Microstructural Changes and Determination of a Continuous Cooling Transformation (CCT) Diagram Using Dilatometric Analysis of M398 High-Alloy Tool Steel Produced by Microclean Powder Metallurgy
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material and Procedure
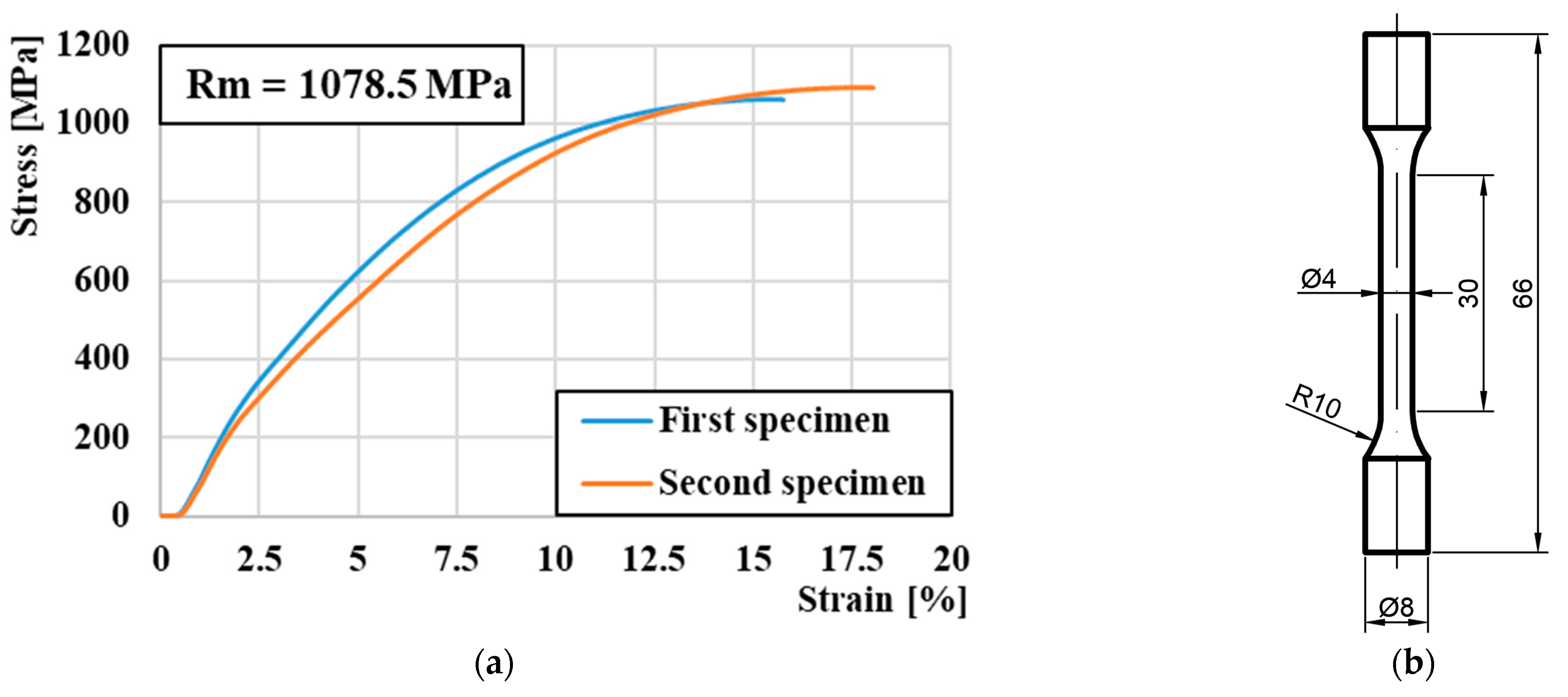
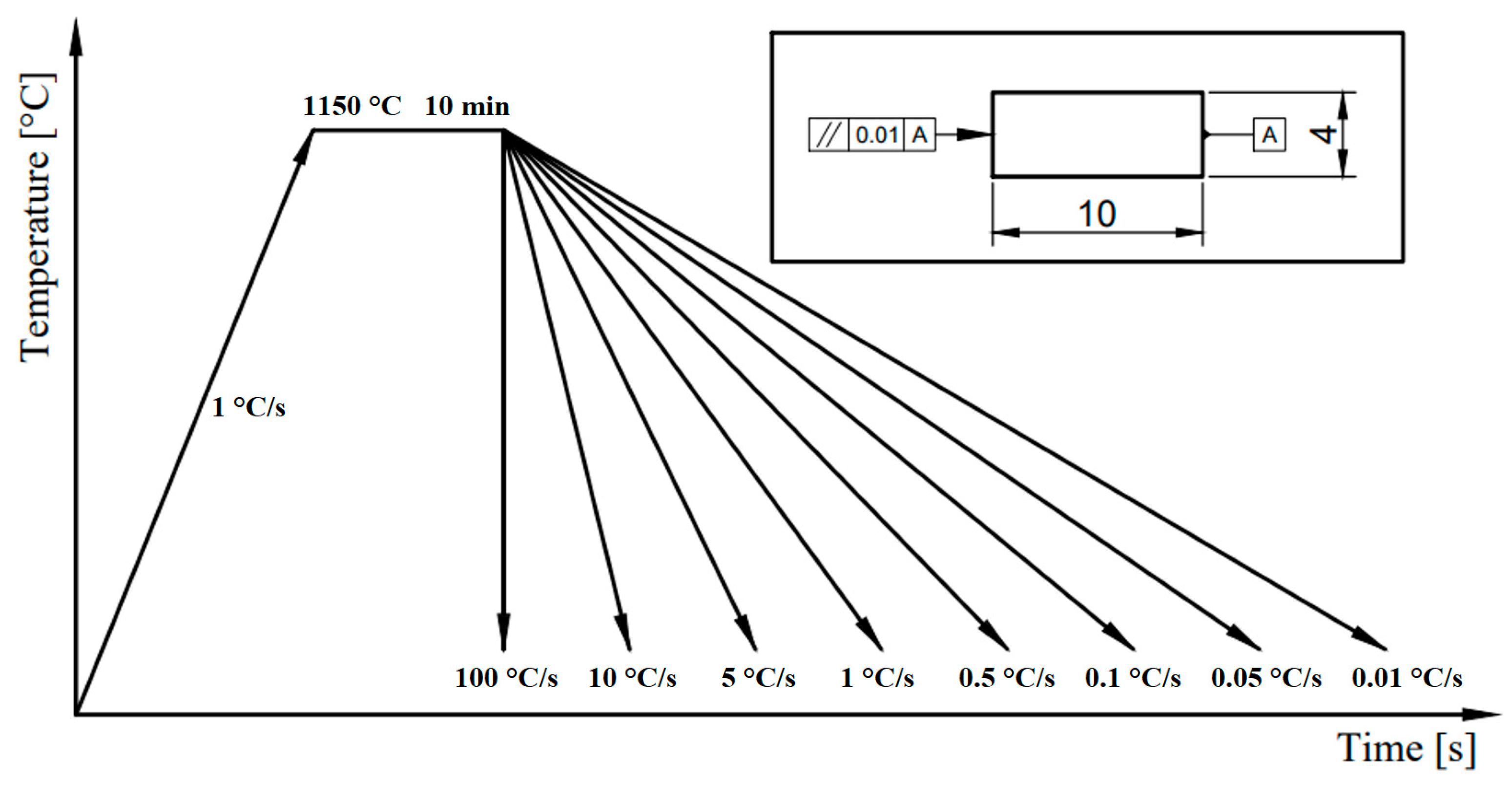
2.1.1. Manufacturing Process
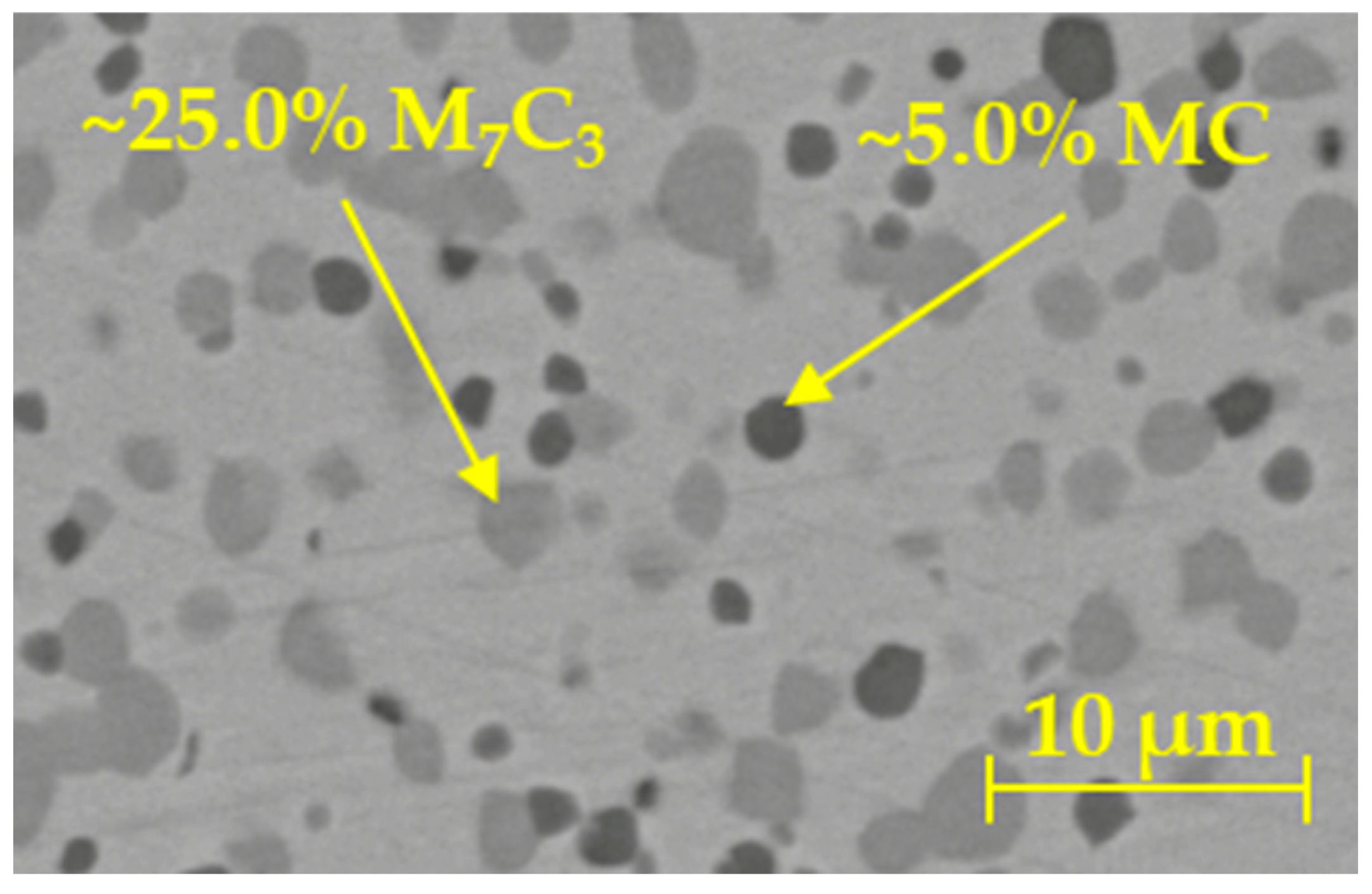
2.1.2. Phase Fraction
3. Results and Discussions
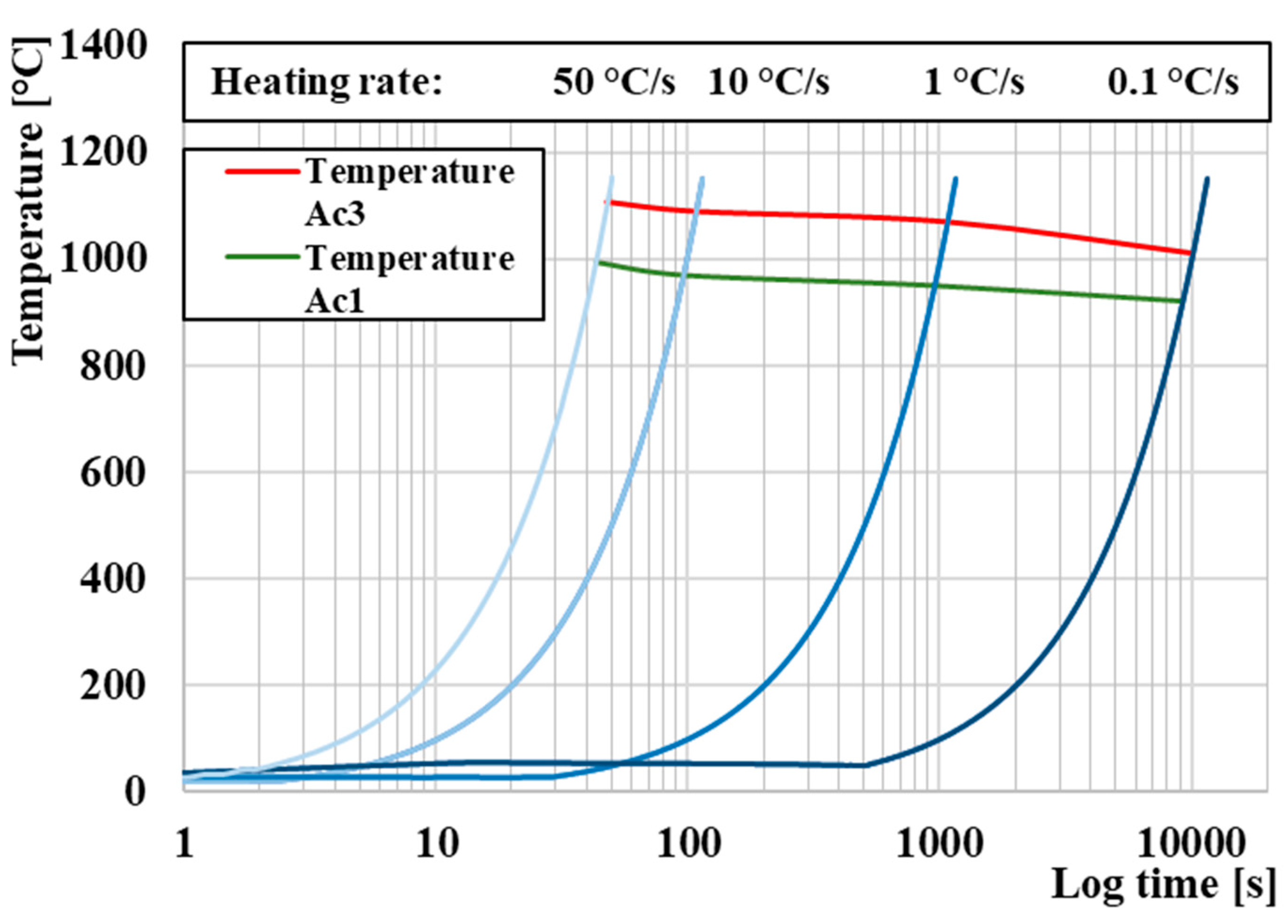
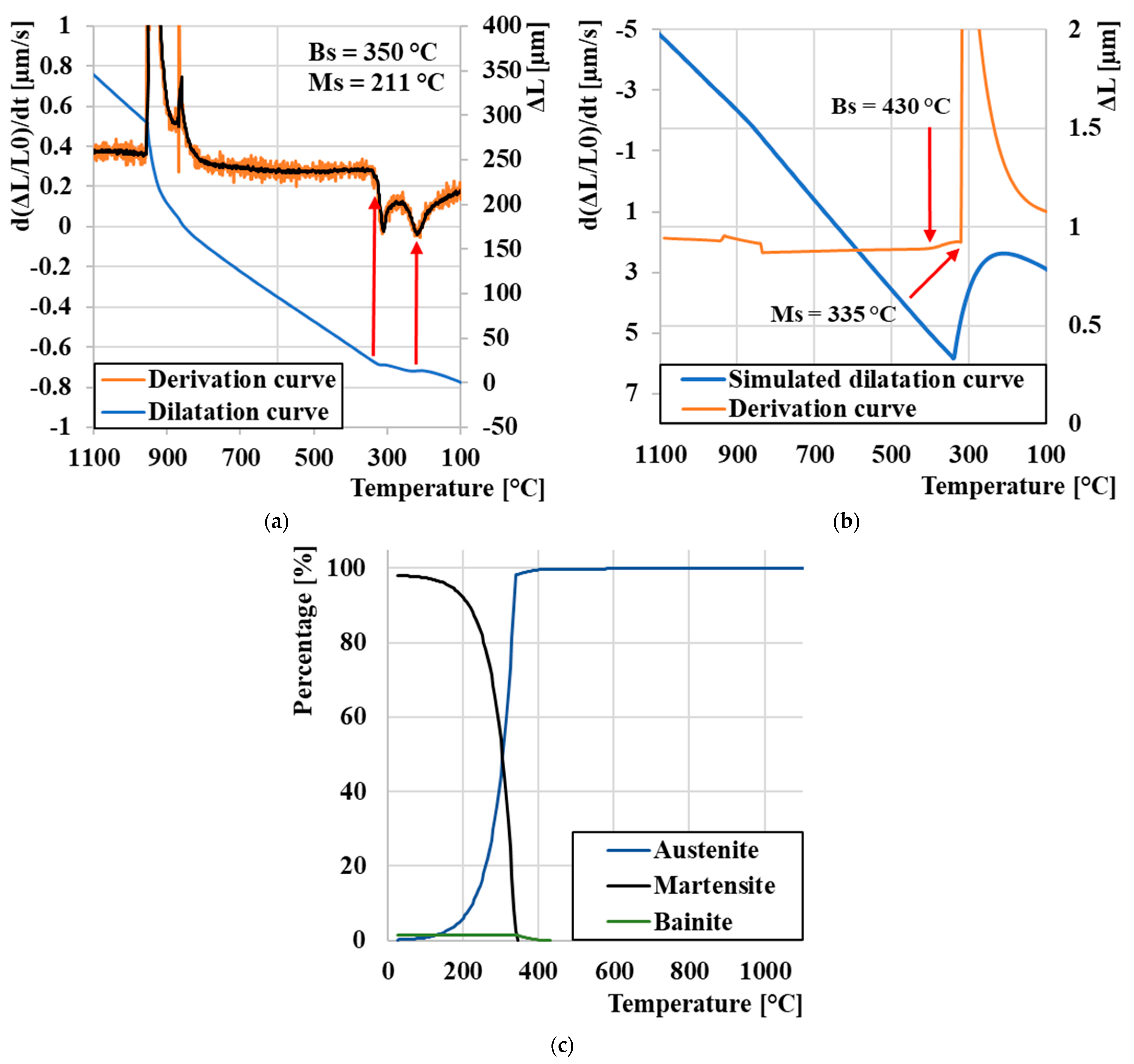
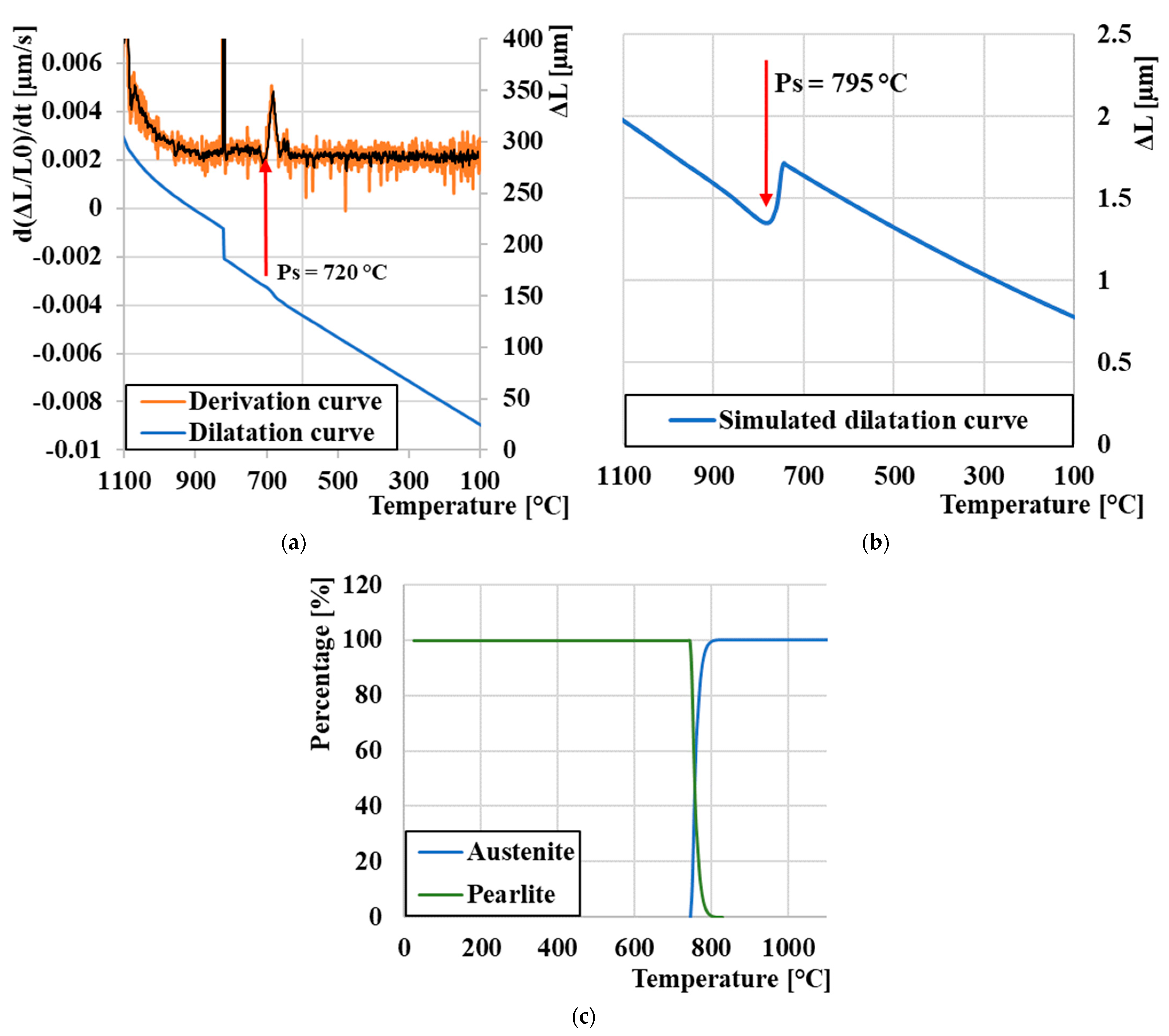
3.1. Determination of Critical Transformation Temperatures during Heating Ac1 and Ac3
3.2. Curie Temperature (TC) Measurement
3.3. Dilatation Curves Analysis
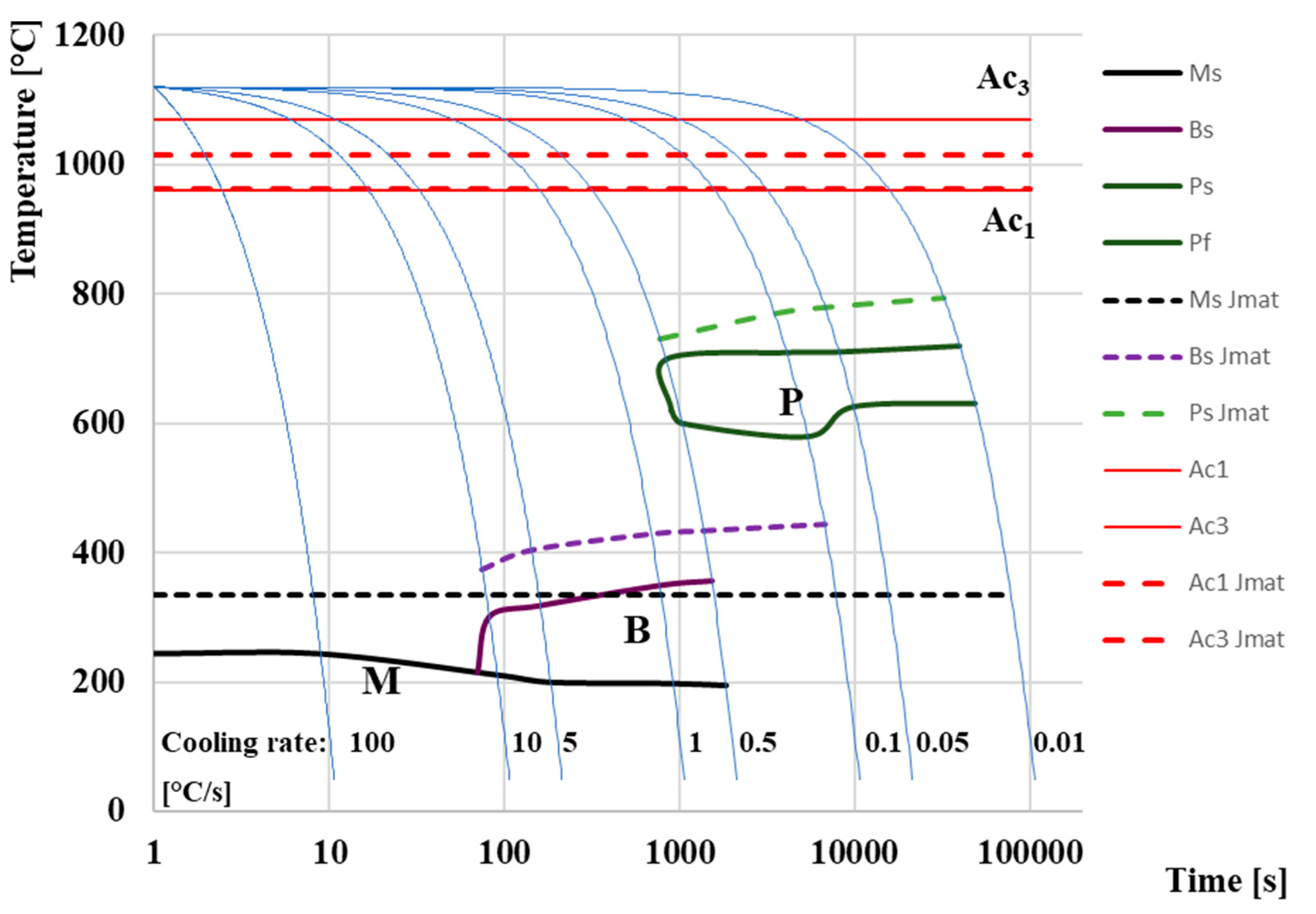
3.4. CCT Diagram
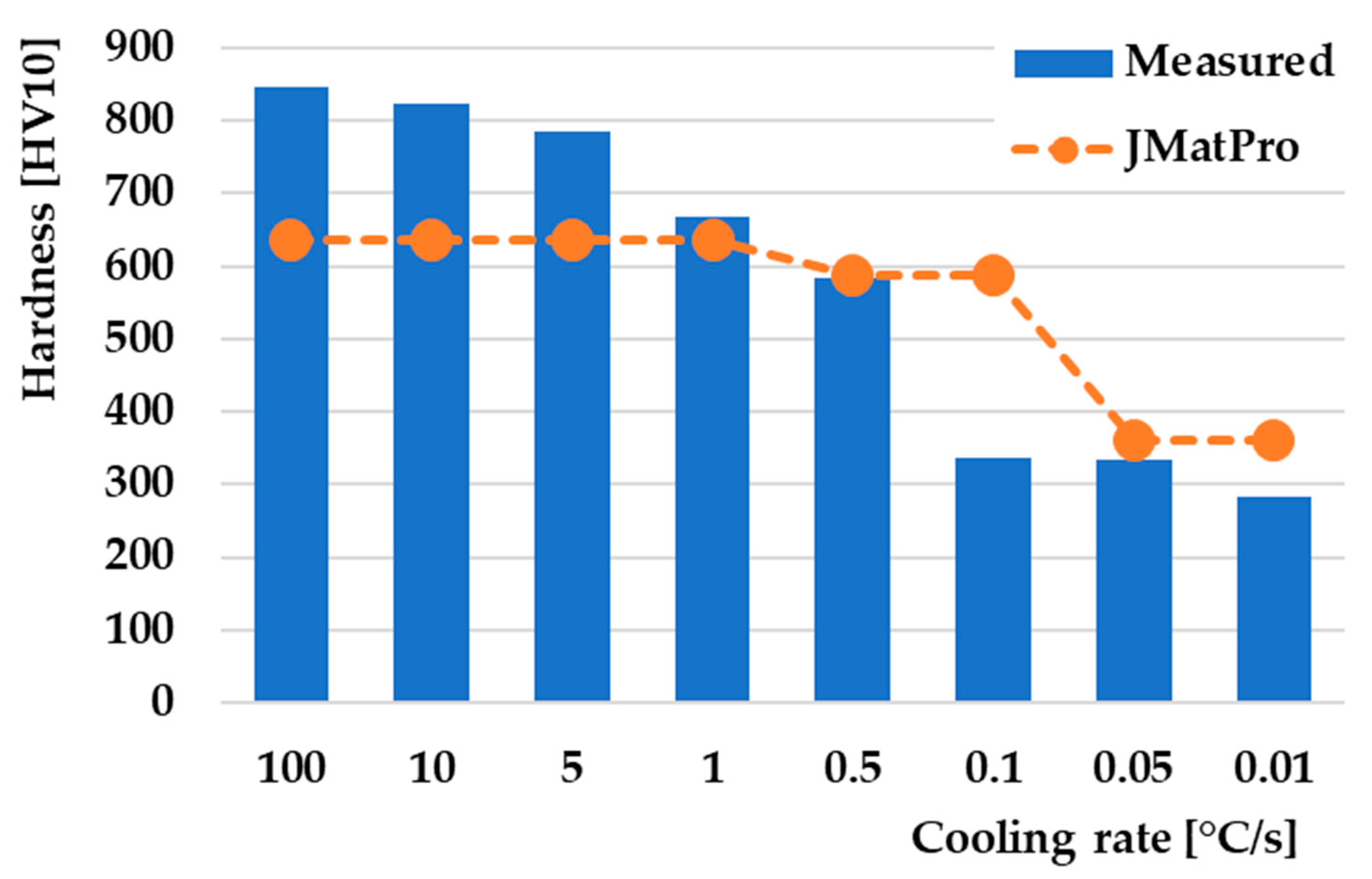
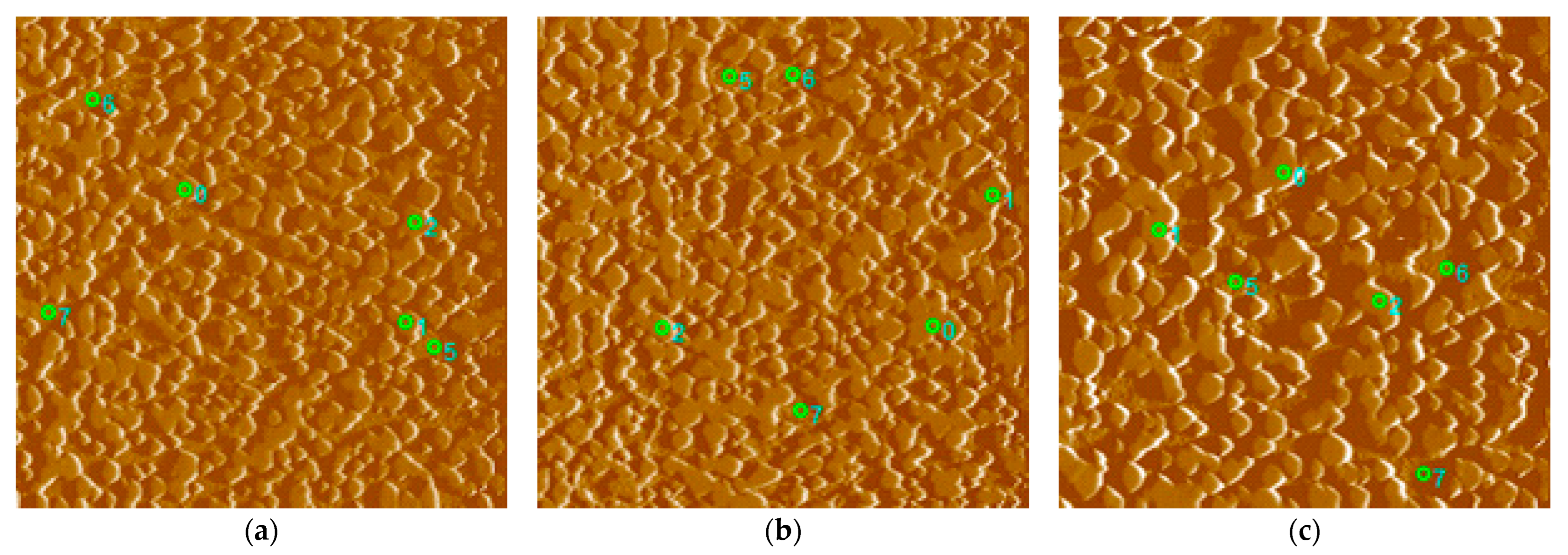
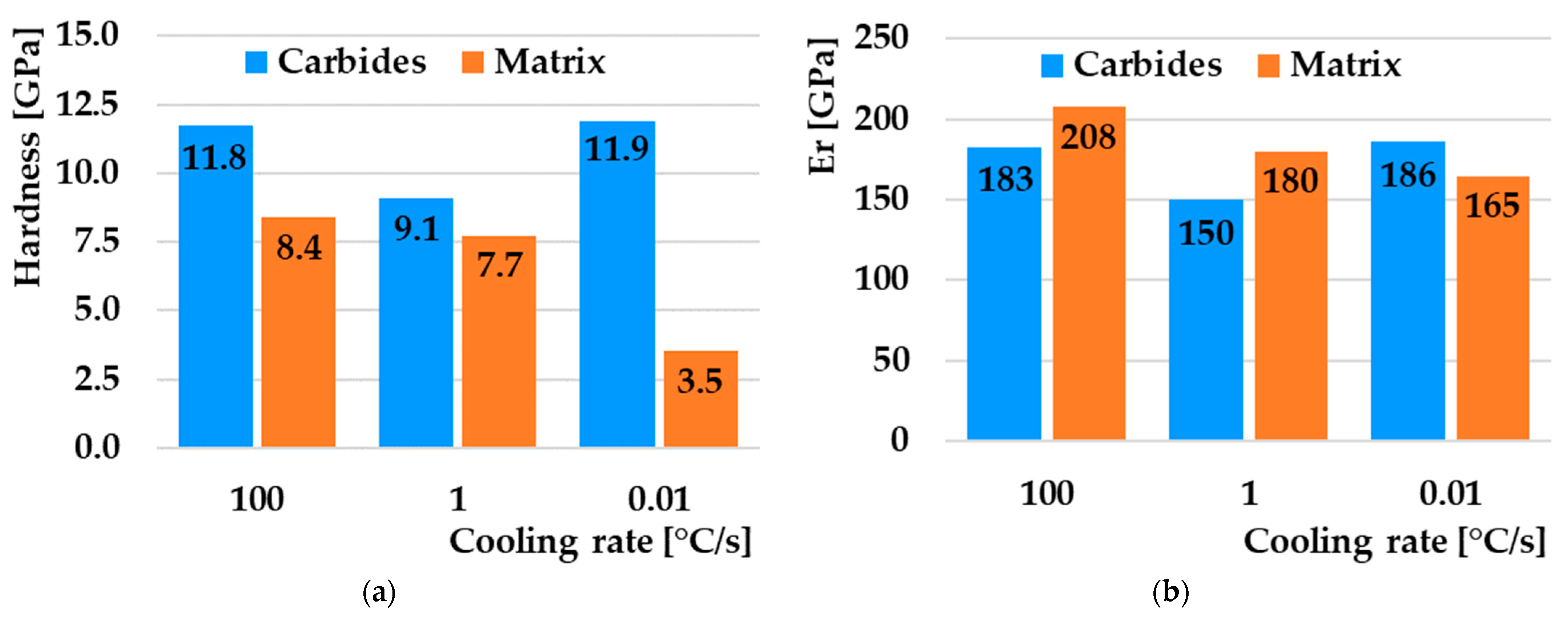
3.5. Hardness in CCT Diagram
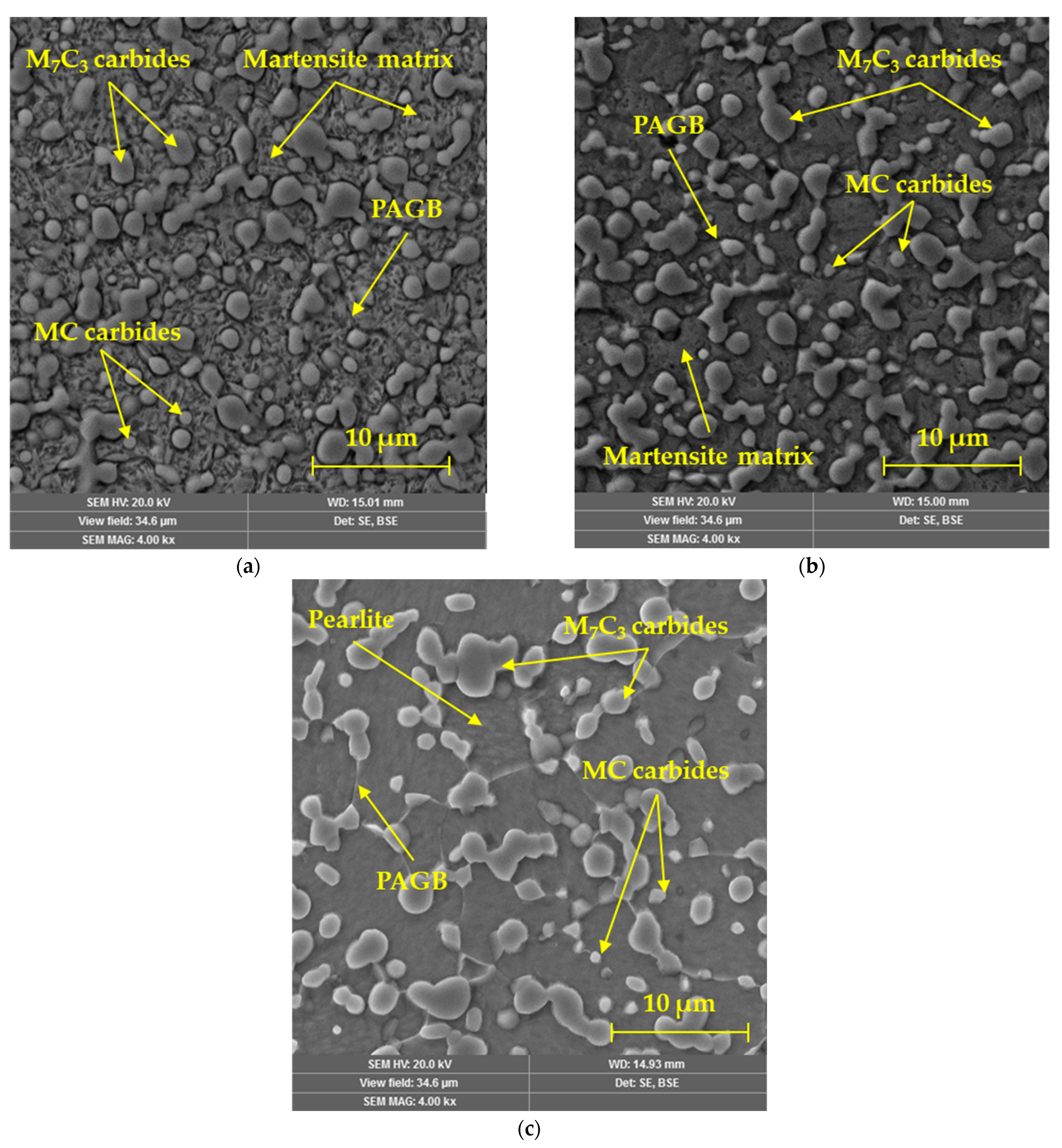
3.6. Microstructure in CCT Diagram
4. Conclusions
- Setting the austenitizing temperature to 1120 °C led to complete austenitization of the entire volume of the sample material, while the temperatures Ac1 and Ac3 were 960 °C and 1070 °C, respectively;
- Heating the samples at a rate of 1 °C/s provides ideal properties due to the optimal setting of the combination of output mechanical and economic properties. Higher heating rates lead to an increase in the temperature Ac1 and Ac3, respectively (increasing PAGS), and conversely, a decrease in temperature leads to an extension of the sample heating time and, thus, to an economic increase in operating costs;
- The actual measured CCT constructed for M398 steel consisted of three different microstructural regions, namely martensitic, bainitic, and pearlitic. The resulting CCT diagram can be used to design various heat treatments. The critical cooling rate must be set to more than 10 °C/s in order to obtain a purely martensitic structure. To reduce the amount of residual austenite, it is necessary that the temperature Mf moves up to negative temperatures due to the high proportion of C in the M398 material;
- The simulation software JMatPro® API v7.0 is a good tool for the approximate determination of the CCT diagram, but it does not present as qualified results as the actual dilatometric measurements. The temperature difference Ms of almost 100 °C is an important factor that confirms this assumption;
- The measured hardness has a decreasing character depending on the decrease in the cooling rate. The highest hardness achieved was 846 HV10 at the highest cooling rate of 100 °C/s, while the lowest hardness was 284 HV10 at the lowest cooling rate of 0.01 °C/s;
- Experimental steel is not suitable for bainitic quenching during anisotemic cooling due to the occurrence of only a small proportion of this structure. An increase in the volume of the bainitic phase would be possible only during isothermal hardening;
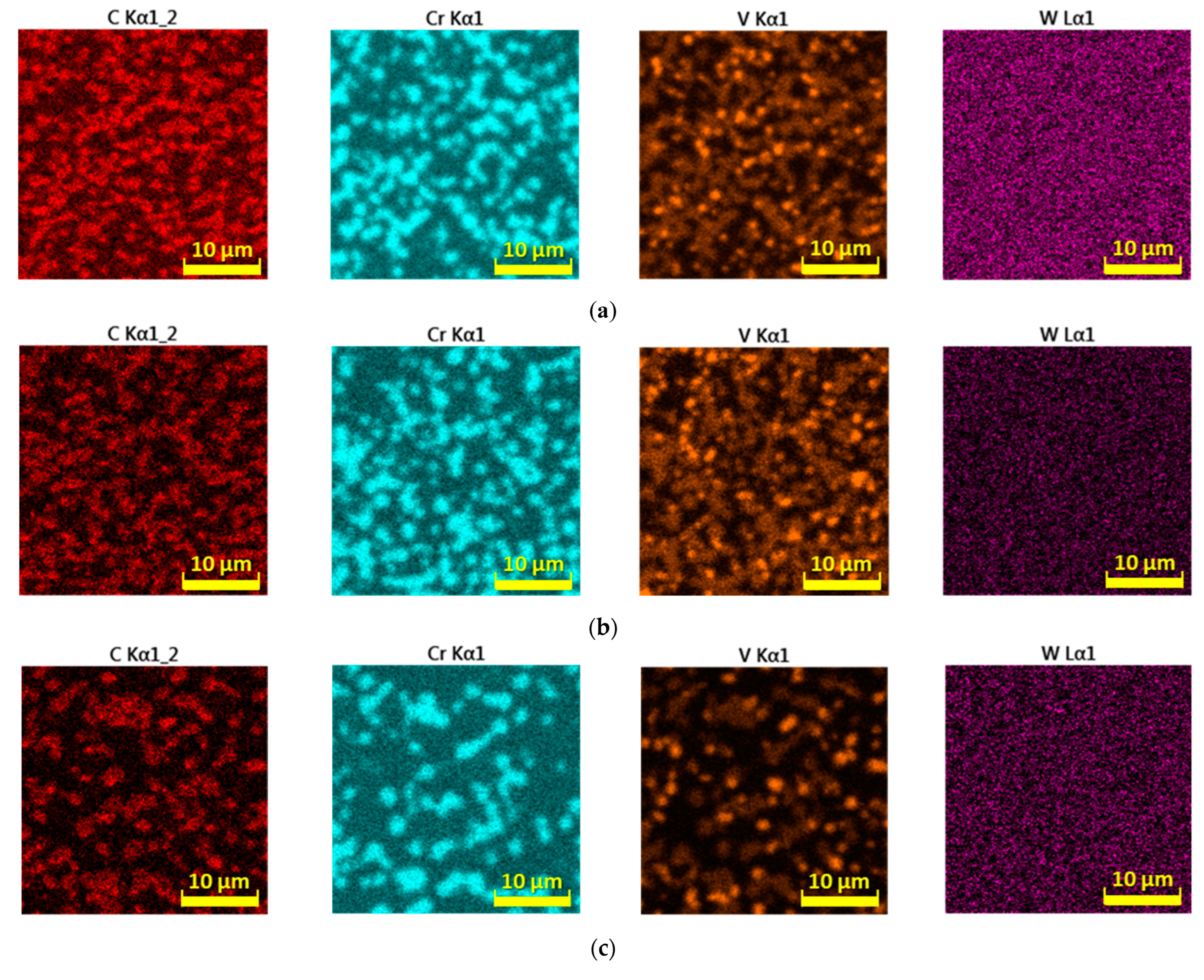
- The distribution of the carbide phase is less uniform when the cooling rate is reduced, which results in a greater representation of the metal matrix in the analyzed area. The chemical element W does not show the ability of such significant agglomeration under the selected cooling conditions as the above-mentioned elements. Molybdenum records the ability to form microregions, but only at higher cooling rates;
- The highest value of nanohardness was achieved by the matrix in the case of cooling at 100 °C/s, where there is a high proportion of the pure martensitic phase, and the lowest in the case of a cooling rate of 0.01 °C/s, where a heterogeneous mixture of pearlite (ferrite + cementite) prevails. The values of the reduced modulus of elasticity also correlate with the given nanohardness results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tulliani, J.M.; Pagès, G.; Fantozzi, G.; Montanaro, L. Dilatometry as a tool to study a new synthesis for calcium hexaluminate. J. Therm. Anal. Calorim. 2003, 72, 1135–1140. [Google Scholar] [CrossRef]
- Gaisford, S.; Kett, V.; Haines, P.; Charsley, E.; Price, D.; Hunter, N.; Gabbot, P.; Priestlay, I.; Royall, P.; Scowen, I.; et al. Principles of Thermal Analysis and Calorimetry; Royal Society of Chemistry: London, UK, 2016; p. 280. [Google Scholar]
- Vorhauer, A.; Pippan, R. On the Onset of a Steady State in Body-Centered Cubic Iron during Severe Plastic Deformation at Low Homologous Temperatures. Metall. Mater. Trans. A 2008, 39, 417–429. [Google Scholar] [CrossRef]
- Ferrari, C.; Forsberg, M.T.; Andersson, A.; Mikula, J.; Beran, P. Effect of Austenitising Temperature and Cooling Rate on Microstructure in a Hot-Work Tool Steel. In Proceedings of the 6th Swedish Production Symposium; 2014; pp. 1–7. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A764703&dswid=2664 (accessed on 19 April 2023).
- Grajcar, A.; Zalecki, W.; Skrzypczyk, P.; Kilarski, A.; Kowalski, A.; Kołodziej, S. Dilatometric study of phase transformations in advanced high-strength bainitic steel. J. Therm. Anal. Calorim. 2014, 118, 739–748. [Google Scholar] [CrossRef]
- Morawiec, M.; Grajcar, A.; Zalecki, W.; Garcia-Mateo, C.; Opiela, M. Dilatometric study of phase transformations in 5 Mn steel subjected to different heat treatments. Materials 2020, 13, 958. [Google Scholar] [CrossRef] [PubMed]
- Hunkel, M.; Surm, H.; Steinbacher, M. Dilatometry. In Handbook of Thermal Analysis and Calorimetry; Elsevier Science BV: Amsterdam, The Netherlands, 2018; Volume 6, pp. 103–129. [Google Scholar] [CrossRef]
- Marchetti, S.; Monaco, P.; Totani, G.; Marchetti, D. In situ tests by seismic dilatometer (SDMT). In From Research to Practice in Geotechnical Engineering; American Society of Civil Engineers: Reston, VA, USA, 2008; pp. 292–311. [Google Scholar] [CrossRef]
- Kop, T.A.; Sietsma, J.; Van Der Zwaag, S. Dilatometric analysis of phase transformations in hypo-eutectoid steels. J. Mater. Sci. 2001, 36, 519–526. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Mesplont, C.; De Cooman, C. Calculation of the phase transformation kinetics from a dilatation curve. J. Mater. Process. Technol. 2002, 129, 345–348. [Google Scholar] [CrossRef]
- Bernasconi, A.; Diella, V.; Pagani, A.; Pavese, A.; Francescon, F.; Young, K.; Tunnicliffe, L. The role of firing temperature, firing time and quartz grain size on phase-formation, thermal dilatation and water absorption in sanitary-ware vitreous bodies. J. Eur. Ceram. Soc. 2011, 31, 1353–1360. [Google Scholar] [CrossRef]
- Choi, S. Model for estimation of transformation kinetics from the dilatation data during a cooling of hypoeutectoid steels. Mater. Sci. Eng. A 2003, 363, 72–80. [Google Scholar] [CrossRef]
- Halmešová, K.; Procházka, R.; Koukolíková, M.; Džugan, J.; Konopík, P.; Bucki, T. Extended continuous cooling transformation (CCT) diagrams determination for additive manufacturing deposited steels. Materials 2022, 15, 3076. [Google Scholar] [CrossRef]
- Rajan, T.V.S.; Sharma, C.P.; Sharma, A. Heat Treatment: Principles and Techniques; PHI Learning Pvt. Ltd.: New Delhi, India, 2011; p. 480. [Google Scholar]
- Navarrete Pino, B.Y.; Torres Castillo, A.A.; Gutiérrez Castañeda, E.J.; Espinosa Zúñiga, L.A.; Hernández Hernández, L.; Salinas Rodríguez, A.; Martínez Sánchez, R. Using Intercritical CCT Diagrams and Multiple Linear Regression for the Development of Low-Alloyed Advanced High-Strength Steels. Metals 2021, 11, 1768. [Google Scholar] [CrossRef]
- Huang, X.; Wang, H.; Xue, W.; Ullah, A.; Xiang, S.; Huang, H.; Zhang, G. A combined machine learning model for the prediction of time-temperature-transformation diagrams of high-alloy steels. J. Alloys Compd. 2020, 823, 153694. [Google Scholar] [CrossRef]
- Hausner, H.H. (Ed.) Modern Developments in Powder Metallurgy: Volume 1: Fundamentals and Methods; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Hausner, H.H. (Ed.) Modern Developments in Powder Metallurgy: Volume 5: Materials and Properties Proceedings of the 1970 International Powder Metallurgy Conference, Sponsored by the Metal Power Industries Federation and the American Powder Metallurgy Institute; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Samal, P.; Newkirk, J. History of powder metallurgy. ASM Handb. 2015, 7, 3–8. [Google Scholar] [CrossRef]
- Quadbeck, P.; Kümmel, K.; Hauser, R.; Standke, G.; Adler, J.; Stephani, G.; Kieback, B. Structural and material design of open-cell powder metallurgical foams. Adv. Eng. Mater. 2011, 13, 1024–1030. [Google Scholar] [CrossRef]
- Kuhn, H. (Ed.) . Powder Metallurgy Processing: The Techniques and Analyses; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Chang, I.; Zhao, Y. (Eds.) . Advances in Powder Metallurgy: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Klar, E.; Samal, P.K. Powder Metallurgy Stainless Steels: Processing, Microstructures, and Properties; ASM International: Detroit, MI, USA, 2007. [Google Scholar]
- Danninger, H.; Gierl-Mayer, C. Advanced powder metallurgy steel alloys. In Advances in Powder Metallurgy; Woodhead Publishing: Sawston, UK, 2013; pp. 149–201. [Google Scholar] [CrossRef]
- Lagutkin, S.; Achelis, L.; Sheikhaliev, S.; Uhlenwinkel, V.; Srivastava, V. Atomization process for metal powder. Mater. Sci. Eng. A 2004, 383, 1–6. [Google Scholar] [CrossRef]
- Popovich, A.; Sufiiarov, V. Metal powder additive manufacturing. In New Trends in 3D Printing; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Beckers, D.; Ellendt, N.; Fritsching, U.; Uhlenwinkel, V. Impact of process flow conditions on particle morphology in metal powder production via gas atomization. Adv. Powder Technol. 2020, 31, 300–311. [Google Scholar] [CrossRef]
- Mandal, S.; Sadeghianjahromi, A.; Wang, C.-C. Experimental and numerical investigations on molten metal atomization techniques–A critical review. Adv. Powder Technol. 2022, 33, 103809. [Google Scholar] [CrossRef]
- Yenwiset, S.; Yenwiset, T. Effect of the molten metal stream’s shape on particle size distribution of water atomized metal powder. Eng. J. 2016, 20, 187–196. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.L.; Friedrich, K. Creep resistant polymeric nanocomposites. Polymer 2004, 45, 3481–3485. [Google Scholar] [CrossRef]
- Müller, M.T.; Krause, B.; Kretzschmar, B.; Pötschke, P. Influence of feeding conditions in twin-screw extrusion of PP/MWCNT composites on electrical and mechanical properties. Compos. Sci. Technol. 2011, 71, 1535–1542. [Google Scholar] [CrossRef]
- Mack, C.; Sathyanarayana, S.; Weiss, P.; Mikonsaari, I.; Hübner, C.; Henning, F.; Elsner, P. Twin-screw extrusion of multi walled carbon nanotubes reinforced polycarbonate composites: Investigation of electrical and mechanical properties. IOP Conf. Ser. Mater. Sci. Eng. 2012, 40, 012020. [Google Scholar] [CrossRef]
- Wang, Y.; Chiao, S.M.; Hung, T.F.; Yang, S.Y. Improvement in toughness and heat resistance of poly (lactic acid)/polycarbonate blend through twin-screw blending: Influence of compatibilizer type. J. Appl. Polym. Sci. 2012, 125, E402–E412. [Google Scholar] [CrossRef]
- Alonso-González, M.; Felix, M.; Guerrero, A.; Romero, A. Effects of mould temperature on rice bran-based bioplastics obtained by injection moulding. Polymers 2021, 13, 398. [Google Scholar] [CrossRef]
- White, J.L.; Potente, H. Screw Extrusion; Hanser Publishers: Munich, Germany, 2001; p. 464. [Google Scholar]
- Yilmazer, U.; Cansever, M. Effects of processing conditions on the fiber length distribution and mechanical properties of glass fiber reinforced nylon-6. Polym. Compos. 2002, 23, 61–71. [Google Scholar] [CrossRef]
- Gaspar-Cunha, A.; Covas, J.A. The design of extrusion screws: An optimization approach. Int. Polym. Process. 2001, 16, 229–240. [Google Scholar] [CrossRef]
- Fernández, J.; Illescas, S.; Guilemany, J.M. Effect of microalloying elements on the austenitic grain growth in a low carbon HSLA steel. Mater. Lett. 2007, 61, 2389–2392. [Google Scholar] [CrossRef]
- Maropoulos, S.; Karagiannis, S.; Ridley, N. The effect of austenitising temperature on prior austenite grain size in a low-alloy steel. Mater. Sci. Eng. A 2008, 483, 735–739. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, F.; Yang, Y.; Jiang, B.; Zhang, C.L.; Liu, Y.Z. The effect of size and distribution of MnS inclusions on the austenite grain growth in a low cost hot forged steel. Steel Res. Int. 2018, 89, 1700270. [Google Scholar] [CrossRef]
- Rahman, K.M.; Vorontsov, V.A.; Dye, D. The effect of grain size on the twin initiation stress in a TWIP steel. Acta Mater. 2015, 89, 247–257. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, J.; Zhang, Z. Austenite grain growth behaviors of La-microalloyed H13 steel and its effect on mechanical properties. Metall. Mater. Trans. A 2020, 51, 4662–4673. [Google Scholar] [CrossRef]
- Gutierrez-Urrutia, I.; Raabe, D. Grain size effect on strain hardening in twinning-induced plasticity steels. Scr. Mater. 2012, 66, 992–996. [Google Scholar] [CrossRef]
- Johannsen, D.L.; Kyrolainen, A.; Ferreira, P.J. Influence of annealing treatment on the formation of nano/submicron grain size AISI 301 austenitic stainless steels. Metall. Mater. Trans. A 2006, 37, 2325–2338. [Google Scholar] [CrossRef]
- Danon, A.; Servant, C.; Alamo, A.; Brachet, J.C. Heterogeneous austenite grain growth in 9Cr martensitic steels: Influence of the heating rate and the austenitization temperature. Mater. Sci. Eng. A 2003, 348, 122–132. [Google Scholar] [CrossRef]
- Liu, Z.B.; Tu, X.; Wang, X.H.; Liang, J.X.; Yang, Z.Y.; Sun, Y.Q.; Wang, C.J. Carbide dissolution and austenite grain growth behavior of a new ultrahigh-strength stainless steel. J. Iron Steel Res. Int. 2020, 27, 732–741. [Google Scholar] [CrossRef]
- Cíger, R.; Barényi, I.; Krbaťa, M. Analysis of heat treatment parameters on the properties of selected tool steels M390 and M398 produced with powder metallurgy. Manuf. Technol. 2021, 21, 774–780. [Google Scholar] [CrossRef]
- Studeny, Z.; Krbata, M.; Dobrocky, D.; Eckert, M.; Ciger, R.; Kohutiar, M.; Mikus, P. Analysis of Tribological Properties of Powdered Tool Steels M390 and M398 in Contact with Al2O3. Materials 2022, 15, 7562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.R.; Niu, H.Z.; Zang, M.C.; Zhang, Y.H.; Liu, S.; Zhang, D.L. Tensile properties and deformation behavior of an extra-low interstitial fine-grained powder metallurgy near alpha titanium alloy by recycling coarse pre-alloyed powder. J. Mater. Sci. Technol. 2022, 129, 96–107. [Google Scholar] [CrossRef]
- Primorac, M.M.; Abad, M.D.; Hosemann, P.; Kreuzeder, M.; Maier, V.; Kiener, D. Elevated temperature mechanical properties of novel ultra-fine grained Cu–Nb composites. Mater. Sci. Eng. A 2015, 625, 296–302. [Google Scholar] [CrossRef]
- Erden, M.A.; Yaşar, N.; Korkmaz, M.E.; Ayvacı, B.; Nimel Sworna Ross, K.; Mia, M. Investigation of microstructure, mechanical and machinability properties of Mo-added steel produced by powder metallurgy method. Int. J. Adv. Manuf. Technol. 2021, 114, 2811–2827. [Google Scholar] [CrossRef]
- Totten, G.E. Steel Heat Treatment Handbook; CRC Press: Boca Raton, FL, USA, 2006; Volume 2. [Google Scholar]
- Angelo, P.C.; Subramanian, R.; Ravisankar, B. Powder Metallurgy: Science, Technology and Applications; PHI Learning Pvt. Ltd.: New Delhi, India, 2022. [Google Scholar]
- Tsutsui, T. Recent technology of powder metallurgy and applications. Hitachi Chem. Tech. Rep. 2012, 54, 12–20. [Google Scholar]
- Krbaťa, M.; Majerik, J.; Barenyi, I.; Eckert, M.; Čep, R.; Sedlak, J.; Samardžić, I. Experimental determination of continuous cooling transformation diagram for high strength steel X155CrMoV12. Metalurgija 2022, 61, 185–188. [Google Scholar]
- Krbaťa, M.; Majerik, J.; Barenyi, I.; Eckert, M.; Čep, R.; Sedlak, J.; Samardžić, I. Dilatometric analysis of cooling curves for high strength steel X155CrMoV12. Metalurgija 2022, 61, 193–196. [Google Scholar]
- Lu, I.R.; Görler, G.P.; Fecht, H.J.; Willnecker, R. Investigation of specific heat and thermal expansion in the glass-transition regime of Pd-based metallic glasses. J. Non-Cryst. Solids 2000, 274, 294–300. [Google Scholar] [CrossRef]
- Mira, J.; Rivas, J.; Hueso, L.E.; Rivadulla, F.; Quintela, M.L.; Rodriguez, M.S.; Ramos, C.A. Strong reduction of lattice effects in mixed-valence manganites related to crystal symmetry. Phys. Rev. B Condens. Matter 2001, 65, 024418. [Google Scholar] [CrossRef]
- Macedo, M.Q.; Cota, A.B.; Araújo, F.G.D.S. The kinetics of austenite formation at high heating rates. Rem Revista Escola de Minas 2011, 64, 163–167. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Wang, Z.; Bao, E. Effect of rare earth addition on continuous heating transformation of a high speed steel for rolls. J. Rare Earths 2012, 30, 84–89. [Google Scholar] [CrossRef]
- Chiriac, C.; Sohmshetty, R. The effects of the heating rate and the incoming microstructure on the phase transformation temperatures of 22MnB5 Steel. CHS2 2017, 6, 403–414. [Google Scholar]
- Hernández-Morale, B.; Vázquez-Gómez, O.; López-Martínez, E.; Vergara-Hernández, H.J.; Olmos, L. Effect of heating rate and silicon content on kinetics of austenite formation during continuous heating. Mater. Sci. Forum 2014, 783, 771–776. [Google Scholar] [CrossRef]
- Jacob, B.P.; Thankachan, S.; Xavier, S.; Mohammed, E.M. Effect of Gd3+ doping on the structural and magnetic properties of nanocrystalline Ni–Cd mixed ferrite. Phys. Scr. 2011, 84, 045702. [Google Scholar] [CrossRef]
- Nath, S.K.; Maria, K.H.; Noor, S.; Sikder, S.S.; Hoque, S.M.; Hakim, M.A. Magnetic ordering in Ni–Cd ferrite. J. Magn. Magn. Mater. 2012, 324, 2116–2120. [Google Scholar] [CrossRef]
- Noor, S.; Hakim, M.A.; Sikder, S.S.; Hoque, S.M.; Maria, K.H.; Nordblad, P. Magnetic behavior of Cd2+ substituted cobalt ferrites. J. Phys. Chem. Solids 2012, 73, 227–231. [Google Scholar] [CrossRef]
- Liu, J.H.; Binot, N.; Delagnes, D.; Jahazi, M. Influence of the cooling rate below Ms on the martensitic transformation in a low alloy medium-carbon steel. J. Mater. Res. Technol. 2021, 12, 234–242. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, C.; Qu, F.; Wang, Y.; Qiao, Z. Martensite transformation kinetics in 9Cr–1.7 W–0.4 Mo–Co ferritic steel. J. Alloy. Compd. 2014, 610, 322–330. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H. Very strong bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 251. [Google Scholar] [CrossRef]
- Fujita, N.; Ishikawa, N.; Roters, F.; Tasan, C.C.; Raabe, D. Experimental–numerical study on strain and stress partitioning in bainitic steels with martensite–austenite constituents. Int. J. Plast. 2018, 104, 39–53. [Google Scholar] [CrossRef]
- Moravec, J.; Nováková, I.; Vondráček, J. Influence of heating rate on the transformation temperature change in selected steel types. Manuf. Technol. 2020, 20, 217–222. [Google Scholar] [CrossRef]
- Zong, Y.; Liu, C.-M. Continuous Cooling Transformation Diagram, Microstructures, and Properties of the Simulated Coarse-Grain Heat-Affected Zone in a Low-Carbon Bainite E550 Steel. Metals 2019, 9, 939. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Fu, R.Y.; Krizan, D.; De Cooman, B.C. Continuous cooling transformation diagrams and properties of micro-alloyed TRIP steels. Mater. Sci. Eng. A 2006, 438, 296–299. [Google Scholar] [CrossRef]
- Geng, X.; Wang, H.; Ullah, A.; Xue, W.; Xiang, S.; Meng, L.; Ma, G. Prediction of Continuous Cooling Transformation Diagrams for Ni-Cr-Mo Welding Steels via Machine Learning Approaches. JOM 2020, 72, 3926–3934. [Google Scholar] [CrossRef]
- Protopopov, E.; Dobrykh, S.; Trofimova, Y.; Malenko, P.; Valter, A.; Protopopov, A. Reflection of strengthening results in values of generalized degrees of metallicity and covalence is principle to new strategy of designing alloys. Sci. Rep. 2020, 10, 2050. [Google Scholar] [CrossRef]
- Xiao, H.; Zheng, S.; Xin, Y.; Xu, J.; Han, K.; Li, H.; Zhai, Q. Characterization of Microstructure in High-Hardness Surface Layer of Low-Carbon Steel. Metals 2020, 10, 995. [Google Scholar] [CrossRef]
- Dubec, A.; Kováčiková, P.; Krmela, J.; Krmelová, V.; Artyukhov, A. Fracture Analysis of High-Strength Screw for Highway Construction. Materials 2021, 14, 1599. [Google Scholar] [CrossRef] [PubMed]
- Kováčiková, P.; Dubec, A.; Kotialiková, D. Examination of surface wear on the timing chain tensioner depending on the engine oil contamination. Manuf. Technol. 2020, 20, 463–467. [Google Scholar] [CrossRef]
- Milenkovic, S.; Sabirov, I.; Lorca, J. Effect of the cooling rate on microstructure and hardness of MAR-M247 Ni-based superalloy. Mater. Lett. 2012, 73, 216–219. [Google Scholar] [CrossRef]
- Boyde, A.; Mccorkell, F.A.; Taylor, G.K.; Bomphrey, R.J.; Doube, M. Iodine vapor staining for atomic number contrast in backscattered electron and X-ray imaging. Microsc. Res. Tech. 2014, 77, 1044–1051. [Google Scholar] [CrossRef]







| Element | C | Mn | Si | Cr | Mo | V | W |
|---|---|---|---|---|---|---|---|
| Böhler | 2.70 | 0.50 | 0.50 | 20.00 | 1.00 | 7.20 | 0.70 |
| Spectral analysis | 2.72 | 0.50 | 0.51 | 20.07 | 1.00 | 7.22 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krbata, M.; Ciger, R.; Kohutiar, M.; Eckert, M.; Barenyi, I.; Trembach, B.; Dubec, A.; Escherova, J.; Gavalec, M.; Beronská, N. Microstructural Changes and Determination of a Continuous Cooling Transformation (CCT) Diagram Using Dilatometric Analysis of M398 High-Alloy Tool Steel Produced by Microclean Powder Metallurgy. Materials 2023, 16, 4473. https://doi.org/10.3390/ma16124473
Krbata M, Ciger R, Kohutiar M, Eckert M, Barenyi I, Trembach B, Dubec A, Escherova J, Gavalec M, Beronská N. Microstructural Changes and Determination of a Continuous Cooling Transformation (CCT) Diagram Using Dilatometric Analysis of M398 High-Alloy Tool Steel Produced by Microclean Powder Metallurgy. Materials. 2023; 16(12):4473. https://doi.org/10.3390/ma16124473
Chicago/Turabian StyleKrbata, Michal, Robert Ciger, Marcel Kohutiar, Maros Eckert, Igor Barenyi, Bohdan Trembach, Andrej Dubec, Jana Escherova, Matúš Gavalec, and Naďa Beronská. 2023. "Microstructural Changes and Determination of a Continuous Cooling Transformation (CCT) Diagram Using Dilatometric Analysis of M398 High-Alloy Tool Steel Produced by Microclean Powder Metallurgy" Materials 16, no. 12: 4473. https://doi.org/10.3390/ma16124473
APA StyleKrbata, M., Ciger, R., Kohutiar, M., Eckert, M., Barenyi, I., Trembach, B., Dubec, A., Escherova, J., Gavalec, M., & Beronská, N. (2023). Microstructural Changes and Determination of a Continuous Cooling Transformation (CCT) Diagram Using Dilatometric Analysis of M398 High-Alloy Tool Steel Produced by Microclean Powder Metallurgy. Materials, 16(12), 4473. https://doi.org/10.3390/ma16124473







