 2023. "Experimental Study on the Physicochemical Properties of Asphalt Modified by Different Anti-Stripping Agents and Their Moisture Susceptibility with Aggregates" Materials 16, no. 13: 4545. https://doi.org/10.3390/ma16134545
APA StyleLu, Z., Chen, A., Wu, S., Li, Y., Zou, Y., Zhu, Y., & Wang, K. (2023). Experimental Study on the Physicochemical Properties of Asphalt Modified by Different Anti-Stripping Agents and Their Moisture Susceptibility with Aggregates. Materials, 16(13), 4545. https://doi.org/10.3390/ma16134545









