Enhanced Hydrogen Generation from Magnesium–Aluminum Scrap Ball Milled with Low Melting Point Solder Alloy
Abstract
1. Introduction
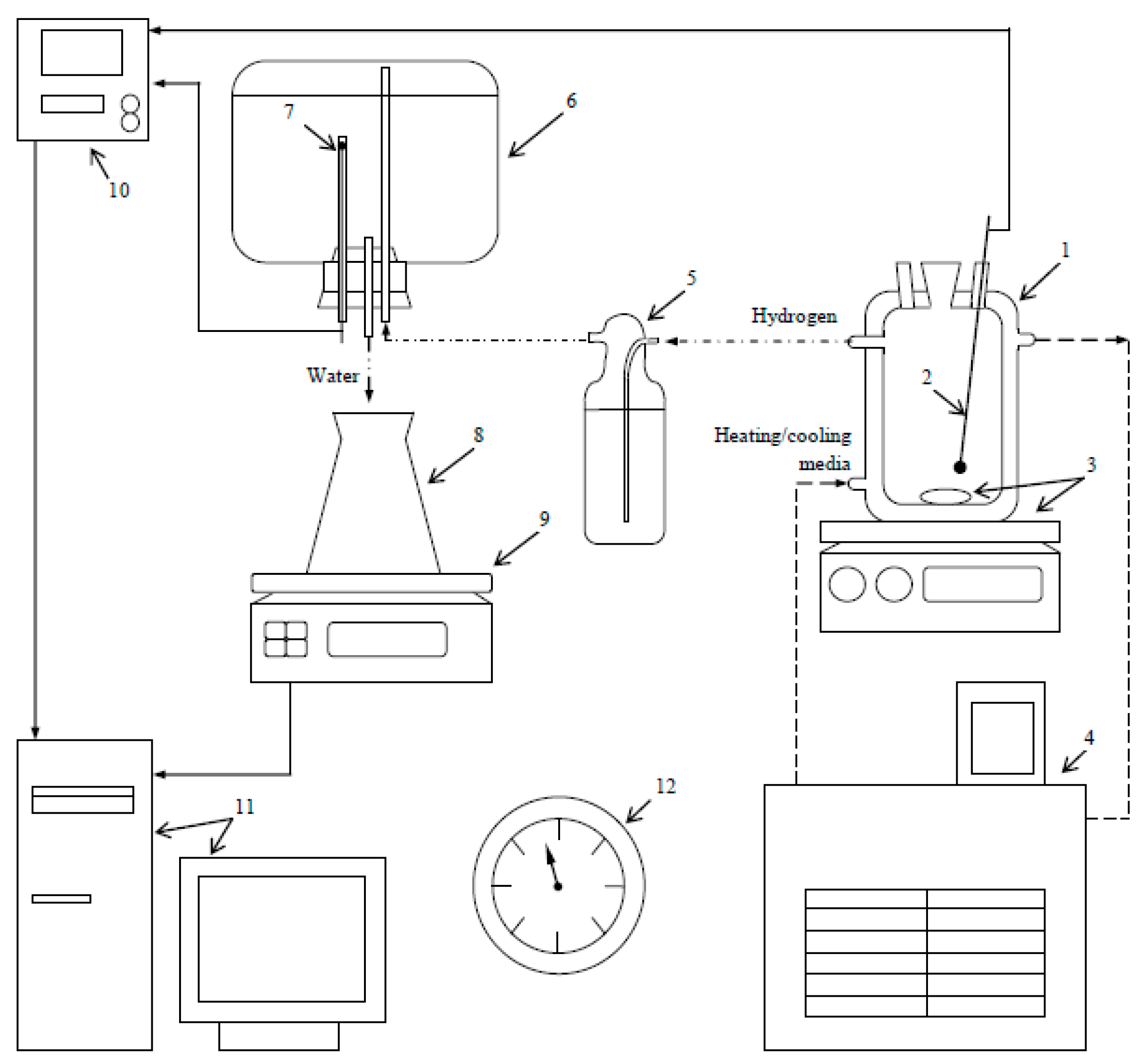
2. Materials and Methods
3. Results and Discussion
3.1. Properties of the Original Materials and Hydroreactive Samples
3.1.1. Microstructure Analysis
3.1.2. Chemical Characterization
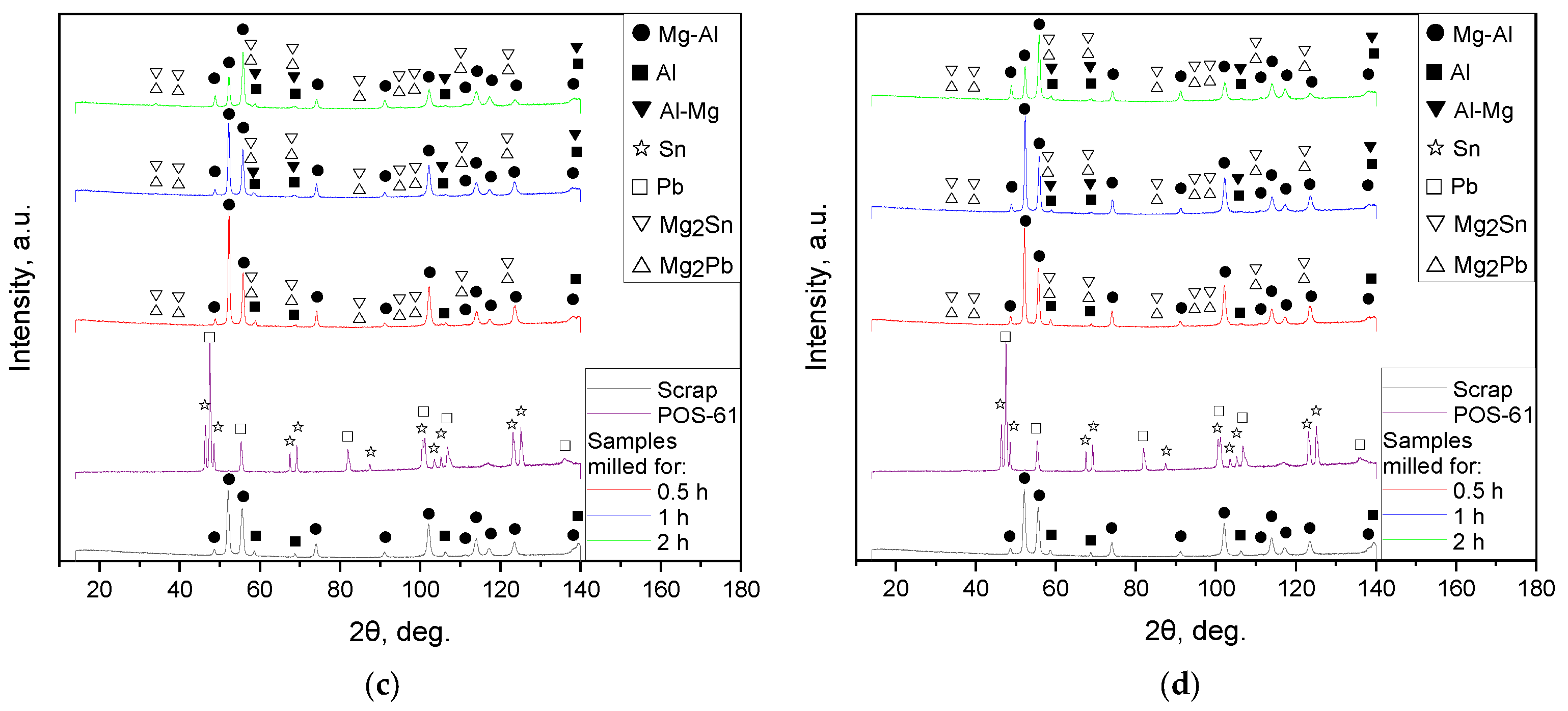
3.1.3. Phase Composition
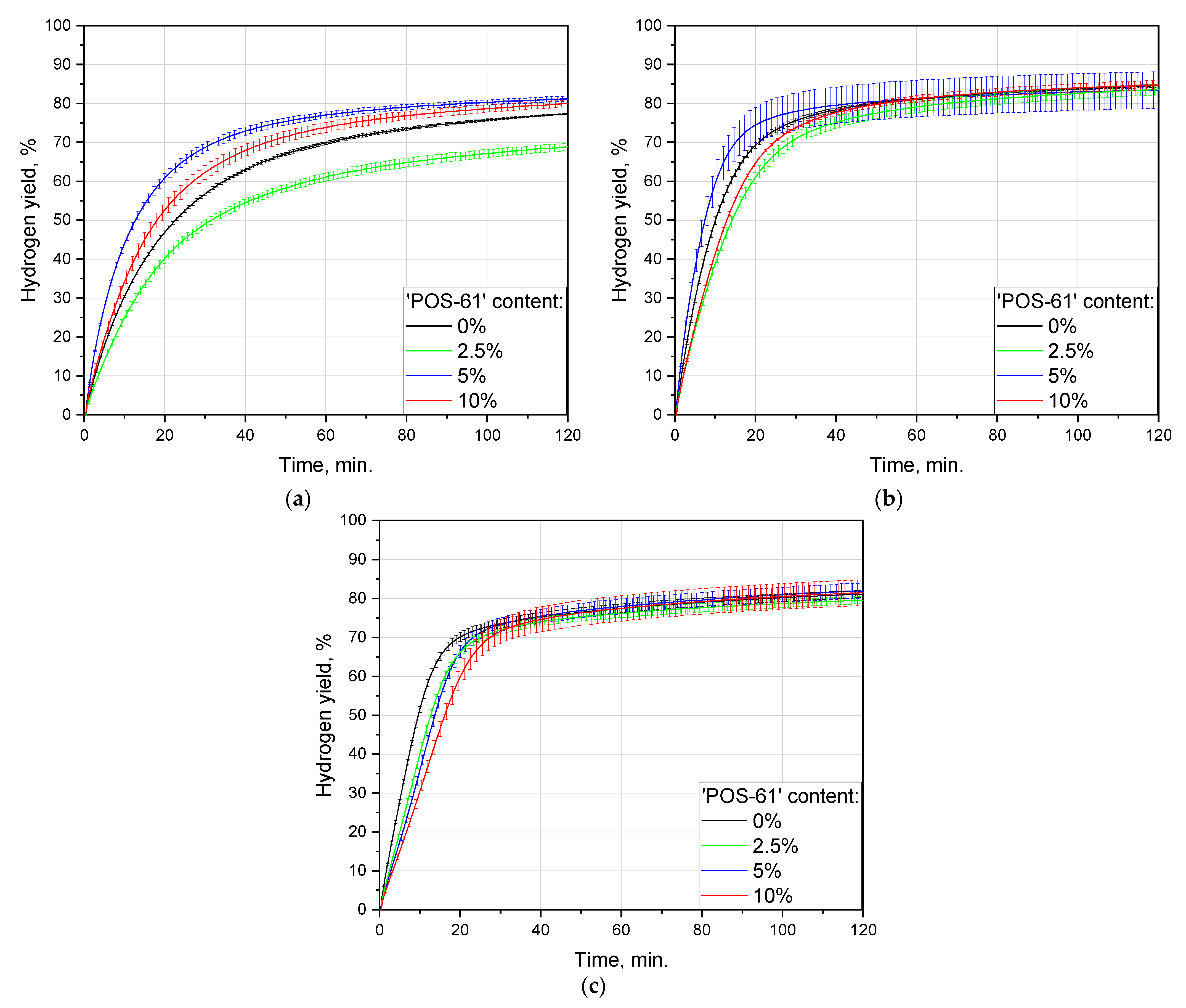
3.2. Tests for Hydrogen Generation Performance
3.2.1. Effect of Ball Milling Duration
3.2.2. Effect of Additive Content
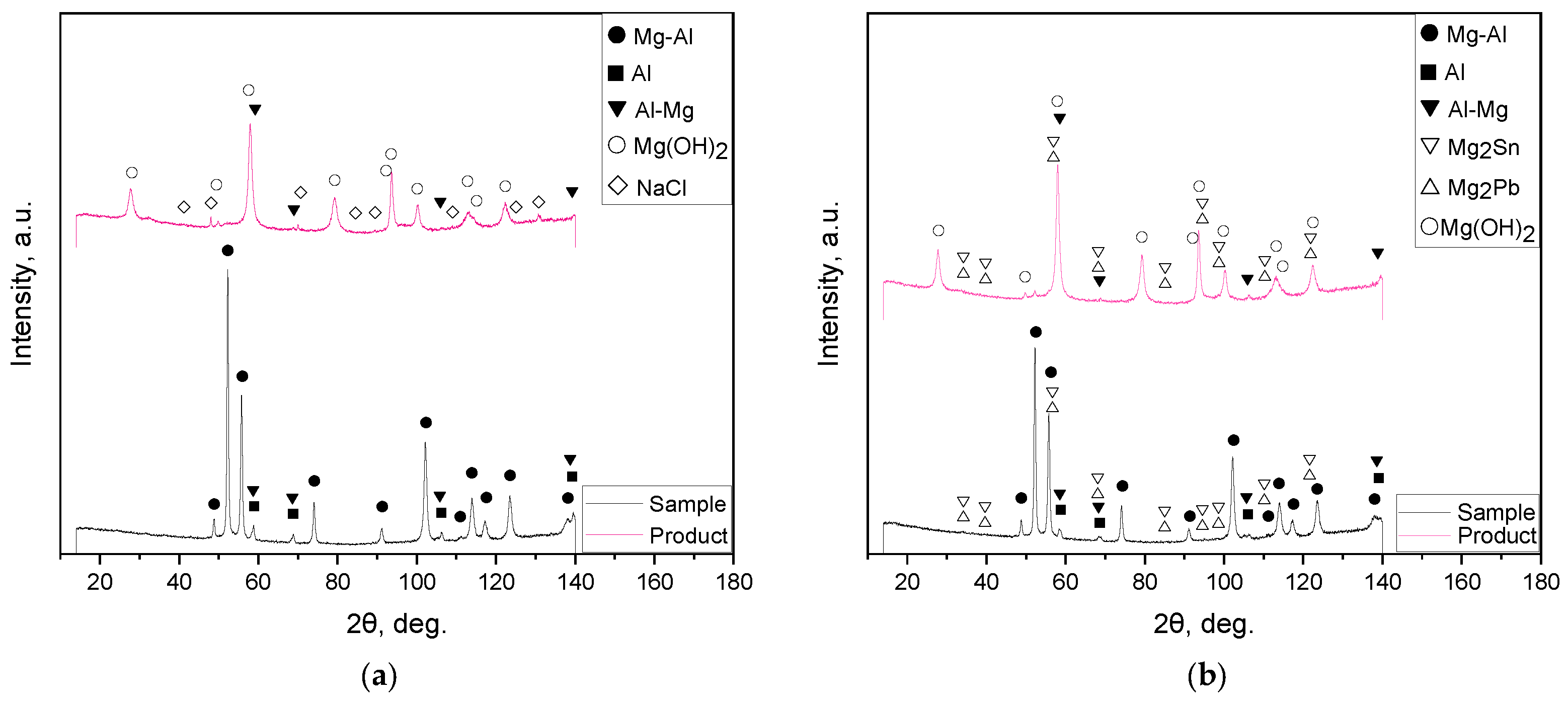
3.3. Characteristics of the Reaction Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Yap, J.; McLellan, B. A Historical Analysis of Hydrogen Economy Research, Development, and Expectations, 1972 to 2020. Environments 2023, 10, 11. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Falcone, P.M.; Hiete, M.; Sapio, A. Hydrogen economy and sustainable development goals: Review and policy insights. Curr. Opin. Green Sustain. Chem. 2021, 31, 100506. [Google Scholar] [CrossRef]
- Tahir, M.B.; Batool, A. Chapter 3—Recent development in sustainable technologies for clean hydrogen evolution: Current scenario and future perspectives. In Sustainable Materials and Green Processing for Energy Conversion; Cheong, K.Y., Apblett, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 97–130. [Google Scholar]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Leng, L.; Zhou, J.; Li, T.; Vlaskin, M.; Zhan, H.; Peng, H.; Huang, H.; Li, H. Nitrogen heterocycles in bio-oil produced from hydrothermal liquefaction of biomass: A review. Fuel 2023, 335, 126995. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Shi, X.; Fan, R. Numerical investigation of the dispersion features of hydrogen gas under various leakage source conditions in a mobile hydrogen refueling station. Int. J. Hydrogen Energy 2023, 48, 9498–9511. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Li, X.-J.; Gao, Z.-X.; Jin, Z.-J. A numerical study of hydrogen leakage and diffusion in a hydrogen refueling station. Int. J. Hydrogen Energy 2020, 45, 14428–14439. [Google Scholar] [CrossRef]
- Mao, X.; Ying, R.; Yuan, Y.; Li, F.; Shen, B. Simulation and analysis of hydrogen leakage and explosion behaviors in various compartments on a hydrogen fuel cell ship. Int. J. Hydrogen Energy 2021, 46, 6857–6872. [Google Scholar] [CrossRef]
- Małachowska, A.; Łukasik, N.; Mioduska, J.; Gębicki, J. Hydrogen Storage in Geological Formations—The Potential of Salt Caverns. Energies 2022, 15, 5038. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, S.; Zhang, Z.; Zheng, J.; Zhao, Y. Numerical study on the fast filling of on-bus gaseous hydrogen storage cylinder. Int. J. Hydrogen Energy 2020, 45, 9241–9251. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, H.; Tong, L.; Wang, L. Research Progress of Cryogenic Materials for Storage and Transportation of Liquid Hydrogen. Metals 2021, 11, 1101. [Google Scholar] [CrossRef]
- Jastrzębski, K.; Kula, P. Emerging Technology for a Green, Sustainable Energy-Promising Materials for Hydrogen Storage, from Nanotubes to Graphene—A Review. Materials 2021, 14, 2499. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Yartys, V.A.; Chidziva, S.; Pasupathi, S.; Lototskyy, M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int. J. Hydrogen Energy 2021, 46, 13647–13657. [Google Scholar] [CrossRef]
- Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Lang, C.; Jia, Y.; Yao, X. Recent advances in liquid-phase chemical hydrogen storage. Energy Storage Mater. 2020, 26, 290–312. [Google Scholar] [CrossRef]
- Wijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Aziz, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrogen Energy 2019, 44, 15026–15044. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Barelli, L.; Baumann, M.; Bidini, G.; Ottaviano, P.A.; Schneider, R.V.; Passerini, S.; Trombetti, L. Reactive Metals as Energy Storage and Carrier Media: Use of Aluminum for Power Generation in Fuel Cell-Based Power Plants. Energy Technol. 2020, 8, 2000233. [Google Scholar] [CrossRef]
- Xie, X.; Ni, C.; Wang, B.; Zhang, Y.; Zhao, X.; Liu, L.; Wang, B.; Du, W. Recent advances in hydrogen generation process via hydrolysis of Mg-based materials: A short review. J. Alloys Compd. 2020, 816, 152634. [Google Scholar] [CrossRef]
- Gao, X.; Wang, C.a.; Bai, W.; Hou, Y.; Che, D. Aluminum-Based Fuels as Energy Carriers for Controllable Power and Hydrogen Generation—A Review. Energies 2023, 16, 436. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, J.; Leng, H.; Xia, G.; Yu, X. Hydrolysis of Mg-based alloys and their hydrides for efficient hydrogen generation. Int. J. Hydrogen Energy 2021, 46, 18988–19000. [Google Scholar] [CrossRef]
- Vostrikov, A.A.; Shishkin, A.V.; Fedyaeva, O.N. Conjugated processes of bulk aluminum and hydrogen combustion in water-oxygen mixtures. Int. J. Hydrogen Energy 2020, 45, 1061–1071. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Shkolnikov, E.I.; Bersh, A.V. Oxidation kinetics of micron-sized aluminum powder in high-temperature boiling water. Int. J. Hydrogen Energy 2011, 36, 6484–6495. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Valyano, G.E.; Zhuk, A.Z.; Shkolnikov, E.I. Oxidation of coarse aluminum in pressured water steam for energy applications. Int. J. Energy Res. 2020, 44, 8689–8715. [Google Scholar] [CrossRef]
- Yavor, Y.; Goroshin, S.; Bergthorson, J.M.; Frost, D.L. Comparative reactivity of industrial metal powders with water for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 1026–1036. [Google Scholar] [CrossRef]
- Sheikhbahaei, V.; Baniasadi, E.; Naterer, G.F. Experimental investigation of solar assisted hydrogen production from water and aluminum. Int. J. Hydrogen Energy 2018, 43, 9181–9191. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Vlaskin, M.S. Hydrogen Recovery from Waste Aluminum–Plastic Composites Treated with Alkaline Solution. Materials 2022, 15, 8699. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Zhang, Z.; Tu, H.; Xiong, J.; Xiang, X.; Wei, C.; Mishra, Y.K. High efficiency Al-based multicomponent composites for low-temperature hydrogen production and its hydrolysis mechanism. Int. J. Hydrogen Energy, 2023, in press. [CrossRef]
- Martínez-Salazar, A.L.; Melo-Banda, J.A.; Coronel-García, M.A.; González-Barbosa, J.J.; Domínguez-Esquivel, J.M. Hydrogen generation by aluminum alloy corrosion in aqueous acid solutions promoted by nanometal: Kinetics study. Renew. Energy 2020, 146, 2517–2523. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Vlaskin, M.S.; Ryzhkova, S.S. Hydrogen production properties of magnesium and magnesium-based materials at low temperatures in reaction with aqueous solutions. J. Alloys Compd. 2019, 785, 136–145. [Google Scholar] [CrossRef]
- Öz, Ç.; Coşkuner Filiz, B.; Kantürk Figen, A. The effect of vinegar–acetic acid solution on the hydrogen generation performance of mechanochemically modified Magnesium (Mg) granules. Energy 2017, 127, 328–334. [Google Scholar] [CrossRef]
- Ambaryan, G.N.; Vlaskin, M.S.; Dudoladov, A.O.; Meshkov, E.A.; Zhuk, A.Z.; Shkolnikov, E.I. Hydrogen generation by oxidation of coarse aluminum in low content alkali aqueous solution under intensive mixing. Int. J. Hydrogen Energy 2016, 41, 17216–17224. [Google Scholar] [CrossRef]
- Al Bacha, S.; Thienpont, A.; Zakhour, M.; Nakhl, M.; Bobet, J.L. Clean hydrogen production by the hydrolysis of magnesium-based material: Effect of the hydrolysis solution. J. Clean. Prod. 2021, 282, 124498. [Google Scholar] [CrossRef]
- Sun, R.; Liu, P.; Qi, H.; Liu, J.; Ding, T. Molecular dynamic simulations of ether-coated aluminum nano-particles as a novel hydrogen source. J. Nanopart. Res. 2019, 21, 72. [Google Scholar] [CrossRef]
- Amberchan, G.; Lopez, I.; Ehlke, B.; Barnett, J.; Bao, N.Y.; Allen, A.L.; Singaram, B.; Oliver, S.R.J. Aluminum Nanoparticles from a Ga–Al Composite for Water Splitting and Hydrogen Generation. ACS Appl. Nano Mater. 2022, 5, 2636–2643. [Google Scholar] [CrossRef]
- Irankhah, A.; Seyed Fattahi, S.M.; Salem, M. Hydrogen generation using activated aluminum/water reaction. Int. J. Hydrogen Energy 2018, 43, 15739–15748. [Google Scholar] [CrossRef]
- Su, M.; Wang, H.; Xu, H.; Chen, F.; Hu, H.; Gan, J. Enhanced hydrogen production properties of a novel aluminum-based composite for instant on-site hydrogen supply at low temperature. Int. J. Hydrogen Energy 2022, 47, 9969–9985. [Google Scholar] [CrossRef]
- Su, M.; Hu, H.; Gan, J.; Ye, W.; Zhang, W.; Wang, H. Thermodynamics, kinetics and reaction mechanism of hydrogen production from a novel Al alloy/NaCl/g-C3N4 composite by low temperature hydrolysis. Energy 2021, 218, 119489. [Google Scholar] [CrossRef]
- Al Bacha, S.; Zakhour, M.; Nakhl, M.; Bobet, J.L. Effect of ball milling in presence of additives (Graphite, AlCl3, MgCl2 and NaCl) on the hydrolysis performances of Mg17Al12. Int. J. Hydrogen Energy 2020, 45, 6102–6109. [Google Scholar] [CrossRef]
- Al Bacha, S.; Pighin, S.A.; Urretavizcaya, G.; Zakhour, M.; Castro, F.J.; Nakhl, M.; Bobet, J.L. Hydrogen generation from ball milled Mg alloy waste by hydrolysis reaction. J. Power Source 2020, 479, 228711. [Google Scholar] [CrossRef]
- He, T.; Chen, W.; Wang, W.; Ren, F.; Stock, H.-R. Effect of different Cu contents on the microstructure and hydrogen production of Al–Cu-Ga-In-Sn alloys for dissolvable materials. J. Alloys Compd. 2020, 821, 153489. [Google Scholar] [CrossRef]
- He, T.; Chen, W.; Wang, W.; Du, S.; Deng, S. Microstructure and hydrogen production of the rapidly solidified Al–Mg-Ga-In-Sn alloy. J. Alloys Compd. 2020, 827, 154290. [Google Scholar] [CrossRef]
- du Preez, S.P.; Bessarabov, D.G. On-demand hydrogen generation by the hydrolysis of ball-milled aluminum composites: A process overview. Int. J. Hydrogen Energy 2021, 46, 35790–35813. [Google Scholar] [CrossRef]
- He, T.; Xiong, Y.; Du, S.; Yuan, Z.; Liang, X.; Huttula, M.; Cao, W. Impact of Li Addition in Al-Rich Alloys on Hydrogen Production in Water. J. Mater. Eng. Perform. 2019, 28, 2459–2464. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. The effects of bismuth and tin on the mechanochemical processing of aluminum-based composites for hydrogen generation purposes. Int. J. Hydrogen Energy 2019, 44, 21896–21912. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. Hydrogen generation of mechanochemically activated AlBiIn composites. Int. J. Hydrogen Energy 2017, 42, 16589–16602. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Y.; Hu, R.; Shi, H.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; Yang, Y. Catalytic effect of EG and MoS2 on hydrolysis hydrogen generation behavior of high-energy ball-milled Mg-10wt.%Ni alloys in NaCl solution—A powerful strategy for superior hydrogen generation performance. Int. J. Energy Res. 2019, 43, 8426–8438. [Google Scholar] [CrossRef]
- Hou, X.; Shi, H.; Yang, L.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; Yang, Y. Comparative investigation on feasible hydrolysis H2 production behavior of commercial Mg-M (M = Ni, Ce, and La) binary alloys modified by high-energy ball milling—Feasible modification strategy for Mg-based hydrogen producing alloys. Int. J. Energy Res. 2020, 44, 11956–11972. [Google Scholar] [CrossRef]
- Hou, X.; Shi, H.; Yang, L.; Hou, K.; Wang, Y.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; Yang, Y. H2 generation kinetics/thermodynamics and hydrolysis mechanism of high-performance La-doped Mg-Ni alloys in NaCl solution—A large-scale and quick strategy to get hydrogen. J. Magnes. Alloy. 2021, 9, 1068–1083. [Google Scholar] [CrossRef]
- Oh, S.; Kim, M.; Eom, K.; Kyung, J.; Kim, D.; Cho, E.; Kwon, H. Design of Mg–Ni alloys for fast hydrogen generation from seawater and their application in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2016, 41, 5296–5303. [Google Scholar] [CrossRef]
- Oh, S.; Cho, T.; Kim, M.; Lim, J.; Eom, K.; Kim, D.; Cho, E.; Kwon, H. Fabrication of Mg–Ni–Sn alloys for fast hydrogen generation in seawater. Int. J. Hydrogen Energy 2017, 42, 7761–7769. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Suleimanov, M.Z.; Vlaskin, M.S.; Kumar, V.; Ambaryan, G.N. Aluminum Scrap to Hydrogen: Complex Effects of Oxidation Medium, Ball Milling Parameters, and Copper Additive Dispersity. Metals 2023, 13, 185. [Google Scholar] [CrossRef]
- Abu-Qdais, H.A.; Kurbatova, A.I. Editorial: Sustainable Municipal Solid Waste Management: A Local Issue with Global Impacts. Sustainability 2022, 14, 11438. [Google Scholar] [CrossRef]
- Abu-Qdais, H.A.; Shatnawi, N.; Al-Shahrabi, R. Modeling the Impact of Fees and Circular Economy Options on the Financial Sustainability of the Solid Waste Management System in Jordan. Resources 2023, 12, 32. [Google Scholar] [CrossRef]
- Abu-Qdais, H.A.; Kurbatova, A.I. The Role of Eco-Industrial Parks in Promoting Circular Economy in Russia: A Life Cycle Approach. Sustainability 2022, 14, 3893. [Google Scholar] [CrossRef]
- Hanko, G.; Antrekowitsch, H.; Ebner, P. Recycling automotive magnesium scrap. JOM 2002, 54, 51–54. [Google Scholar] [CrossRef]
- Ercoli, R.; Laskowska, D.; Nguyen, V.V.; Le, V.S.; Louda, P.; Łoś, P.; Ciemnicka, J.; Prałat, K.; Renzulli, A.; Paris, E.; et al. Mechanical and Thermal Properties of Geopolymer Foams (GFs) Doped with By-Products of the Secondary Aluminum Industry. Polymers 2022, 14, 703. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Wang, B.; Pei, X.; Li, J.; Li, M.; Xu, K. Study on the influence of Mg content on the risk of hydrogen production from waste alloy dust in wet dust collector. Int. J. Hydrogen Energy 2021, 46, 38563–38573. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, K.; Liu, B.; Ge, J. Conversion coating technology for recovery and reuse of waste Mg alloy polishing powder. J. Clean. Prod. 2022, 368, 133181. [Google Scholar] [CrossRef]
- Elsarrag, E.; Elhoweris, A.; Alhorr, Y. The production of hydrogen as an alternative energy carrier from aluminium waste. Energy Sustain. Soc. 2017, 7, 9. [Google Scholar] [CrossRef]
- Ercoli, R.; Orlando, A.; Borrini, D.; Tassi, F.; Bicocchi, G.; Renzulli, A. Hydrogen-Rich Gas Produced by the Chemical Neutralization of Reactive By-Products from the Screening Processes of the Secondary Aluminum Industry. Sustainability 2021, 13, 12261. [Google Scholar] [CrossRef]
- Tekade, S.P.; Pednekar, A.S.; Jadhav, G.R.; Kalekar, S.E.; Shende, D.Z.; Wasewar, K.L. Hydrogen generation through water splitting reaction using waste aluminum in presence of gallium. Int. J. Hydrogen Energy 2020, 45, 23954–23965. [Google Scholar] [CrossRef]
- Al Bacha, S.; Aubert, I.; Zakhour, M.; Nakhl, M.; Bobet, J.L. Valorization of AZ91 by the hydrolysis reaction for hydrogen production (Electrochemical approach). J. Magnes. Alloy. 2021, 9, 1942–1953. [Google Scholar] [CrossRef]
- Yu, S.-H.; Uan, J.-Y.; Hsu, T.-L. Effects of concentrations of NaCl and organic acid on generation of hydrogen from magnesium metal scrap. Int. J. Hydrogen Energy 2012, 37, 3033–3040. [Google Scholar] [CrossRef]
- Kantürk Figen, A.; Coşkuner, B.; Pişkin, S. Hydrogen generation from waste Mg based material in various saline solutions (NiCl2, CoCl2, CuCl2, FeCl3, MnCl2). Int. J. Hydrogen Energy 2015, 40, 7483–7489. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Luo, A.A.; Peng, L.; Zhang, P. Fatigue behavior and life prediction of cast magnesium alloys. Mater. Sci. Eng. A 2015, 647, 113–126. [Google Scholar] [CrossRef]
- Viswanadhapalli, B.; Bupesh Raja, V.K. Application of Magnesium Alloys in Automotive Industry—A Review. In Emerging Trends in Computing and Expert Technology; Springer: Cham, Switzerland, 2020; pp. 519–531. [Google Scholar]
- Oslanec, P.; Iždinský, K.; Simančík, F. Possibilities of magnesium recycling. Mater. Sci. Technol. 2008, 4, 83–88. [Google Scholar]
- Gouty, Q.; Castro, F.J.; Urretavizcaya, G.; Sabatier, J.; Bobet, J.L. Experimental and theoretical approach of the hydrolysis of pelleted magnesium alloys scraps. J. Alloys Compd. 2022, 919, 165784. [Google Scholar] [CrossRef]
- Duan, Y.H.; Sun, Y.; He, J.H.; Guo, Z.Z.; Fang, D.S. Corrosion Behavior of As-Cast Pb-Mg-Al Alloys in 3.5% NaCl Solution. Corrosion 2012, 68, 822–826. [Google Scholar] [CrossRef]
- Gabalcová, Z.; Gogola, P.; Gerhátová, Ž.; Palcut, M. Corrosion Behavior of an Mg2Sn Alloy. Materials 2022, 15, 2025. [Google Scholar] [CrossRef]
- Periyapperuma, K.; Tran, T.T.; Purcell, M.I.; Obrovac, M.N. The Reversible Magnesiation of Pb. Electrochim. Acta 2015, 165, 162–165. [Google Scholar] [CrossRef]
- Babayigit, A.; Duy Thanh, D.; Ethirajan, A.; Manca, J.; Muller, M.; Boyen, H.-G.; Conings, B. Assessing the toxicity of Pb- and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 2016, 6, 18721. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Seo, S.; Lee, S.I.; Kim, D.W.; Kim, M.J. A study on pyro-hydrometallurgical process for selective recovery of Pb, Sn and Sb from lead dross. J. Hazard. Mater. 2021, 417, 126071. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Guo, Y.; Yang, R.; Li, J. Hydrogen generation from hydrolysis of activated magnesium/low-melting-point metals alloys. Int. J. Hydrogen Energy 2019, 44, 1366–1373. [Google Scholar] [CrossRef]
- Gallagher, P.K.; Gyorgy, E.M. Curie temperature standards for thermogravimetry: The effect of magnetic field strength and comparison with melting point standards using Ni and Pb. Thermochim. Acta 1986, 109, 193–206. [Google Scholar] [CrossRef]
- Wildner, W.; Pfannes, H.-D.; Gonser, U. Anomalies at the Sn melting point. J. Phys. Colloq. 1974, 35, C6-381–C6-382. [Google Scholar] [CrossRef]
- D’Amelia, R.P.; Clark, D.; Nirode, W. An Undergraduate Experiment Using Differential Scanning Calorimetry: A Study of the Thermal Properties of a Binary Eutectic Alloy of Tin and Lead. J. Chem. Educ. 2012, 89, 548–551. [Google Scholar] [CrossRef]
- Karakaya, I.; Thompson, W.T. The Pb−Sn (Lead-Tin) system. J. Phase Equilibria 1988, 9, 144–152. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Zhu, Z.; Jiang, Y.; Zhang, J.; Niu, J.; Mao, L.; Yuan, G. In vitro degradation and biocompatibility of Mg-Nd-Zn-Zr alloy. Chin. Sci. Bull. 2012, 57, 2163–2170. [Google Scholar] [CrossRef]
- Ballerini, G.; Bardi, U.; Bignucolo, R.; Ceraolo, G. About some corrosion mechanisms of AZ91D magnesium alloy. Corros. Sci. 2005, 47, 2173–2184. [Google Scholar] [CrossRef]
- GOST 2856–79; Casting Magnesium Alloys. Grades. IPK Standards Publishing House: Moscow, Russia, 1981.
- GOST 21930-76; Tin-Lead Solders in Pigs. Specifications. IPK Standards Publishing House: Moscow, Russia, 1978.
- GOST 4233-77; Reagents. Sodium Chloride. Specifications. IPK Standards Publishing House: Moscow, Russia, 1978.
- GOST 10157-79; Gaseous and Liquid Argon. Specifications. IPK Standards Publishing House: Moscow, Russia, 1980.
- Buryakovskaya, O.A.; Kurbatova, A.I.; Vlaskin, M.S.; Valyano, G.E.; Grigorenko, A.V.; Ambaryan, G.N.; Dudoladov, A.O. Waste to Hydrogen: Elaboration of Hydroreactive Materials from Magnesium-Aluminum Scrap. Sustainability 2022, 14, 4496. [Google Scholar] [CrossRef]
- DIN 1343-1990; Reference Conditions, Normal Conditions, Normal Volume; Concepts and Values. Deutsche Institut für Normung: Berlin, Grmany, 1990.
- Buryakovskaya, O.A.; Vlaskin, M.S. Microstructural Transformation and Hydrogen Generation Performance of Magnesium Scrap Ball Milled with Devarda’s Alloy. Materials 2022, 15, 8058. [Google Scholar]
- Bobrynina, E.; Koltsova, T.; Larionova, T. Investigation of Copper–Carbon Composite Microstructure and Properties. Metals 2023, 13, 1052. [Google Scholar]
- Tang, L.; Peng, X.; Huang, J.; Ma, A.; Deng, Y.; Xu, G. Microstructure and mechanical properties of severely deformed Al-Mg-Sc-Zr alloy and their evolution during annealing. Mater. Sci. Eng. A 2019, 754, 295–308. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Effect of structural evolution of aluminum powder during ball milling on hydrogen generation in aluminum–water reaction. Int. J. Hydrogen Energy 2013, 38, 795–806. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Effect of ball size on steady state of aluminum powder and efficiency of impacts during milling. Powder Technol. 2015, 284, 149–158. [Google Scholar] [CrossRef]
- Grosjean, M.H.; Zidoune, M.; Roué, L.; Huot, J.Y. Hydrogen production via hydrolysis reaction from ball-milled Mg-based materials. Int. J. Hydrogen Energy 2006, 31, 109–119. [Google Scholar] [CrossRef]
- Ardelean, H.; Seyeux, A.; Zanna, S.; Prima, F.; Frateur, I.; Marcus, P. Corrosion processes of Mg–Y–Nd–Zr alloys in Na2SO4 electrolyte. Corros. Sci. 2013, 73, 196–207. [Google Scholar] [CrossRef]
- Qian, X.; Dong, Z.; Jiang, B.; Lei, B.; Yang, H.; He, C.; Liu, L.; Wang, C.; Yuan, M.; Yang, H.; et al. Influence of alloying element segregation at grain boundary on the microstructure and mechanical properties of Mg-Zn alloy. Mater. Des. 2022, 224, 111322. [Google Scholar] [CrossRef]
- Czerwinski, F. The reactive element effect on high-temperature oxidation of magnesium. Int. Mater. Rev. 2015, 60, 264–296. [Google Scholar] [CrossRef]
- Feng, H.; Liu, S.; Du, Y.; Lei, T.; Zeng, R.; Yuan, T. Effect of the second phases on corrosion behavior of the Mg-Al-Zn alloys. J. Alloys Compd. 2017, 695, 2330–2338. [Google Scholar] [CrossRef]
- Candan, S.; Candan, E. Comparative study on corrosion behaviors of Mg-Al-Zn alloys. Trans. Nonferrous Met. Soc. China 2018, 28, 642–650. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Wei, Q.; Wang, N.; Hou, B. Electrochemical behavior of Mg-Al-Zn-In alloy as anode materials in 3.5wt.% NaCl solution. Electrochim. Acta 2017, 238, 156–167. [Google Scholar] [CrossRef]
- Ren, T.; Yang, S.; Wu, S.; Wang, M.; Xue, Y. High-energy ball milling enhancing the reactivity of microscale zero-valent aluminum toward the activation of persulfate and the degradation of trichloroethylene. Chem. Eng. J. 2019, 374, 100–111. [Google Scholar] [CrossRef]
- Wang, C.; Yang, T.; Liu, Y.; Ruan, J.; Yang, S.; Liu, X. Hydrogen generation by the hydrolysis of magnesium–aluminum–iron material in aqueous solutions. Int. J. Hydrogen Energy 2014, 39, 10843–10852. [Google Scholar] [CrossRef]
- Wan, D.-Q.; Hu, Y.-L.; Ye, S.-T.; Wang, H.-B.; Wei, S.-M. Effects of Pb on microstructure, mechanical properties and corrosion resistance of as-cast Mg97Zn1Y2 alloys. China Foundry 2018, 15, 443–448. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, W.; Yang, Y.; Zhan, H.; Yan, K.; Zeng, G. Effects of Mg2Sn precipitation on the age-hardening and deformation behaviour of a Mg-Sn-Al-Zn alloy. Mater. Sci. Eng. A 2023, 867, 144714. [Google Scholar] [CrossRef]
- Jung, I.-H.; Kim, J. Thermodynamic modeling of the Mg–Ge–Si, Mg–Ge–Sn, Mg–Pb–Si and Mg–Pb–Sn systems. J. Alloys Compd. 2010, 494, 137–147. [Google Scholar] [CrossRef]
- Birchal, V.; Rocha, S.; Mansur, M.; Ciminelli, V. A simplified mechanistic analysis of magnesia hydration. Can. J. Chem. Eng. 2001, 79, 507–511. [Google Scholar] [CrossRef]
- Zou, M.-S.; Yang, R.-J.; Guo, X.-Y.; Huang, H.-T.; He, J.-Y.; Zhang, P. The preparation of Mg-based hydro-reactive materials and their reactive properties in seawater. Int. J. Hydrogen Energy 2011, 36, 6478–6483. [Google Scholar] [CrossRef]
- Wang, C.-H.; Guo, X.-Y.; Zou, M.-S.; Yang, R.-J. Preparation and hydro-reactivity of ball-milled aluminum-based hydro-reactive metal materials. Acta Armamentarii 2016, 37, 817–822. [Google Scholar] [CrossRef]
- Al Bacha, S.; Aubert, I.; Devos, O.; Zakhour, M.; Nakhl, M.; Bobet, J.L. Corrosion of pure and milled Mg17Al12 in “model” seawater solution. Int. J. Hydrogen Energy 2020, 45, 15805–15813. [Google Scholar] [CrossRef]
- Rodríguez, M.; Niro, F.; Urretavizcaya, G.; Bobet, J.-L.; Castro, F.J. Hydrogen production from hydrolysis of magnesium wastes reprocessed by mechanical milling under air. Int. J. Hydrogen Energy 2022, 47, 5074–5084. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.P.; Guo, Y.C.; Yang, Z.; Xia, F. Effect of Tin on Microstructure and Electrochemistrical Properties of Mg-Al-Sn-Zn Magnesium Alloys Anodic Materials. Adv. Mater. Res. 2011, 197–198, 1129–1134. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Guo, Y.; Yang, Z.; Xia, F.; Wang, J. Effect of tin addition on microstructure and electrochemical properties of rolled AZ61-Sn magnesium anodic materials. Rare Met. 2011, 30, 639. [Google Scholar] [CrossRef]
- Ping, W.; Jianping, L.; Yongchun, G.; Zhong, Y.; Feng, X.; Jianli, W. Effect of Sn on microstructure and electrochemical properties of Mg alloy anode materials. Rare Met. Mater. Eng. 2012, 41, 2095–2099. [Google Scholar] [CrossRef]
- Song, G.-L. Effect of tin modification on corrosion of AM70 magnesium alloy. Corros. Sci. 2009, 51, 2063–2070. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Jihua, C.; Zhenhua, C.; Hongge, Y.; Fuquan, Z.; Yingliang, C. Effects of Sn and Ca additions on microstructure, mechanical properties, and corrosion resistance of the as-cast Mg-Zn-Al-based alloy. Mater. Corros. 2008, 59, 934–941. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kang, J.-Y.; Yang, J.; Yim, C.D.; You, B.S. Role of Sn in corrosion and passive behavior of extruded Mg-5 wt% Sn alloy. Corros. Sci. 2016, 102, 355–362. [Google Scholar] [CrossRef]
- Tong, F.; Chen, X.; Wei, S.; Malmström, J.; Vella, J.; Gao, W. Microstructure and battery performance of Mg-Zn-Sn alloys as anodes for magnesium-air battery. J. Magnes. Alloy. 2021, 9, 1967–1976. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Huang, S.; Zhou, X. Study of the corrosion behavior and the corrosion films formed on the surfaces of Mg–xSn alloys in 3.5wt.% NaCl solution. Appl. Surf. Sci. 2014, 317, 1143–1150. [Google Scholar] [CrossRef]
- Cain, T.W.; Glover, C.F.; Scully, J.R. The corrosion of solid solution Mg-Sn binary alloys in NaCl solutions. Electrochim. Acta 2019, 297, 564–575. [Google Scholar] [CrossRef]
- Wang, N.-G.; Wang, R.-C.; Peng, C.-q.; Yan, F.; Zhang, X.-Y. Influence of aluminium and lead on activation of magnesium as anode. Trans. Nonferrous Met. Soc. China 2010, 20, 1403–1411. [Google Scholar] [CrossRef]
- Wang, N.-G.; Wang, R.-C.; Peng, C.-Q.; Feng, Y.; Zhang, X.-Y. Corrosion behavior of Mg-Al-Pb and Mg-Al-Pb-Zn-Mn alloys in 3.5% NaCl solution. Trans. Nonferrous Met. Soc. China 2010, 20, 1936–1943. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.; Peng, C.; Feng, Y.; Chen, B. Effect of hot rolling and subsequent annealing on electrochemical discharge behavior of AP65 magnesium alloy as anode for seawater activated battery. Corros. Sci. 2012, 64, 17–27. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.; Peng, C.; Peng, B.; Feng, Y.; Hu, C. Discharge behaviour of Mg-Al-Pb and Mg-Al-Pb-In alloys as anodes for Mg-air battery. Electrochim. Acta 2014, 149, 193–205. [Google Scholar] [CrossRef]
- Südholz, A.D.; Birbilis, N.; Bettles, C.J.; Gibson, M.A. Corrosion behaviour of Mg-alloy AZ91E with atypical alloying additions. J. Alloys Compd. 2009, 471, 109–115. [Google Scholar] [CrossRef]
- Çiçek, B.; Sun, Y. A study on the mechanical and corrosion properties of lead added magnesium alloys. Mater. Des. 2012, 37, 369–372. [Google Scholar] [CrossRef]
- Li, B.; Duan, Y.; Zheng, S.; Li, M.; Peng, M.; Qi, H. Microstructure evolution and corrosion properties of ECAPed Mg–Pb-9.2Al-0.8B alloys. J. Mater. Res. Technol. 2023, 24, 6048–6064. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Feng, Y.; Deng, M.; Wang, N. Effect of Al and Pb Contents on the Corrosion Electrochemical Properties and Activation of Mg-Al-Pb Alloy Anode. J. Electrochem. Soc. 2017, 164, A438. [Google Scholar] [CrossRef]
- Liu, R.L.; Scully, J.R.; Williams, G.; Birbilis, N. Reducing the corrosion rate of magnesium via microalloying additions of group 14 and 15 elements. Electrochim. Acta 2018, 260, 184–195. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.-H.; Kim, Y.M.; You, B.S. Improving mechanical properties of extruded Mg–Al alloy with a bimodal grain structure through alloying addition. J. Alloys Compd. 2015, 646, 932–936. [Google Scholar] [CrossRef]
- Bae, S.W.; Kim, S.-H.; Lee, J.U.; Jo, W.-K.; Hong, W.-H.; Kim, W.; Park, S.H. Improvement of mechanical properties and reduction of yield asymmetry of extruded Mg-Al-Zn alloy through Sn addition. J. Alloys Compd. 2018, 766, 748–758. [Google Scholar] [CrossRef]
- Wen, L.; Yu, K.; Fang, H.-J.; Xiong, H.-Q.; Yin, X.; Zhu, H.-L.; Ma, J.-J.; Jiang, D.-Y. Electrochemical behavior of Mg-Al-Pb alloy in 3.5% NaCl solution. J. Cent. South Univ. 2016, 23, 2475–2482. [Google Scholar] [CrossRef]

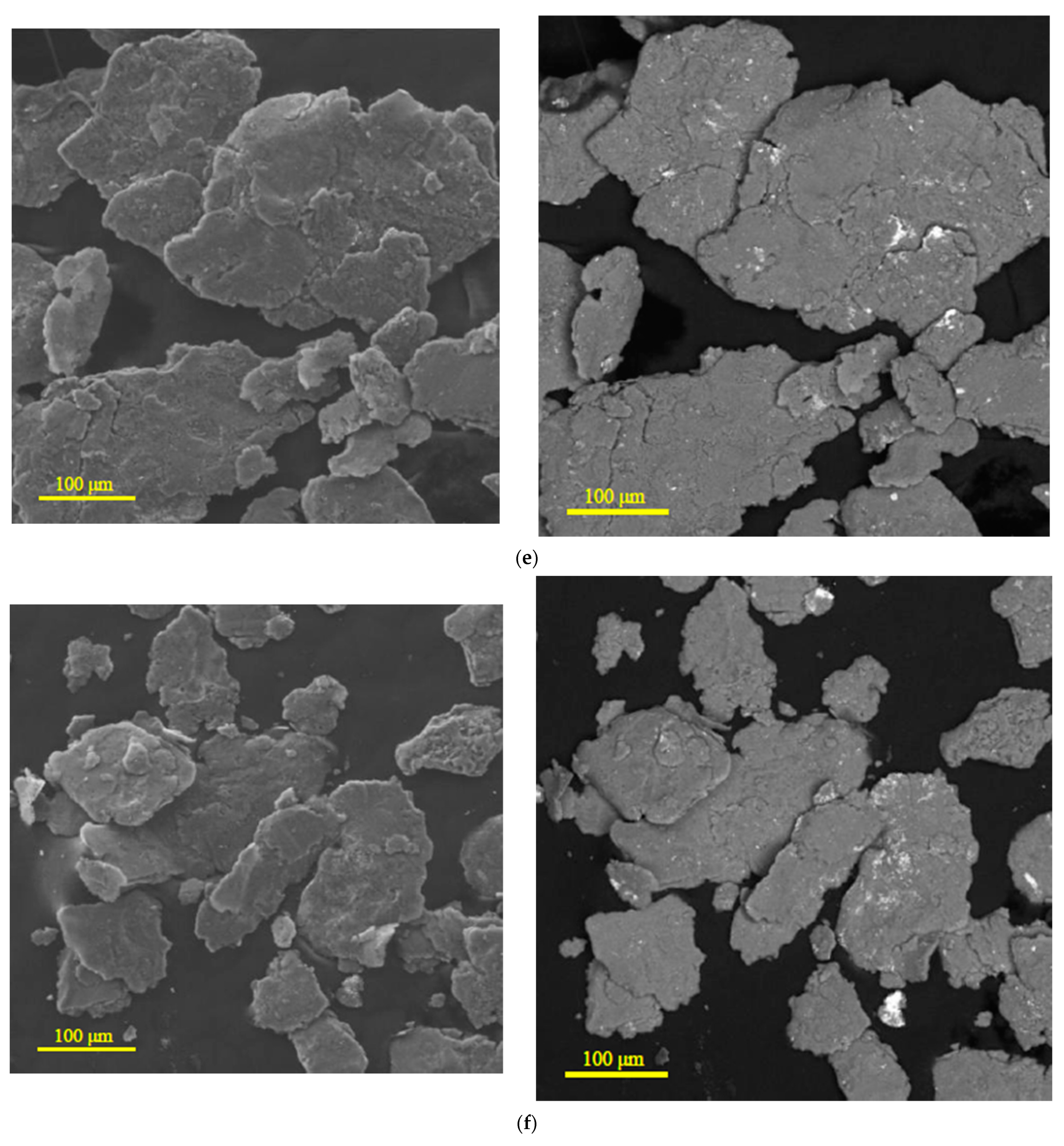
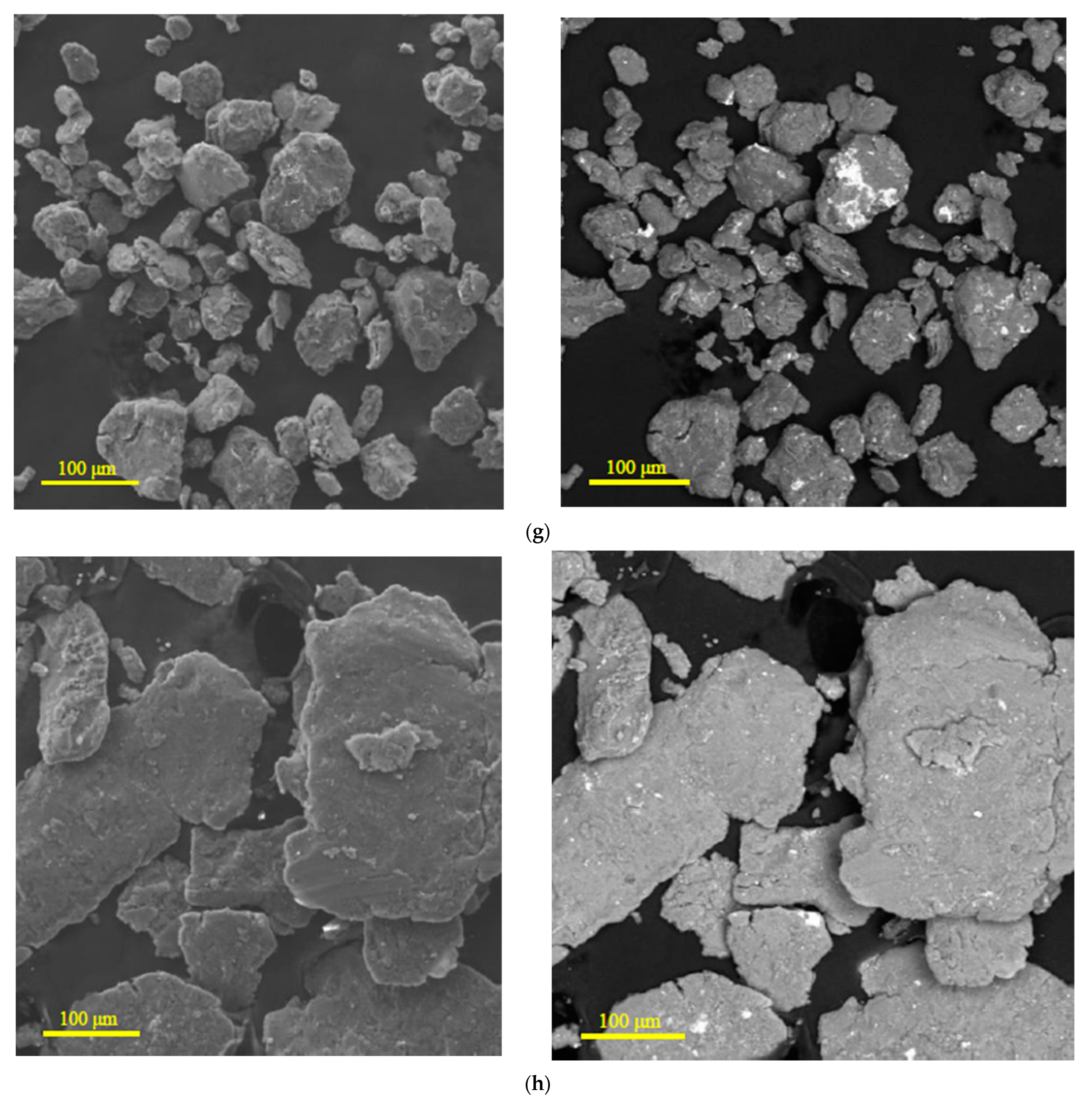
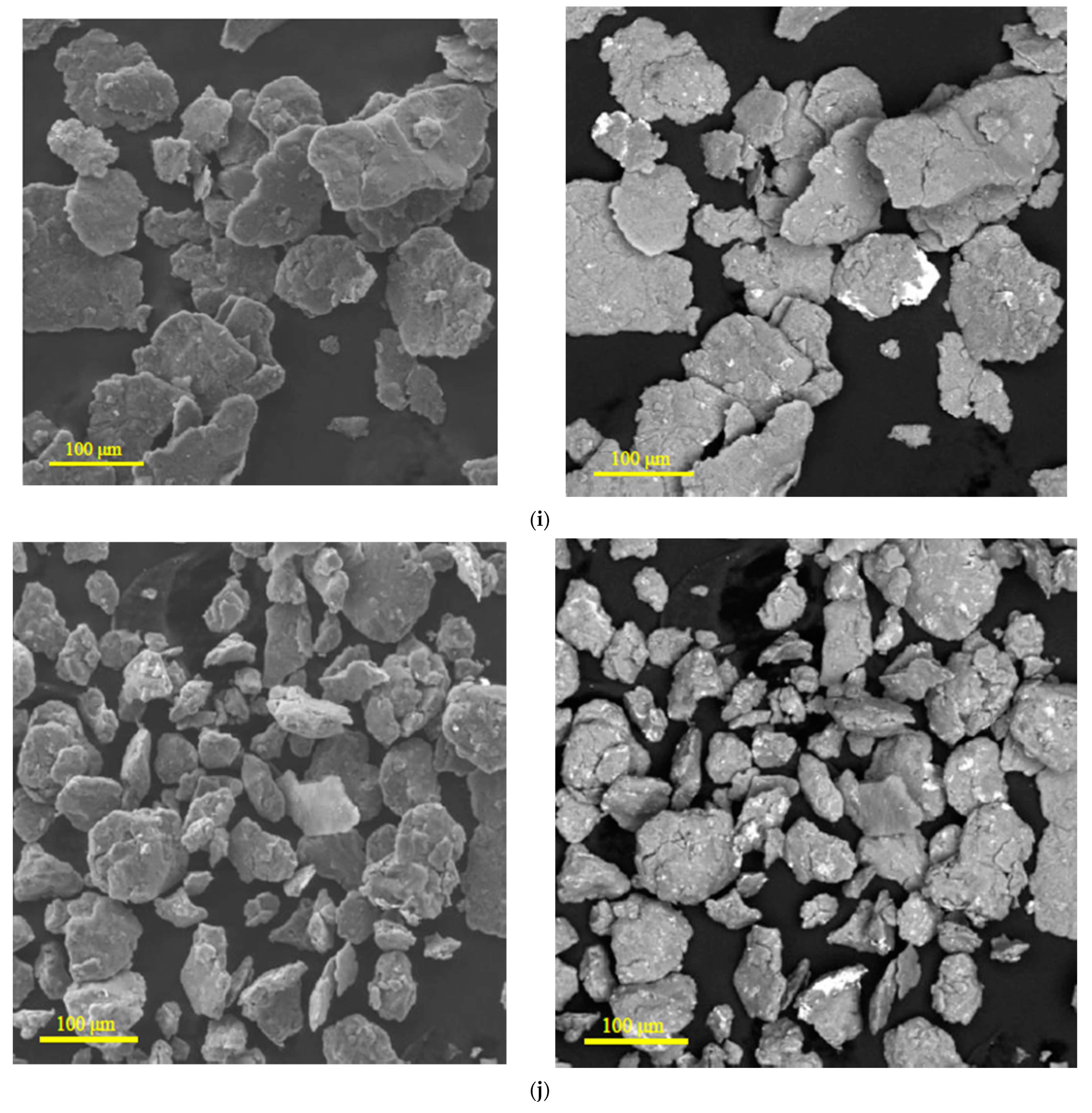
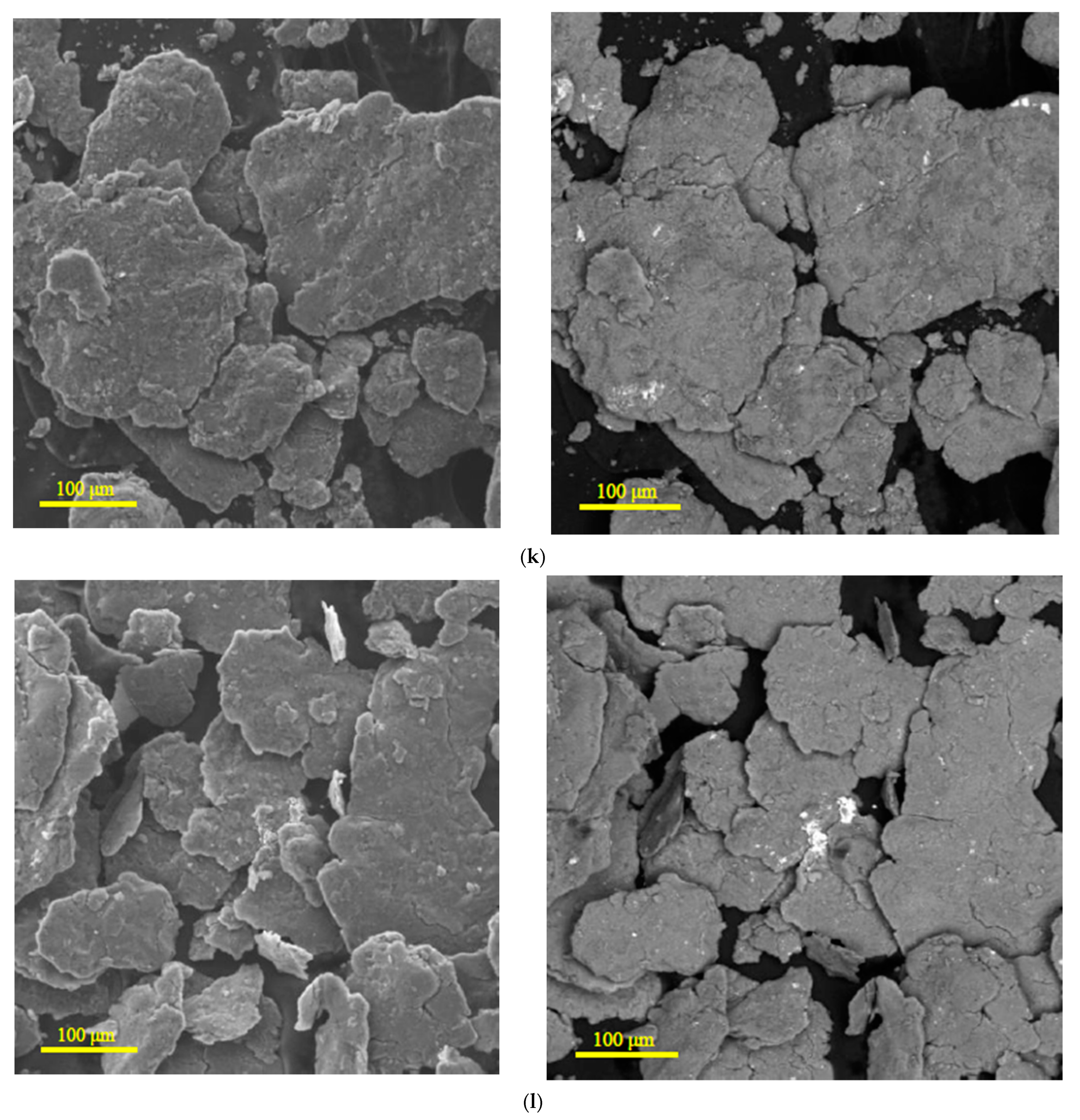




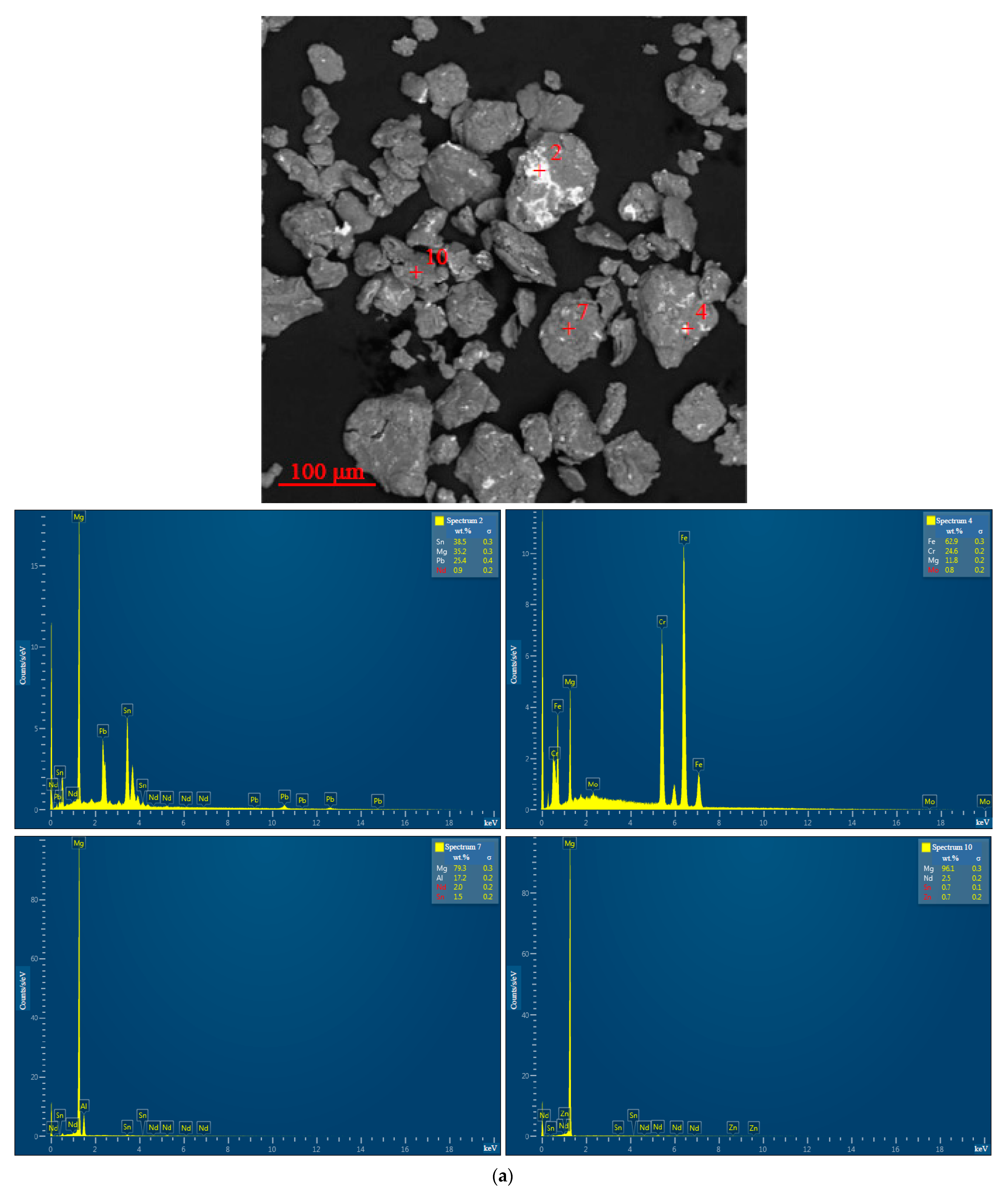
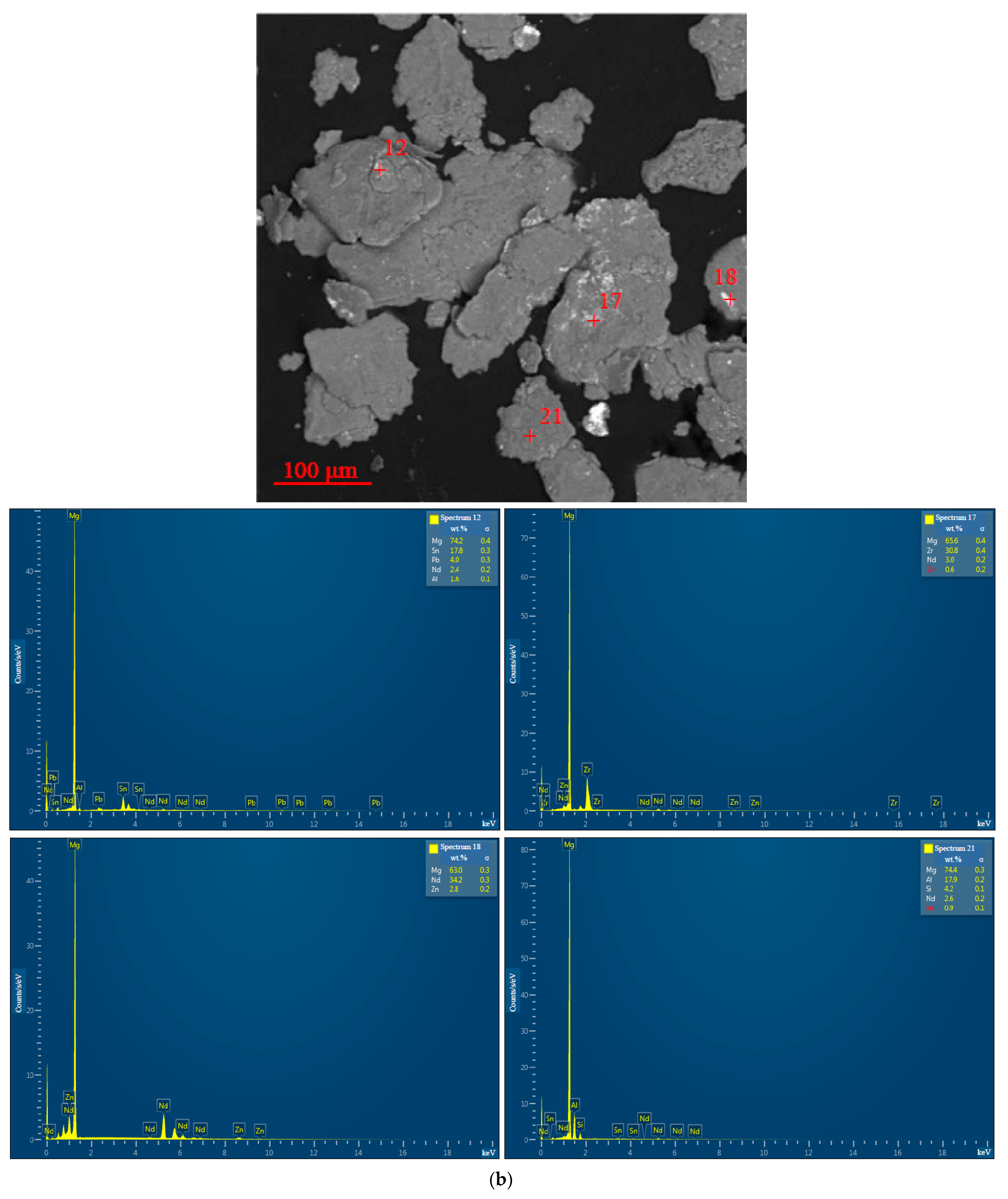
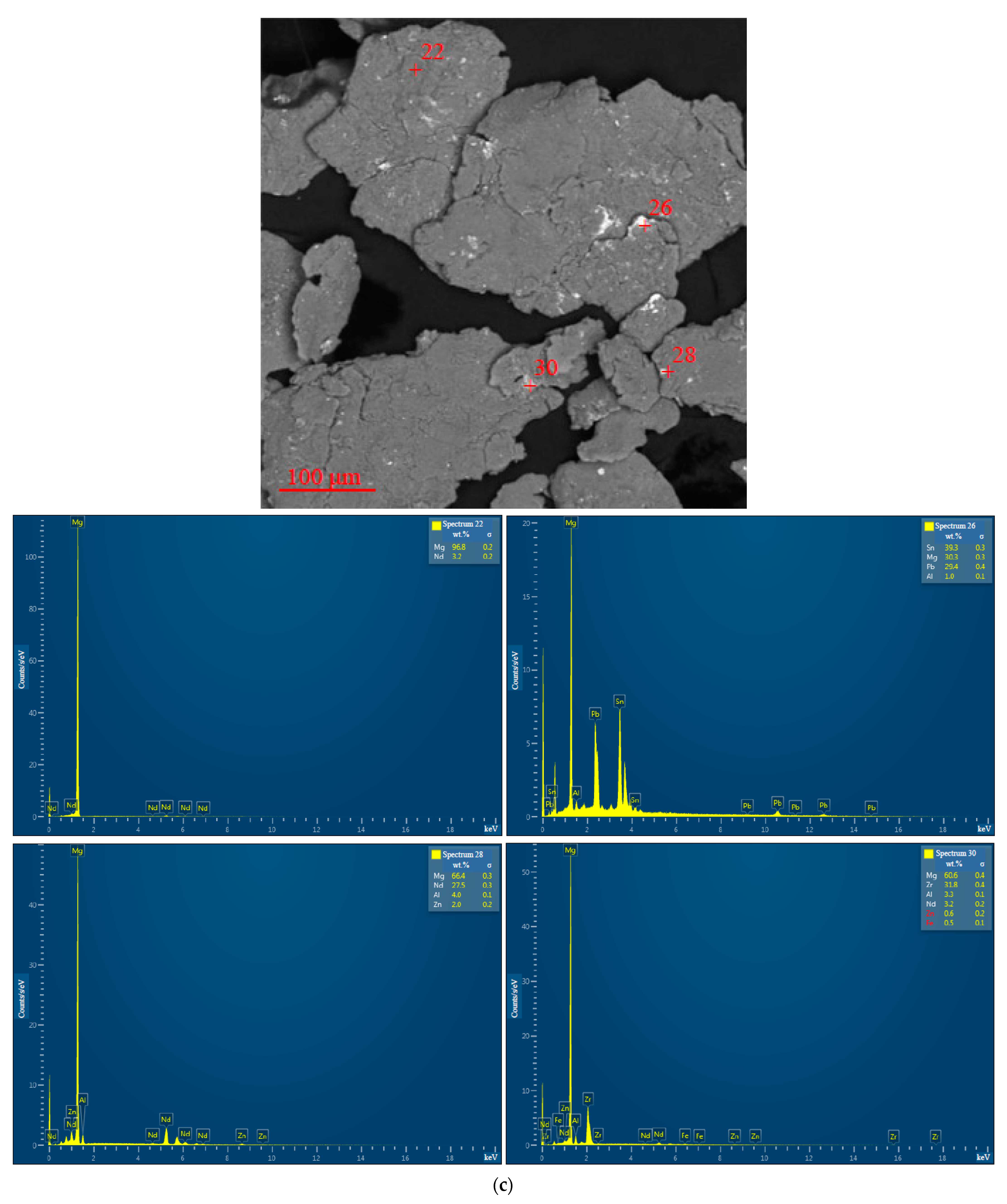
| Sample | Spectra No. | Mg | Al | Zn | Nd | Zr | Pb | Sn | Fe | Cr | Mo | Si | Cu | Sb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 wt.% ‘POS-61’, 2 h | 2 | 35.2 ± 0.3 | - | - | 0.9 ± 0.2 | - | 25.4 ± 0.4 | 38.5 ± 0.3 | - | - | - | - | - | - |
| 4 | 11.8 ± 0.2 | - | - | - | - | - | - | 62.9 ± 0.3 | 24.6 ± 0.2 | 0.8 ± 0.2 | - | - | - | |
| 10 wt.% ‘POS-61’, 1 h | 17 | 65.6 ± 0.4 | - | 0.6 ± 0.2 | 3.0 ± 0.2 | 30.8 ± 0.4 | - | - | - | - | - | - | - | - |
| 21 | 74.4 ± 0.3 | 17.9 ± 0.2 | - | 2.6 ± 0.2 | - | - | 0.9 ± 0.1 | - | - | - | 4.2 ± 0.1 | - | - | |
| 10 wt.% ‘POS-61’, 0.5 h | 28 | 66.4 ± 0.3 | 4.0 ± 0.1 | 2.0 ± 0.2 | 27.5 ± 0.3 | - | - | - | - | - | - | - | - | - |
| 30 | 60.6 ± 0.4 | 3.3 ± 0.1 | 0.6 ± 0.2 | 3.2 ± 0.2 | 31.8 ± 0.4 | - | - | 0.5 ± 0.1 | - | - | - | - | - | |
| 5 wt.% ‘POS-61′, 2 h | 36 | 47.9 ± 0.3 | - | - | 1.8 ± 0.2 | - | 17.3 ± 0.4 | 32.1 ± 0.3 | - | - | - | - | 0.9 ± 0.2 | - |
| 5 wt.% ‘POS-61′, 1 h | 46 | 15.1 ± 0.2 | 23.0 ± 0.2 | - | - | - | 14.1 ± 0.4 | 46.6 ± 0.3 | - | - | - | - | - | 1.2 ± 0.3 |
| Composition | Ball Milling Time, h | Hydrogen Yield, % | Maximum Reaction Rate, mL/g/min. |
|---|---|---|---|
| Scrap without additives | 0.5 | 77.3 ± 0.2 | 56 |
| 1 | 84.6 ± 0.4 | 86 | |
| 2 | 81.2 ± 0.9 | 77 | |
| Scrap + 2.5 wt.% ‘POS-61’ | 0.5 | 68.9 ± 1.0 | 45 |
| 1 | 83.7 ± 1.5 | 63 | |
| 2 | 79.6 ± 0.9 | 58 | |
| Scrap + 5 wt.% ‘POS-61’ | 0.5 | 81.2 ± 0.7 | 83 |
| 1 | 83.5 ± 4.7 | 108 | |
| 2 | 82.0 ± 1.9 | 52 | |
| Scrap + 10 wt.% ‘POS-61’ | 0.5 | 79.9 ± 0.8 | 56 |
| 1 | 84.8 ± 1.2 | 60 | |
| 2 | 81.6 ± 3.3 | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buryakovskaya, O.A.; Ambaryan, G.N.; Suleimanov, M.Z.; Tarasenko, A.B.; Vlaskin, M.S. Enhanced Hydrogen Generation from Magnesium–Aluminum Scrap Ball Milled with Low Melting Point Solder Alloy. Materials 2023, 16, 4450. https://doi.org/10.3390/ma16124450
Buryakovskaya OA, Ambaryan GN, Suleimanov MZ, Tarasenko AB, Vlaskin MS. Enhanced Hydrogen Generation from Magnesium–Aluminum Scrap Ball Milled with Low Melting Point Solder Alloy. Materials. 2023; 16(12):4450. https://doi.org/10.3390/ma16124450
Chicago/Turabian StyleBuryakovskaya, Olesya A., Grayr N. Ambaryan, Musi Zh. Suleimanov, Alexey B. Tarasenko, and Mikhail S. Vlaskin. 2023. "Enhanced Hydrogen Generation from Magnesium–Aluminum Scrap Ball Milled with Low Melting Point Solder Alloy" Materials 16, no. 12: 4450. https://doi.org/10.3390/ma16124450
APA StyleBuryakovskaya, O. A., Ambaryan, G. N., Suleimanov, M. Z., Tarasenko, A. B., & Vlaskin, M. S. (2023). Enhanced Hydrogen Generation from Magnesium–Aluminum Scrap Ball Milled with Low Melting Point Solder Alloy. Materials, 16(12), 4450. https://doi.org/10.3390/ma16124450






