Synthesis of Mesoporous and Hollow SiO2@ Eu(TTA)3phen with Enhanced Fluorescence Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silica Spheres
2.3. Preparation of Hollow Silica
2.4. Preparation of Mesoporous Silica
2.5. Preparation of Mesoporous Hollow SiO2
2.6. Preparation of SiO2@Eu(TTA)3phen with Four Morphologies
2.7. Preparation of SiO2@Eu(TTA)3phen with Three Morphologies
2.8. Characterizations
3. Results and Discussion
3.1. Preparation Process Analysis
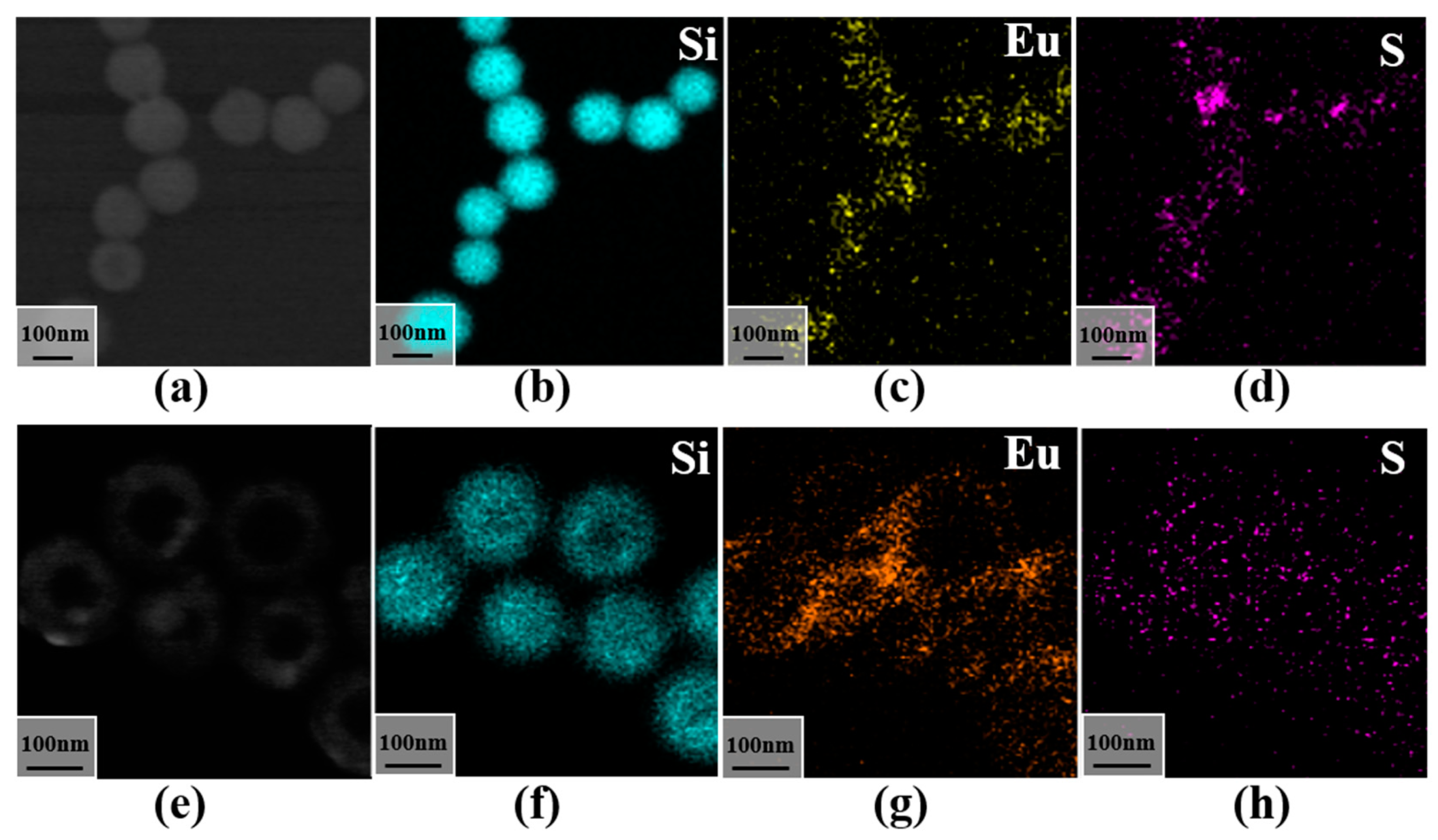
3.2. Morphological Analysis of Silica-Doped Europium Complexes
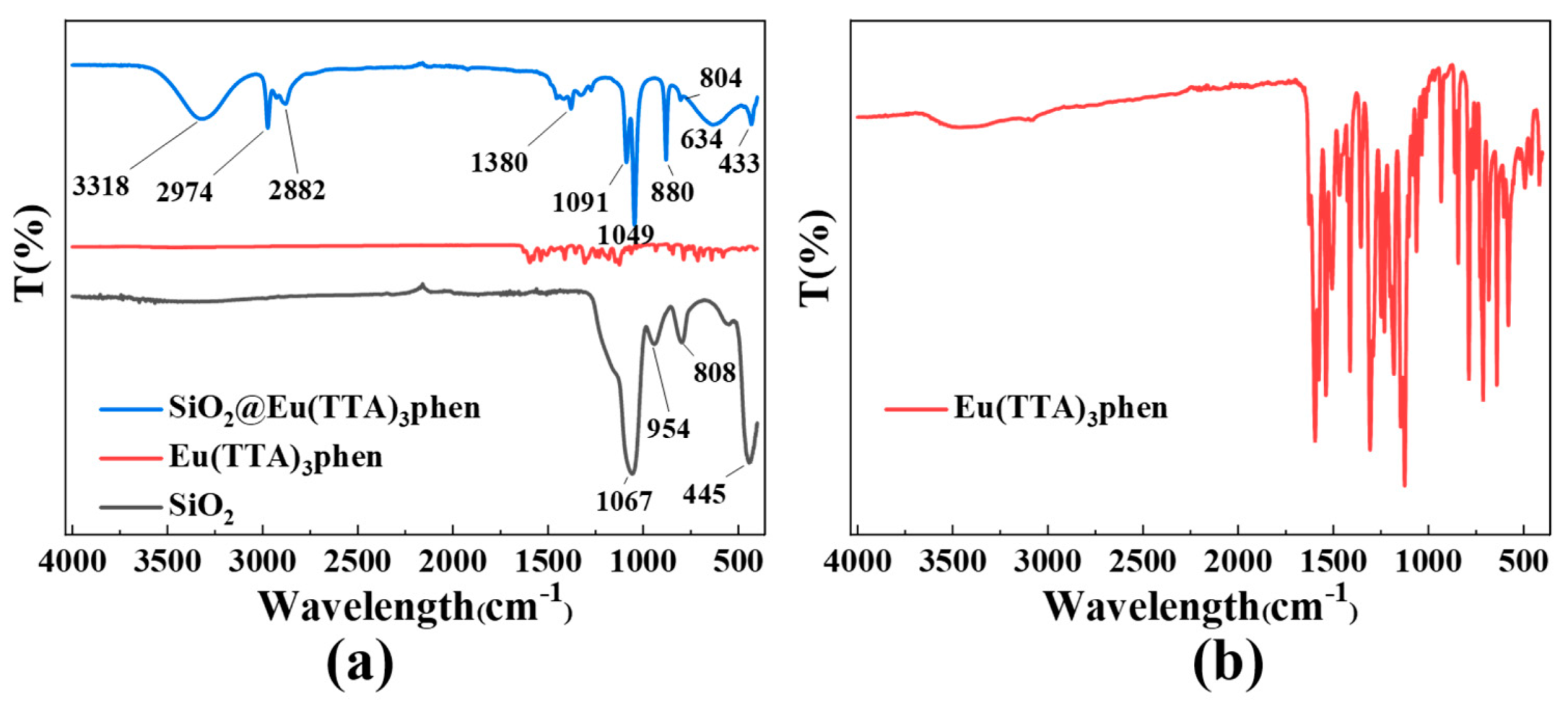
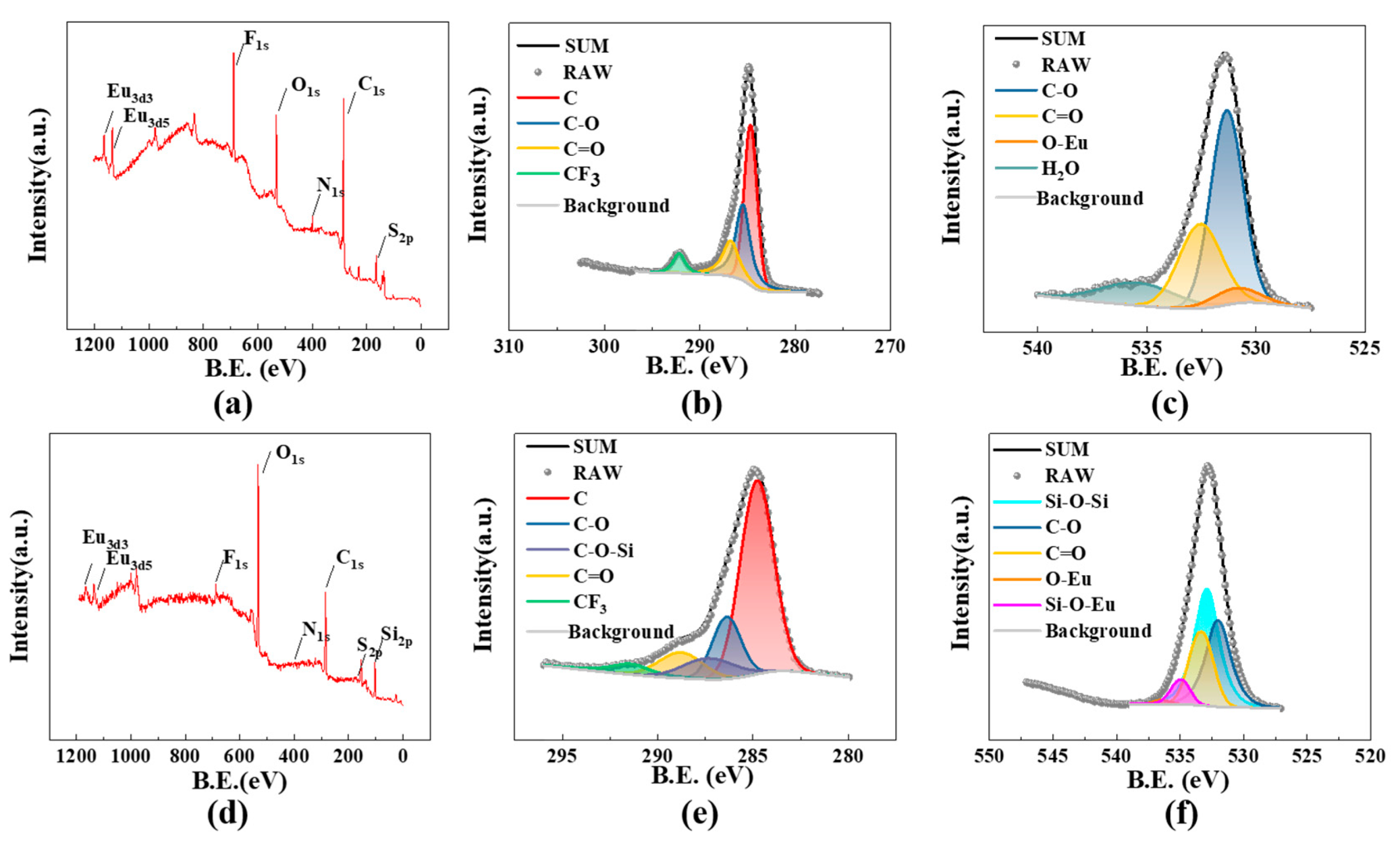
3.3. Analysis of the Binding Mechanism of S@Eu
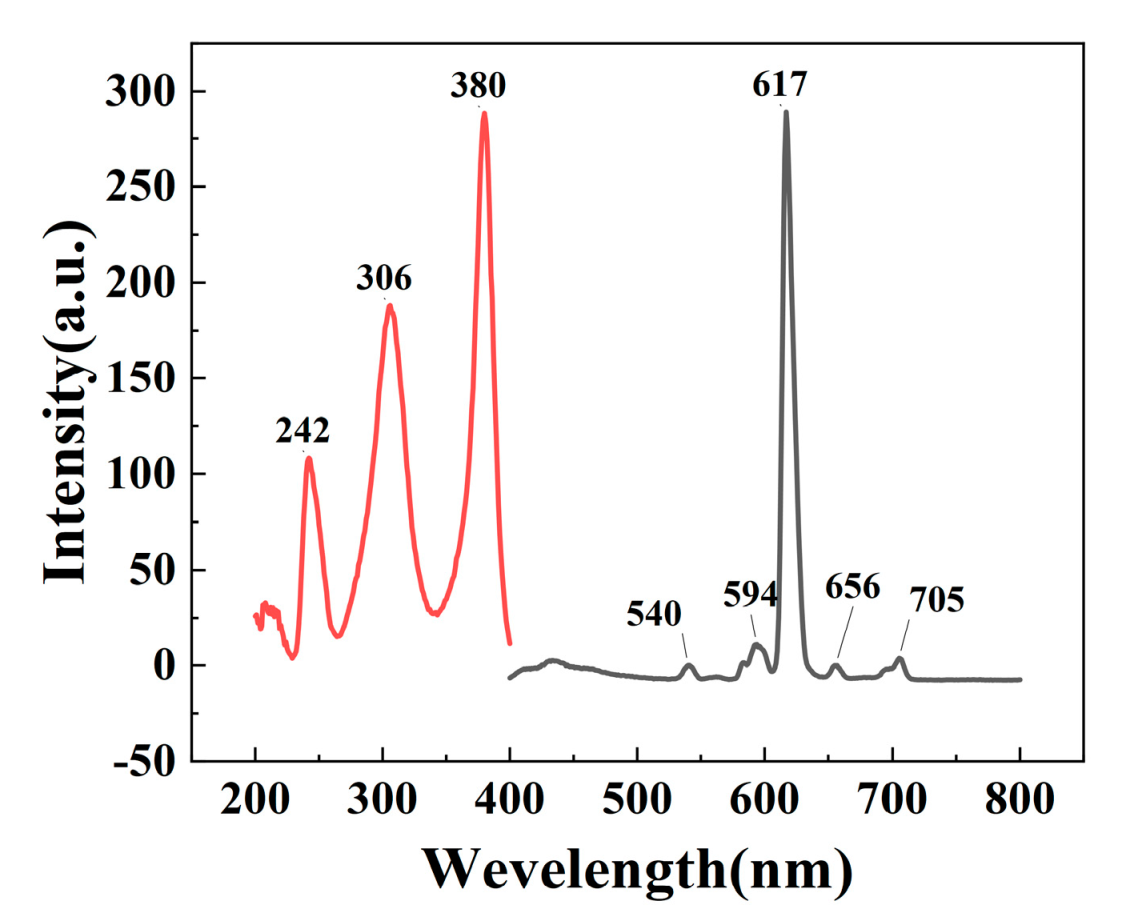
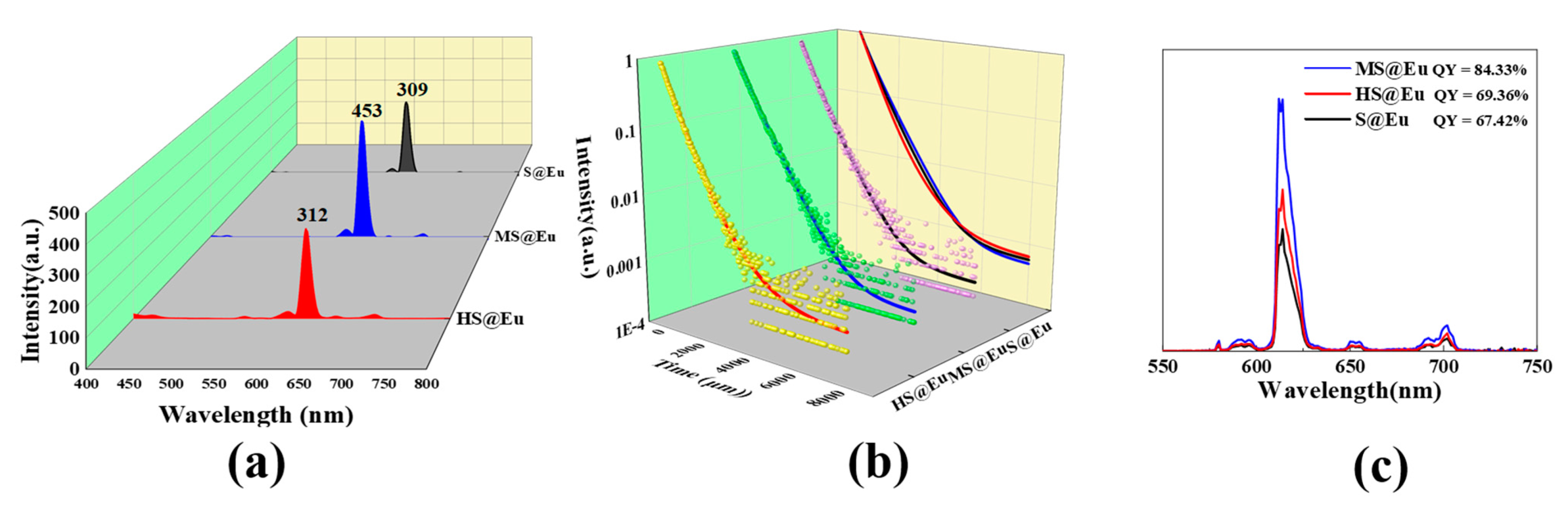
3.4. Analysis of the Fluorescence Properties of S@Eu
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Das, A.; Mohanty, S.; Kumar, R.; Kuanr, B.K. Tailoring the Design of a Lanthanide Complex/Magnetic Ferrite Nanocomposite for Efficient Photoluminescence and Magnetic Hyperthermia Performance. ACS Appl. Mater. Interfaces 2020, 12, 42016–42029. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Tan, X.; Yu, Y.; Li, Y.; Wang, P.; Liang, Y.; Yan, Y. Effectively auto-regulated adsorption and recovery of rare earth elements via an engineered E. coli. J. Hazard. Mater. 2022, 424, 127642. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Liu, L.; Zhang, H.; Tang, S.; Chi, R. A facile solvent-free and one-step route to prepare amino-phosphonic acid functionalized hollow mesoporous silica nanospheres for efficient Gd(III) removal. J. Clean. Prod. 2020, 243, 118688. [Google Scholar] [CrossRef]
- Yang, J.; Feng, M.; Zhang, K.; Chen, Z.; Xu, R.; Khan, A.; Li, Y.; Xie, J.; Liu, L.; Song, F.; et al. All-Inorganic Functional Phosphor–Glass Composites by Light Curing Induced 3D Printing for Next-Generation Modular Lighting Devices. Adv. Opt. Mater. 2022, 10, 2201110. [Google Scholar] [CrossRef]
- Zhang, T.; Kitagawa, Y.; Moriake, R.; Ferreira da Rosa, P.P.; Islam, M.J.; Yoneda, T.; Inokuma, Y.; Fushimi, K.; Hasegawa, Y. Hybrid Eu(III) Coordination Luminophore Standing on Two Legs on Silica Nanoparticles for Enhanced Luminescence. Chemistry 2021, 27, 14438–14443. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, H.; Ran, G.; Mangel, D.N.; Yao, Y.; Zhang, R.; Tan, J.; Zhang, W.; Song, J.; Sessler, J.L.; et al. Metal Modulation: An Easy-to-Implement Tactic for Tuning Lanthanide Phototheranostics. J. Am. Chem. Soc. 2021, 143, 7541–7552. [Google Scholar] [CrossRef]
- Szpikowska-Sroka, B.; Pawlik, N.; Goryczka, T.; Pisarski, W.A. Effect of the initial reagents concentration on final crystals size and luminescence properties of PbF2:Eu3+ phosphors. J. Alloys Compd. 2018, 730, 150–160. [Google Scholar] [CrossRef]
- De Azevedo, L.A.; Gamonal, A.; Maier-Queiroz, R.; Silva, C.S.; Ferro, J.N.S.; Oliveira, P.d.A.S.C.; Barreto, E.O.; da Luz, L.L.; Alves Júnior, S. Multi-stimuli-responsive luminescent MCM48 hybrid for advanced anti-counterfeiting applications. J. Mater. Chem. C 2021, 9, 9261–9270. [Google Scholar] [CrossRef]
- Ansari, A.A. Photochemical studies of monodispersed YPO4: Eu microspheres: The role of surface modification on structural and luminescence properties. J. Photochem. Photobiol. A Chem. 2017, 343, 126–132. [Google Scholar] [CrossRef]
- Patten, J.T.; Byrne, R.H. Assessment of Fe(III) and Eu(III) complexation by silicate in aqueous solutions. Geochim. Cosmochim. Acta 2017, 202, 361–373. [Google Scholar] [CrossRef]
- Novotný, M.; Vondráček, M.; Marešová, E.; Fitl, P.; Bulíř, J.; Pokorný, P.; Havlová, Š.; Abdellaoui, N.; Pereira, A.; Hubík, P.; et al. Optical and structural properties of ZnO:Eu thin films grown by pulsed laser deposition. Appl. Surf. Sci. 2019, 476, 271–275. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Cao, J.; Wei, Z.; Guo, Y.; Wang, Q.; Peng, Q.; Li, Y.; He, X. Excellent flexibility of high-temperature-treated SiO2-TiO2 hybrid fibres and their enhanced luminescence with Eu3+ doping. Ceram. Int. 2017, 43, 12710–12717. [Google Scholar] [CrossRef]
- Liu, S.; Cui, H.; Liu, M.; Chen, J.; Wen, M.; Li, S.; Wang, W.; Li, J.-G.; Sun, X. Spherical red-emitting X1-Y2SiO5:Eu and α-Y2Si2O7:Eu phosphors with high color purity: The evolution of morphology, phase and photoluminescence upon annealing. Ceram. Int. 2022, 48, 8641–8652. [Google Scholar] [CrossRef]
- Mason, H.E.; Begg, J.D.; Maxwell, R.S.; Kersting, A.B.; Zavarin, M. A novel solid-state NMR method for the investigation of trivalent lanthanide sorption on amorphous silica at low surface loadings. Environ. Sci. Process. Impacts 2016, 18, 802–809. [Google Scholar] [CrossRef]
- Armelao, L.; Belli Dell’Amico, D.; Bellucci, L.; Bottaro, G.; Labella, L.; Marchetti, F.; Samaritani, S. Smart Grafting of Lanthanides onto Silica via N,N-Dialkylcarbamato Complexes. Inorg. Chem. 2016, 55, 939–947. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Hu, C.; Li, W.; Lei, B.; Liu, Y.; Zhuang, J. Construction of NaYF(4):Eu@carbon dots nanocomposites for multifunctional applications. J. Colloid. Interface Sci. 2019, 543, 156–163. [Google Scholar] [CrossRef]
- Lin, C.; Song, Y.; Gao, F.; Zhang, H.; Sheng, Y.; Zheng, K.; Shi, Z.; Xu, X.; Zou, H. Synthesis and luminescence properties of Eu(III)-doped silica nanorods based on the sol–gel process. J. Sol-Gel Sci. Technol. 2013, 69, 536–543. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Zhou, X.; Sun, P.; Fu, L.; You, Y.; Xu, M.; You, Z.; Kai, G.; He, C. Tumor-targeted biodegradable multifunctional nanoparticles for cancer theranostics. Chem. Eng. J. 2019, 378, 122171. [Google Scholar] [CrossRef]
- Dalmis, R.; Birlik, I.; Ak Azem, N.F.; Celik, E. Structurally colored silica photonic crystal coatings modified by Ce or Eu rare-earth dopants. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125138. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, H.; Lin, Y.; Yang, Z.; Cheng, X.; Huang, J. Photoluminescent Lanthanide-Doped Silica Nanotubes: Sol−Gel Transcription from Functional Template. J. Phys. Chem. C 2011, 115, 7323–7330. [Google Scholar] [CrossRef]
- Cheng, C.-A.; Chen, W.; Zhang, L.; Wu, H.H.; Zink, J.I. A Responsive Mesoporous Silica Nanoparticle Platform for Magnetic Resonance Imaging-Guided High-Intensity Focused Ultrasound-Stimulated Cargo Delivery with Controllable Location, Time, and Dose. J. Am. Chem. Soc. 2019, 141, 17670–17684. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.; Yao, P.; Li, J.; Chen, F.; Liu, Y. Sink-in/pile-up formation and crack nucleation mechanisms of high purity fused silica and soda-lime silica glass during nanoindentation experiments. Ceram. Int. 2020, 46, 24698–24709. [Google Scholar] [CrossRef]
- Garcia, E.; Arnold, C.; Hermier, J.P.; D’Amico, M. Correction: Gold plasmonic enhanced luminescence of silica encapsulated semiconductor hetero-nanoplatelets. Nanoscale Adv. 2021, 3, 5968. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, H.; Wang, W.; Wang, L.; Zhang, L.; Liu, T.; Sheng, J.; Zhu, S.; He, D.; Lin, L.; et al. Atomic origins of the strong metal-support interaction in silica supported catalysts. Chem. Sci. 2021, 12, 12651–12660. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Z.; Li, X.; Jiangyin, X.; Djati Utomo, H. Development of water-based thermal insulation paints using silica aerogel made from incineration bottom ash. Energy Build. 2022, 259, 111866. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Xu, B.; Chen, Y. Fabrication and Characterization of Porous Core–Shell Graphene/SiO2 Nanocomposites for the Removal of Cationic Neutral Red Dye. Appl. Sci. 2020, 10, 8529. [Google Scholar] [CrossRef]
- Alftessi, S.A.; Othman, M.H.D.; Adam, M.R.; Farag, T.M.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Habib, M.A.; Raji, Y.O.; Hubadillah, S.K. Novel silica sand hollow fibre ceramic membrane for oily wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 104975. [Google Scholar] [CrossRef]
- Kong, S.; Wu, S.; Tang, Z.; Guo, B. Hydrogen bond-containing oligomer as a facile interfacial mediator in rubber/silica composites. Compos. Commun. 2023, 37, 101413. [Google Scholar] [CrossRef]
- Shams, M.; Alam, I.; Chowdhury, I. Interactions of nanoscale plastics with natural organic matter and silica surfaces using a quartz crystal microbalance. Water Res. 2021, 197, 117066. [Google Scholar] [CrossRef]
- Wang, M.; Kickhoefer, V.A.; Lan, E.H.; Dunn, B.S.; Rome, L.H.; Mahendra, S. Vault nanocapsule-mediated biomimetic silicification for efficient and robust immobilization of proteins in silica composites. Chem. Eng. J. 2021, 418, 129406. [Google Scholar] [CrossRef]
- Dahal, R.H.; Nguyen, T.M.; Shim, D.S.; Kim, J.Y.; Lee, J.; Kim, J. Development of Multifunctional Cosmetic Cream Using Bioactive Materials from Streptomyces sp. T65 with Synthesized Mesoporous Silica Particles SBA-15. Antioxidants 2020, 9, 278. [Google Scholar] [CrossRef]
- Yu, J.; Kim, Y.H.; Kim, H.M.; Oh, J.M.; Kim, Y.R.; Choi, S.J. Determination of the fate and biological responses of food additive silica particles in commercial foods. Food Chem. 2020, 331, 127304. [Google Scholar] [CrossRef]
- Errington, E.; Guo, M.; Heng, J.Y.Y. Synthetic amorphous silica: Environmental impacts of current industry and the benefit of biomass-derived silica. Green. Chem. 2023, 25, 4244–4259. [Google Scholar] [CrossRef]
- Porrang, S.; Davaran, S.; Rahemi, N.; Allahyari, S.; Mostafavi, E. How Advancing are Mesoporous Silica Nanoparticles? A Comprehensive Review of the Literature. Int. J. Nanomed. 2022, 17, 1803–1827. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Naumova, A.O.; Alexandrovskaya, A.Y.; Zaitsev, N.K. Optimizing Production Conditions for a Composite Optical Oxygen Sensor Using Mesoporous SiO2. Nanotechnologies Russ. 2019, 13, 602–608. [Google Scholar] [CrossRef]
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis Methods of Mesoporous Silica Materials. Mater. Today Proc. 2017, 4, 350–357. [Google Scholar] [CrossRef]
- Sharma, J.; Polizos, G. Hollow Silica Particles: Recent Progress and Future Perspectives. Nanomaterials 2020, 10, 1599. [Google Scholar] [CrossRef]
- Li, K.; Yang, C.; Yu, H.; Xiao, T.; Guan, W.; Ding, P.; Yin, H.; Cohen Stuart, M.A.; Wang, J.; Zhou, S. Coordination-Enhanced Synthesis for Hollow Mesoporous Silica Nanoreactors. Chem. Mater. 2020, 32, 2086–2096. [Google Scholar] [CrossRef]
- Allouche, J.; Le Beulze, A.; Dupin, J.-C.; Ledeuil, J.-B.; Blanc, S.; Gonbeau, D. Hybrid spiropyran–silica nanoparticles with a core-shell structure: Sol–gel synthesis and photochromic properties. J. Mater. Chem. 2010, 20, 9370–9378. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; Li, M.; Tian, A.; Wang, C.; Fu, S. Controlling morphology and particle size of hollow poly(styrene-divinylbenzene) microspheres fabricated by template-based method. J. Saudi Chem. Soc. 2018, 22, 644–653. [Google Scholar] [CrossRef]
- Zhao, C.; Ge, Z.; Jiang, Z.; Yan, S.; Shu, J.; Wang, M.; Ge, X. Study on the morphological regulation mechanism of hollow silica microsphere prepared via emulsion droplet template. Chin. Chem. Lett. 2023, 34, 107499. [Google Scholar] [CrossRef]
- Ye, L.; Li, L.; Wang, X.; Zhang, Y.; Yan, L. Template-free synthesis of uniform hollow silica nanoparticles for controllable antireflection coatings. Ceram. Int. 2020, 46, 7453–7458. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Z.; Ren, D.; Xiang, X.; Chen, N.; Li, X.; Ye, Z.; Chen, Q.; Ma, S. Preparation of hollow nanoparticles with controllable diameter by one-step controlled etching of microporous silica particles using an ammonia-based etchant. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124579. [Google Scholar] [CrossRef]
- Yuan, N.; Sun, Y.-N.; Liu, Z.-W.; Han, B.-H. Facile synthesis of diamine-functionalized hollow mesoporous silica sphere with self-templating method. J. Porous Mater. 2018, 25, 1715–1721. [Google Scholar] [CrossRef]
- Watanabe, K.; Kuroda, K.; Nagao, D. Polyethylenimine-assisted synthesis of hollow silica spheres without shape deformation. Mater. Chem. Phys. 2021, 262, 124267. [Google Scholar] [CrossRef]
- Wu, X.; Nan, Z. Degradation of rhodamine B by a novel Fe3O4/SiO2 double-mesoporous-shelled hollow spheres through photo-Fenton process. Mater. Chem. Phys. 2019, 227, 302–312. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, E.; Yu, L.; Wang, R.; Zhu, Y.; Fu, Y.; Ni, Q. Self-templated route to synthesis bowl-like and deflated balloon-like hollow silica spheres. Mater. Lett. 2017, 206, 150–153. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tagaya, M.; Kataoka, T.; Chatani, S.; Inui, M.; Higa, Y.; Motozuka, S. Synthesis and photoluminescence properties of the Eu(III)-containing silica nanoparticles via a mechanochemical solid-state reaction between SiO2 and EuCl3·6H2O. Adv. Powder Technol. 2020, 31, 3650–3656. [Google Scholar] [CrossRef]
- Guillaume, C.; Frieiro, J.L.; Blázquez, O.; Labbé, C.; López-Vidrier, J.; Garrido, B.; Hernández, S.; Liu, B.; Khomenkova, L.; Frilay, C.; et al. Influence of post annealing treatments on the luminescence of rare earth ions in ZnO: Tb, Eu/Si heterojunction. Appl. Surf. Sci. 2021, 556, 149754. [Google Scholar] [CrossRef]
- Liang, Y.; Noh, H.M.; Park, S.H.; Choi, B.C.; Jeong, J.H. Colloidal GdVO4:Eu3+ @SiO2 nanocrystals for highly selective and sensitive detection of Cu2+ ions. Appl. Surf. Sci. 2018, 433, 381–387. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Y.; Li, B.; Huang, Z.; Gao, Z.; Mao, X.; Wang, W.; Zhu, Z.; Kipper, M.J.; Huang, L.; et al. Polyacrylonitrile fluorescent nanofibers for selective and reversible copper detection in aqueous solutions. Appl. Surf. Sci. 2022, 602, 154302. [Google Scholar] [CrossRef]
- Alcaraz, L.; Isasi, J. Synthesis and study of Y0.9Ln0.1VO4 nanophosphors and Y0.9Ln0.1VO4@SiO2 luminescent nanocomposites with Ln=Eu, Dy, Er. Ceram. Int. 2017, 43, 5311–5318. [Google Scholar] [CrossRef]
- Chen, P.; Mao, Y.; Hou, S.; Chen, Y.; Liu, X.; Lou, Y.; Chen, A.; Yang, L.; Li, J.; Dai, N. Effects of In2O3 nanoparticles doping on the photoluminescent properties of Eu2+/Eu3+ ions in silica glasses. Ceram. Int. 2019, 45, 233–238. [Google Scholar] [CrossRef]
- De Pablos-Martín, A.; Oberleiter, B.; Thieme, C.; Rainer, T.; Höche, T. Nanostructure investigation of Eu3+-containing porous silica glasses: Influence of pore size and sintering conditions. Ceram. Int. 2018, 44, 14625–14630. [Google Scholar] [CrossRef]
- Jia, M.; Wen, J.; Pan, X.; Zhang, L.; Yuan, J.; Huang, Y.; Zhang, X.; He, L.; Pang, F.; Wang, T. Flexible Scintillation Silica Fiber with Engineered Nanocrystals for Remote Real-Time X-ray Detection. ACS Appl. Mater. Interfaces 2022, 14, 1362–1372. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Sung, S.-J.; Chang, C.-H. Downshifting and antireflective thin films for solar module power enhancement. Mater. Des. 2021, 201, 109454. [Google Scholar] [CrossRef]
- Li, H.; Kang, J.; Yang, J.; Wu, B. Fabrication of Aunanoparticle@mSiO2@Y2O3:Eu nanocomposites with enhanced fluorescence. J. Alloys Compd. 2016, 673, 283–288. [Google Scholar] [CrossRef]
- Han, Y.; Guo, Z.; Teng, S.; Xia, H.; Wang, D.; Han, M.-Y.; Yang, W. Rationalized Fabrication of Structure-Tailored Multishelled Hollow Silica Spheres. Chem. Mater. 2019, 31, 7470–7477. [Google Scholar] [CrossRef]
- Liu, L.; Lu, Y.L.; He, L.; Zhang, W.; Yang, C.; Liu, Y.D.; Zhang, L.Q.; Jin, R.G. Novel Europium-Complex/Nitrile-Butadiene Rubber Composites. Adv. Funct. Mater. 2005, 15, 309–314. [Google Scholar] [CrossRef]






| Sample | y0 | A1 | t1 | A2 | t2 | τ |
|---|---|---|---|---|---|---|
| HS@Eu | 6.04 × 10−4 | 1.23 | 460,387.78 | 0.10 | 950,775.77 | 460 μs |
| Ms@Eu | 4.71 × 10−4 | 0.51 | 391,854.01 | 0.77 | 712,052.92 | 557 μs |
| S@Eu | 5.47 × 10−4 | 0.85 | 436,486.49 | 0.47 | 730,546.66 | 549 μs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Hu, X.; Yang, Y.; Wang, W.; Wang, Y.; Gong, X.; Geng, C.; Tang, J. Synthesis of Mesoporous and Hollow SiO2@ Eu(TTA)3phen with Enhanced Fluorescence Properties. Materials 2023, 16, 4501. https://doi.org/10.3390/ma16134501
Wang Z, Hu X, Yang Y, Wang W, Wang Y, Gong X, Geng C, Tang J. Synthesis of Mesoporous and Hollow SiO2@ Eu(TTA)3phen with Enhanced Fluorescence Properties. Materials. 2023; 16(13):4501. https://doi.org/10.3390/ma16134501
Chicago/Turabian StyleWang, Zhiheng, Xiaoli Hu, Yinqi Yang, Wei Wang, Yao Wang, Xuezhong Gong, Caiyun Geng, and Jianguo Tang. 2023. "Synthesis of Mesoporous and Hollow SiO2@ Eu(TTA)3phen with Enhanced Fluorescence Properties" Materials 16, no. 13: 4501. https://doi.org/10.3390/ma16134501
APA StyleWang, Z., Hu, X., Yang, Y., Wang, W., Wang, Y., Gong, X., Geng, C., & Tang, J. (2023). Synthesis of Mesoporous and Hollow SiO2@ Eu(TTA)3phen with Enhanced Fluorescence Properties. Materials, 16(13), 4501. https://doi.org/10.3390/ma16134501






