Novel Concepts for Graphene-Based Nanomaterials Synthesis for Phenol Removal from Palm Oil Mill Effluent (POME)
Abstract
1. Introduction
2. Characteristics of Phenol Content in POME
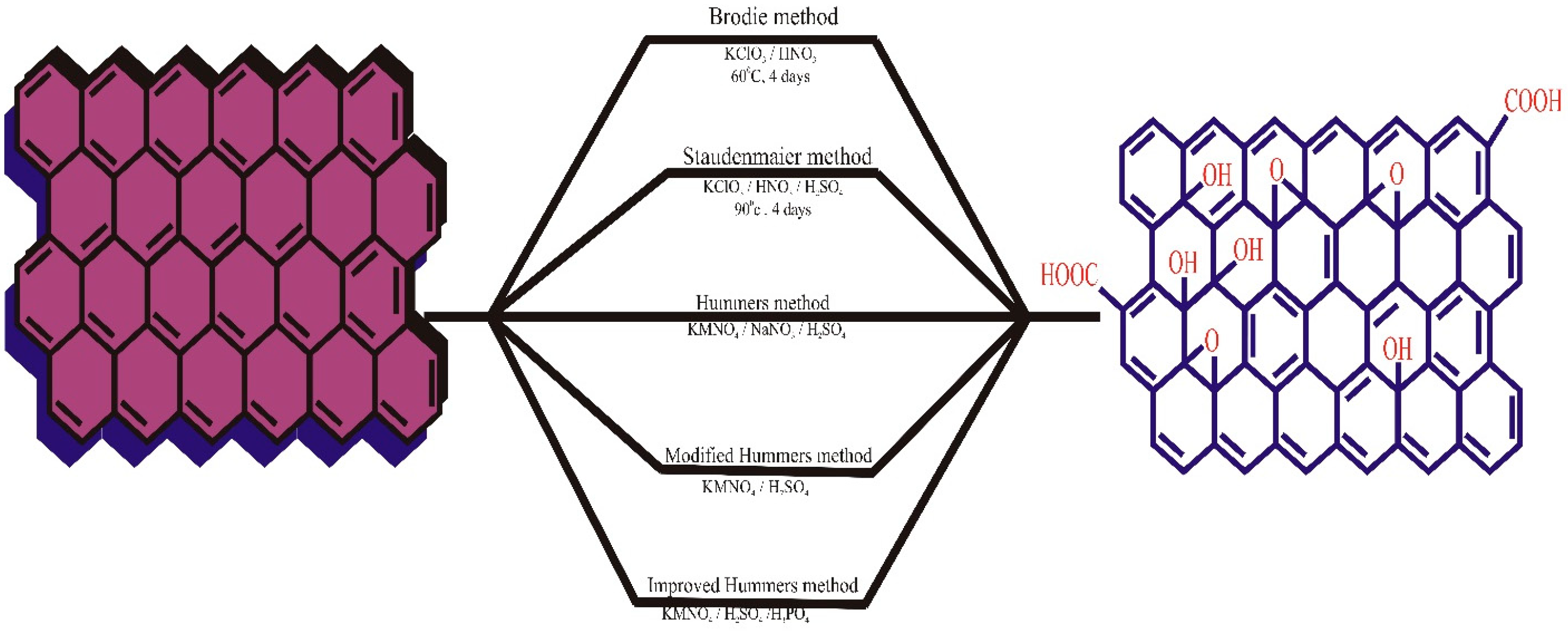
3. Synthesis and Characterization of GOs
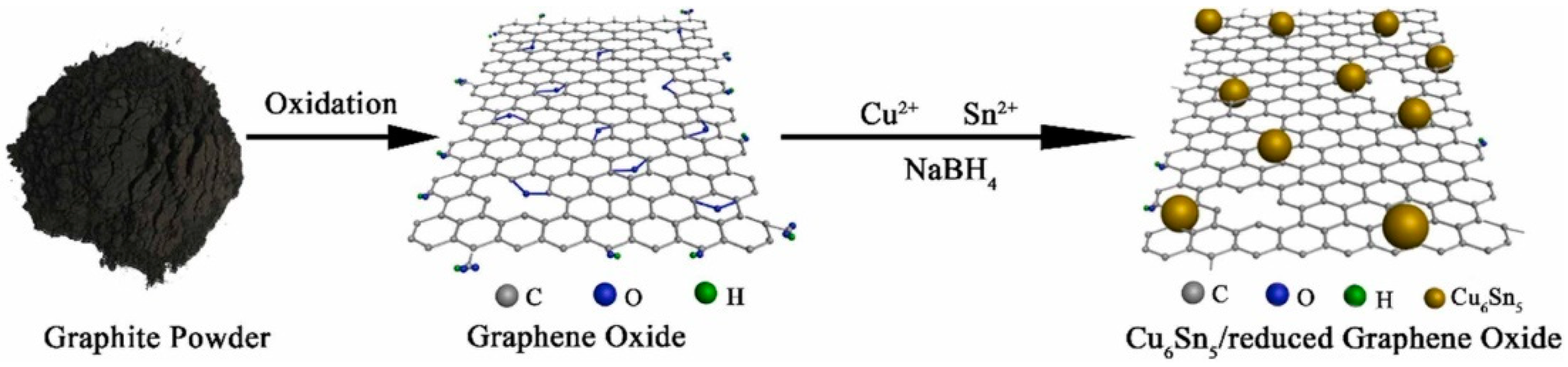
4. Mechanism of Graphene Nanomaterial Formation
5. GO-Based Phenol Treatment
| Adsorbents | Experimental Conditions | Adsorption Capacity (mg/g) | Isotherm Models | Kinetics Models | Adsorption Mechanisms | References | ||
|---|---|---|---|---|---|---|---|---|
| pH | Time (min) | Temp. (°C) | ||||||
| GO1 | 2 | - | 35 | 0.9 | Langmuir | - | Hydrophobic effect, electrostatic interaction, H-bonding, π-π-interaction, and van der Waals forces | [9] |
| GO2 | 6 | - | 25 | 20.2 | ||||
| GO-Fe3O4/PRD | 7 | 15 | 40 | 191 | Langmuir | PSO | - | [78] |
| GO-PAA | 2 | - | 25 | 84 | Langmuir | - | π-π interaction, electrostatic, and hydrophobic interaction, H-bonding, dispersion by van der Waals forces | [26] |
| GO-coated biochar | 7 | 60 | 35 | 23.47 | Langmuir | PSO | - | [81] |
| GO-PNIPAM | 7 | - | 25 | 12.74 | Langmuir | - | H-bonding | [82] |
| GO-(O-MWCNTs)-Fe3O4 | 6 | 60 | - | 224.21 | Langmuir | PSO | H-bonding, electrostatic interaction, hydrophobic, and π-π interaction | [79] |
| GO/PPy | 5 | 1440 | - | 7.75 | Langmuir | PSO | Ion exchange, π-π -electron donor acceptor (EDA) interaction, hydrophobic interaction, and Lewis’s acid-base interaction | [83] |
| -N-RGO | 6 | 2160 | 30 | 155.82 | - | PSO | Electrostatic, hydrophobic, and π-π interactions | [27] |
| GO-Fe3O4 | 4 | 70 | - | 657.9 | Langmuir | PSO | - | [80] |
| GO | 7 | - | 30 | 10.23 | Langmuir | PSO | - | [84] |
| GO-CTES-β-CD/PNIPAM | 7 | - | 25 | 131.64 | Freundlich | PSO | Hydrogen bonding | [24] |
6. Novel Graphene Nanomaterial-Based Concepts for Phenol Treatment
7. Future Perspective
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment technologies of palm oil mill effluent (POME) and olive mill wastewater (OMW): A brief review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Lawal, A.A.; Hassan, M.A.; Farid, M.A.A.; Yasim-Anuar, T.A.T.; Yusoff, M.Z.M.; Zakaria, M.R.; Roslan, A.M.; Mokhtar, M.N.; Shirai, Y. One-step steam pyrolysis for the production of mesoporous biochar from oil palm frond to effectively remove phenol in facultatively treated palm oil mill effluent. Environ. Technol. Innov. 2020, 18, 100730. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chong, C.C.; Lam, M.K.; Ayoub, M.; Cheng, C.K.; Lim, J.W.; Yusup, S.; Tang, Y.; Bai, J. Holistic process evaluation of non-conventional palm oil mill effluent (POME) treatment technologies: A conceptual and comparative review. J. Hazard. Mater. 2021, 409, 124964. [Google Scholar] [CrossRef] [PubMed]
- Choong, Y.Y.; Chou, K.W.; Norli, I. Strategies for improving biogas production of palm oil mill effluent (POME) anaerobic digestion: A critical review. Renew. Sustain. Energy Rev. 2018, 82, 2993–3006. [Google Scholar] [CrossRef]
- Chantho, P.; Musikavong, C.; Suttinun, O. Removal of phenolic compounds from palm oil mill effluent by thermophilic Bacillus thermoleovorans strain A2 and their effect on anaerobic digestion. Int. Biodeterior. Biodegrad. 2016, 115, 293–301. [Google Scholar] [CrossRef]
- Khadaroo, S.N.B.A.; Poh, P.E.; Gouwanda, D.; Grassia, P. Applicability of various pretreatment techniques to enhance the anaerobic digestion of Palm oil Mill effluent (POME): A review. J. Environ. Chem. Eng. 2019, 7, 103310. [Google Scholar] [CrossRef]
- Tirapanampai, C.; Toewiwat, N.; Weeranoppanant, N.; Chaiyen, P.; Wongnate, T. Processing of palm oil mill effluent (POME) into food waste digesting microbes: An investigation of acclimatization strategies. Sustain. Energy Technol. Assess. 2022, 52, 102287. [Google Scholar] [CrossRef]
- Wang, W.; Gong, Q.; Chen, Z.; Wang, W.D.; Huang, Q.; Song, S.; Chen, J.; Wang, X. Adsorption and competition investigation of phenolic compounds on the solid-liquid interface of three-dimensional foam-like graphene oxide. Chem. Eng. J. 2019, 378, 122085. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Sayma, J.; Munira, N.; Mohamed, D.; Da’na, D.A.; Qiblawey, H.; Alkhouzaam, A. Effective removal of phenol from wastewater using a hybrid process of graphene oxide adsorption and UV-irradiation. Environ. Technol. Innov. 2022, 27, 102525. [Google Scholar] [CrossRef]
- Nunes, F.B.; da Silva Bruckmann, F.; da Rosa Salles, T.; Rhoden, C.R.B. Study of phenobarbital removal from the aqueous solutions employing magnetite-functionalized chitosan. Environ. Sci. Pollut. Res. 2023, 30, 12658–12671. [Google Scholar] [CrossRef]
- Esmaeilion, F. Hybrid renewable energy systems for desalination. Appl. Water Sci. 2020, 10, 84. [Google Scholar] [CrossRef]
- Jiménez, S.M.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- Rashtbari, Y.; Hazrati, S.; Azari, A.; Afshin, S.; Fazlzadeh, M.; Vosoughi, M. A novel, eco-friendly and green synthesis of PPAC-ZnO and PPAC-nZVI nanocomposite using pomegranate peel: Cephalexin adsorption experiments, mechanisms, isotherms and kinetics. Adv. Powder Technol. 2020, 31, 1612–1623. [Google Scholar] [CrossRef]
- Sayadi, S.; Allouche, N.; Jaoua, M.; Aloui, F. Detrimental effects of high molecular-mass polyphenols on olive mill wastewater biotreatment. Process Biochem. 2000, 35, 725–735. [Google Scholar] [CrossRef]
- Beccari, M.; Carucci, G.; Lanz, A.M.; Majone, M.; Petrangeli Papini, M. Removal of molecular weight fractions of COD and phenolic compounds in an integrated treatment of olive oil mill effluents. Biodegradation 2002, 13, 401–410. [Google Scholar] [CrossRef]
- Mohammad, S.; Baidurah, S.; Kobayashi, T.; Ismail, N.; Leh, C.P. Palm oil mill effluent treatment processes—A review. Processes 2021, 9, 739. [Google Scholar] [CrossRef]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects—A review. Environ. Rev. 2010, 18, 255–278. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Abu-Nada, A.; Abdala, A.; McKay, G. Removal of phenols and dyes from aqueous solutions using graphene and graphene composite adsorption: A review. J. Environ. Chem. Eng. 2021, 9, 105858. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; Kazan-Kaya, E.S.; El Messaoudi, N.; Fernine, Y.; Américo-Pinheiro, J.H.P.; Jada, A. Remediation of tetracycline from aqueous solution through adsorption on g-C3N4-ZnO-BaTiO3 nanocomposite: Optimization, modeling, and theoretical calculation. J. Mol. Liq. 2023, 369, 120866. [Google Scholar] [CrossRef]
- El Mouden, A.; El Messaoudi, N.; El Guerraf, A.; Bouich, A.; Mehmeti, V.; Lacherai, A.; Jada, A.; Sher, F. Multifunctional cobalt oxide nanocomposites for efficient removal of heavy metals from aqueous solutions. Chemosphere 2023, 317, 137922. [Google Scholar] [CrossRef] [PubMed]
- Farhan, A.; Rashid, E.U.; Waqas, M.; Ahmad, H.; Nawaz, S.; Munawar, J.; Rahdar, A.; Varjani, S.; Bilal, M. Multifunctional graphene-based nanocomposites and nanohybrids for the abatement of agro-industrial pollutants in aqueous environments―A review. Environ. Pollut. 2022, 308, 119557. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, J.; Xu, J.; Dong, F.; Xiong, Y.; Chen, Q. In-situ formed Cyclodextrin-functionalized graphene oxide/poly (N-isopropylacrylamide) nanocomposite hydrogel as an recovery adsorbent for phenol and microfluidic valve. J. Colloid Interface Sci. 2022, 607, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.Z.N.; Salleh, W.N.W.; Ismail, A.F.; Yusof, N.; Yusop, M.Z.M.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef] [PubMed]
- Bibi, A.; Bibi, S.; Abu-Dieyeh, M.; Al-Ghouti, M.A. New material of polyacrylic acid-modified graphene oxide composite for phenol remediation from synthetic and real wastewater. Environ. Technol. Innov. 2022, 27, 102795. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Ji, J.; Wang, Q.; Li, G.; Wu, T.; Zhang, B. Efficient removal of phenol and p-nitrophenol using nitrogen-doped reduced graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125866. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic compounds in water: Sources, reactivity, toxicity and treatment methods. In Phenolic Compounds-Natural Sources, Importance and Applications; IntechOpen: Rijeka, Croatia, 2017; pp. 419–443. [Google Scholar]
- Mohammed, R.R.; Chong, M.F. Treatment and decolorization of biologically treated Palm Oil Mill Effluent (POME) using banana peel as novel biosorbent. J. Environ. Manag. 2014, 132, 237–249. [Google Scholar] [CrossRef]
- Kafle, B.P. Application of UV–VIS spectrophotometry for chemical analysis. In Chemical Analysis and Material Characterization by Spectrophotometry; Elsevier: New York, NY, USA, 2020; pp. 79–145. [Google Scholar]
- Abd Gami, A.; Shukor, M.Y.; Khalil, K.A.; Dahalan, F.A.; Khalid, A.; Ahmad, S.A. Phenol and its toxicity. J. Environ. Microbiol. Toxicol. 2014, 2, 11–23. [Google Scholar] [CrossRef]
- Møller, L.M.; Larsen, P.B.; Fotel, F.L.; Slothuus, T.; Boyd, H.B. Survey of Phenol. 2014. Available online: https://www2.mst.dk/Udgiv/publications/2014/01/978-87-93026-89-6.pdf (accessed on 4 June 2023).
- Scholze, M.; Knorr, W.; Arnell, N.W.; Prentice, I.C. A climate-change risk analysis for world ecosystems. Proc. Natl. Acad. Sci. USA 2006, 103, 13116–13120. [Google Scholar] [CrossRef]
- Delfino, J.J.; Dube, D.J. Persistent contamination of ground water by phenol. J. Environ. Sci. Health Part A 1976, 11, 345–355. [Google Scholar] [CrossRef]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A short review of techniques for phenol removal from wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Ahmed, I.; Kazmi, S.A.H. Phenol toxicity. J. Coll. Physicians Surg. Pak. 2000, 10, 344–345. [Google Scholar]
- Harborne, J.B. Do natural plant phenols play a role in ecology? Acta Hortic. 1994, 381, 36–45. [Google Scholar] [CrossRef]
- Kulkarni, S.J.; Kaware, J.P. Review on research for removal of phenol from wastewater. Int. J. Sci. Res. Publ. 2013, 3, 1–5. [Google Scholar]
- Said, K.A.M.; Ismail, A.F.; Karim, Z.A.; Abdullah, M.S.; Hafeez, A. A review of technologies for the phenolic compounds recovery and phenol removal from wastewater. Process Saf. Environ. Prot. 2021, 151, 257–289. [Google Scholar] [CrossRef]
- Oliveira, M.F.; da Silva, M.G.C.; Vieira, M.G.A. Equilibrium and kinetic studies of caffeine adsorption from aqueous solutions on thermally modified Verde-lodo bentonite. Appl. Clay Sci. 2019, 168, 366–373. [Google Scholar] [CrossRef]
- de Andrade, J.R.; Vieira, M.G.A.; da Silva, M.G.C.; Wang, S. Oxidative degradation of pharmaceutical losartan potassium with N-doped hierarchical porous carbon and peroxymonosulfate. Chem. Eng. J. 2020, 382, 122971. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, J.C.G.E. Advanced oxidation processes coupled with nanomaterials for water treatment. Nanomaterials 2021, 11, 2045. [Google Scholar] [CrossRef]
- Beker, U.; Ganbold, B.; Dertli, H.; Gülbayir, D.D. Adsorption of phenol by activated carbon: Influence of activation methods and solution pH. Energy Convers. Manag. 2010, 51, 235–240. [Google Scholar] [CrossRef]
- Matias, T.; Marques, J.; Quina, M.J.; Gando-Ferreira, L.; Valente, A.J.M.; Portugal, A.; Durães, L. Silica-based aerogels as adsorbents for phenol-derivative compounds. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 260–269. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Saleh, M.; El-Gendy, R.A.; Saleh, M.R.; Thabet, S.M. High adsorption capacity of phenol and methylene blue using activated carbon derived from lignocellulosic agriculture wastes. Sci. Rep. 2022, 12, 5499. [Google Scholar] [CrossRef] [PubMed]
- Yousef, R.I.; El-Eswed, B.; Ala’a, H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Daffalla, S.B.; Mukhtar, H.; Shaharun, M.S. Preparation and characterization of rice husk adsorbents for phenol removal from aqueous systems. PLoS ONE 2020, 15, e0243540. [Google Scholar] [CrossRef] [PubMed]
- Haydari, I.; Aziz, K.; Kaya, S.; Daştan, T.; Ouazzani, N.; Mandi, L.; Aziz, F. Green synthesis of reduced graphene oxide and their use on column adsorption of phenol from olive mill wastewater. Process Saf. Environ. Prot. 2023, 170, 1079–1091. [Google Scholar] [CrossRef]
- Shin, D.S.; Kim, H.G.; Ahn, H.S.; Jeong, H.Y.; Kim, Y.-J.; Odkhuu, D.; Tsogbadrakh, N.; Kim, B.H. Distribution of oxygen functional groups of graphene oxide obtained from low-temperature atomic layer deposition of titanium oxide. RSC Adv. 2017, 7, 13979–13984. [Google Scholar] [CrossRef]
- Brodie, B. Note sur un nouveau procédé pour la purification et la désagrégation du graphite. Ann. Chim. Phys. 1855, 45, 351–353. [Google Scholar]
- Brodie, B.C. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced graphene oxide molecular sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-wrapped sulfur particles as a rechargeable lithium–sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef]
- Jankovský, O.; Nováček, M.; Luxa, J.; Sedmidubský, D.; Boháčová, M.; Pumera, M.; Sofer, Z. Concentration of nitric acid strongly influences chemical composition of graphite oxide. Chem. Eur. J. 2017, 23, 6432–6440. [Google Scholar] [CrossRef] [PubMed]
- Perumal, D.; Albert, E.L.; Abdullah, C.A. Green Reduction of Graphene Oxide Involving Extracts of Plants from Different Taxonomy Groups. J. Compos. Sci. 2022, 6, 58. [Google Scholar] [CrossRef]
- Obayomi, K.S.; Lau, S.Y.; Danquah, M.; Chiong, T.; Takeo, M. Advances in graphene oxide based nanobiocatalytic technology for wastewater treatment. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100647. [Google Scholar] [CrossRef]
- Li, L.; Zhou, M.; Jin, L.; Liu, L.; Mo, Y.; Li, X.; Mo, Z.; Liu, Z.; You, S.; Zhu, H. Research progress of the liquid-phase exfoliation and stable dispersion mechanism and method of graphene. Front. Mater. 2019, 6, 325. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, H.; Zou, G.; Shi, W.; Shuai, H.; Li, J.; Ji, X. Electrochemical exfoliation of graphene-like two-dimensional nanomaterials. Nanoscale 2019, 11, 16–33. [Google Scholar] [CrossRef] [PubMed]
- El-Hallag, I.S.; El-Nahass, M.N.; Youssry, S.M.; Kumar, R.; Abdel-Galeil, M.M.; Matsuda, A. Facile in-situ simultaneous electrochemical reduction and deposition of reduced graphene oxide embedded palladium nanoparticles as high performance electrode materials for supercapacitor with excellent rate capability. Electrochim. Acta 2019, 314, 124–134. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical vapour deposition of graphene—Synthesis, characterisation, and applications: A review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Niavol, S.S.; Budde, M.; Papadogianni, A.; Heilmann, M.; Moghaddam, H.M.; Aldao, C.M.; Ligorio, G.; List-Kratochvil, E.J.W.; Lopes, J.M.J.; Barsan, N. Conduction mechanisms in epitaxial NiO/Graphene gas sensors. Sens. Actuators B Chem. 2020, 325, 128797. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Chen, Z. Graphene-related nanomaterials: Tuning properties by functionalization. Nanoscale 2013, 5, 4541–4583. [Google Scholar] [CrossRef]
- Coroş, M.; Pogăcean, F.; Măgeruşan, L.; Socaci, C.; Pruneanu, S. A brief overview on synthesis and applications of graphene and graphene-based nanomaterials. Front. Mater. Sci. 2019, 13, 23–32. [Google Scholar] [CrossRef]
- Gerosa, R.M.; Steinberg, D.; Nagaoka, D.A.; Zapata, J.D.; Domingues, S.H.; Thoroh de Souza, E.A.; Saito, L.A.M. Liquid phase exfoliated black phosphorus and reduced graphene oxide polymer-based saturable absorbers fabrication using the droplet method for mode-locking applications. Opt. Laser Technol. 2018, 106, 107–112. [Google Scholar] [CrossRef]
- Randhir Singh, B. Enhancing Liquid Phase Exfoliation of Graphene in Organic Solvents with Additives. In Graphene and Its Derivatives; Ishaq, A., Fabian, I.E., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 3. [Google Scholar] [CrossRef]
- Chang, J.-L.; Liao, C.-W.; Arthisree, D.; Senthil Kumar, A.; Zen, J.-M. A Size-Controlled Graphene Oxide Materials Obtained by One-Step Electrochemical Exfoliation of Carbon Fiber Cloth for Applications to In Situ Gold Nanoparticle Formation and Electrochemical Sensors—A Preliminary Study. Biosensors 2022, 12, 360. [Google Scholar]
- Ikram, R.; Jan, B.M.; Ahmad, W. An overview of industrial scalable production of graphene oxide and analytical approaches for synthesis and characterization. J. Mater. Res. Technol. 2020, 9, 11587–11610. [Google Scholar] [CrossRef]
- Lin, C.; Liu, H.; Guo, M.; Zhao, Y.; Su, X.; Zhang, P.; Zhang, Y. Plasmon-induced broad spectrum photocatalytic overall water splitting: Through non-noble bimetal nanoparticles hybrid with reduced graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 128962. [Google Scholar] [CrossRef]
- Zhou, A.a.; Bai, J.; Hong, W.; Bai, H. Electrochemically reduced graphene oxide: Preparation, composites, and applications. Carbon 2022, 191, 301–332. [Google Scholar] [CrossRef]
- Xu, Q.; Lin, X.; Gan, L.; Owens, G.; Chen, Z. Green reduction of graphene oxide using Bacillus sphaericus. J. Colloid Interface Sci. 2022, 605, 881–887. [Google Scholar] [CrossRef]
- Ullah, S.; Liu, Y.; Hasan, M.; Zeng, W.; Shi, Q.; Yang, X.; Fu, L.; Ta, H.Q.; Lian, X.; Sun, J.; et al. Direct synthesis of large-area Al-doped graphene by chemical vapor deposition: Advancing the substitutionally doped graphene family. Nano Res. 2022, 15, 1310–1318. [Google Scholar] [CrossRef]
- Wan, X.; Huang, Y.; Chen, Y. Focusing on energy and optoelectronic applications: A journey for graphene and graphene oxide at large scale. Acc. Chem. Res. 2012, 45, 598–607. [Google Scholar] [CrossRef]
- Rodner, M.; Icardi, A.; Kodu, M.; Jaaniso, R.; Schütze, A.; Eriksson, J. Metal Oxide Nanolayer-Decorated Epitaxial Graphene: A Gas Sensor Study. Nanomaterials 2020, 10, 2168. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Yadav, A.; Olabi, A.G. Graphene Synthesis Techniques and Environmental Applications. Materials 2022, 15, 7804. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Hu, Y. Improved synthesis of graphene/β-cyclodextrin composite for highly efficient dye adsorption and removal. J. Mol. Liq. 2017, 242, 181–189. [Google Scholar] [CrossRef]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Kang, S.; Jung, K.H.; Mhin, S.; Son, Y.; Lee, K.; Kim, W.R.; Choi, H.; Ryu, J.H.; Han, H.; Kim, K.M. Fundamental Understanding of the Formation Mechanism for Graphene Quantum Dots Fabricated by Pulsed Laser Fragmentation in Liquid: Experimental and Theoretical Insight. Small 2020, 16, 2003538. [Google Scholar] [CrossRef]
- Parvin, N.; Babapoor, A.; Nematollahzadeh, A.; Mousavi, S.M. Removal of phenol and β-naphthol from aqueous solution by decorated graphene oxide with magnetic iron for modified polyrhodanine as nanocomposite adsorbents: Kinetic, equilibrium and thermodynamic studies. React. Funct. Polym. 2020, 156, 104718. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, J.; Xiao, Y.; Zhao, Z.; Zhang, M.; Wang, L.; Zhang, X.; Zhou, C. High-efficiency adsorption of and competition between phenol and hydroquinone in aqueous solution on highly cationic amino-poly (vinylamine)-functionalized GO-(o-MWCNTs) magnetic nanohybrids. Chem. Eng. J. 2020, 389, 124223. [Google Scholar] [CrossRef]
- Badhai, P.; Kashyap, S.; Behera, S.K. Adsorption of phenol red onto GO-Fe3O4 hybrids in aqueous media. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100282. [Google Scholar] [CrossRef]
- Manna, S.; Prakash, S.; Das, P. Synthesis of graphene oxide nano-materials coated bio-char using carbonaceous industrial waste for phenol separation from water. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123818. [Google Scholar] [CrossRef]
- Gong, Z.; Li, S.; Han, W.; Wang, J.; Ma, J.; Zhang, X. Recyclable graphene oxide grafted with poly (N-isopropylacrylamide) and its enhanced selective adsorption for phenols. Appl. Surf. Sci. 2016, 362, 459–468. [Google Scholar] [CrossRef]
- Hu, R.; Dai, S.; Shao, D.; Alsaedi, A.; Ahmad, B.; Wang, X. Efficient removal of phenol and aniline from aqueous solutions using graphene oxide/polypyrrole composites. J. Mol. Liq. 2015, 203, 80–89. [Google Scholar] [CrossRef]
- Mukherjee, M.; Goswami, S.; Banerjee, P.; Sengupta, S.; Das, P.; Banerjee, P.K.; Datta, S. Ultrasonic assisted graphene oxide nanosheet for the removal of phenol containing solution. Environ. Technol. Innov. 2019, 13, 398–407. [Google Scholar] [CrossRef]
- Maio, A.; Gammino, M.; Gulino, E.F.; Megna, B.; Fara, P.; Scaffaro, R. Rapid One-Step Fabrication of Graphene Oxide-Decorated Polycaprolactone Three-Dimensional Templates for Water Treatment. ACS Appl. Polym. Mater. 2020, 2, 4993–5005. [Google Scholar] [CrossRef]
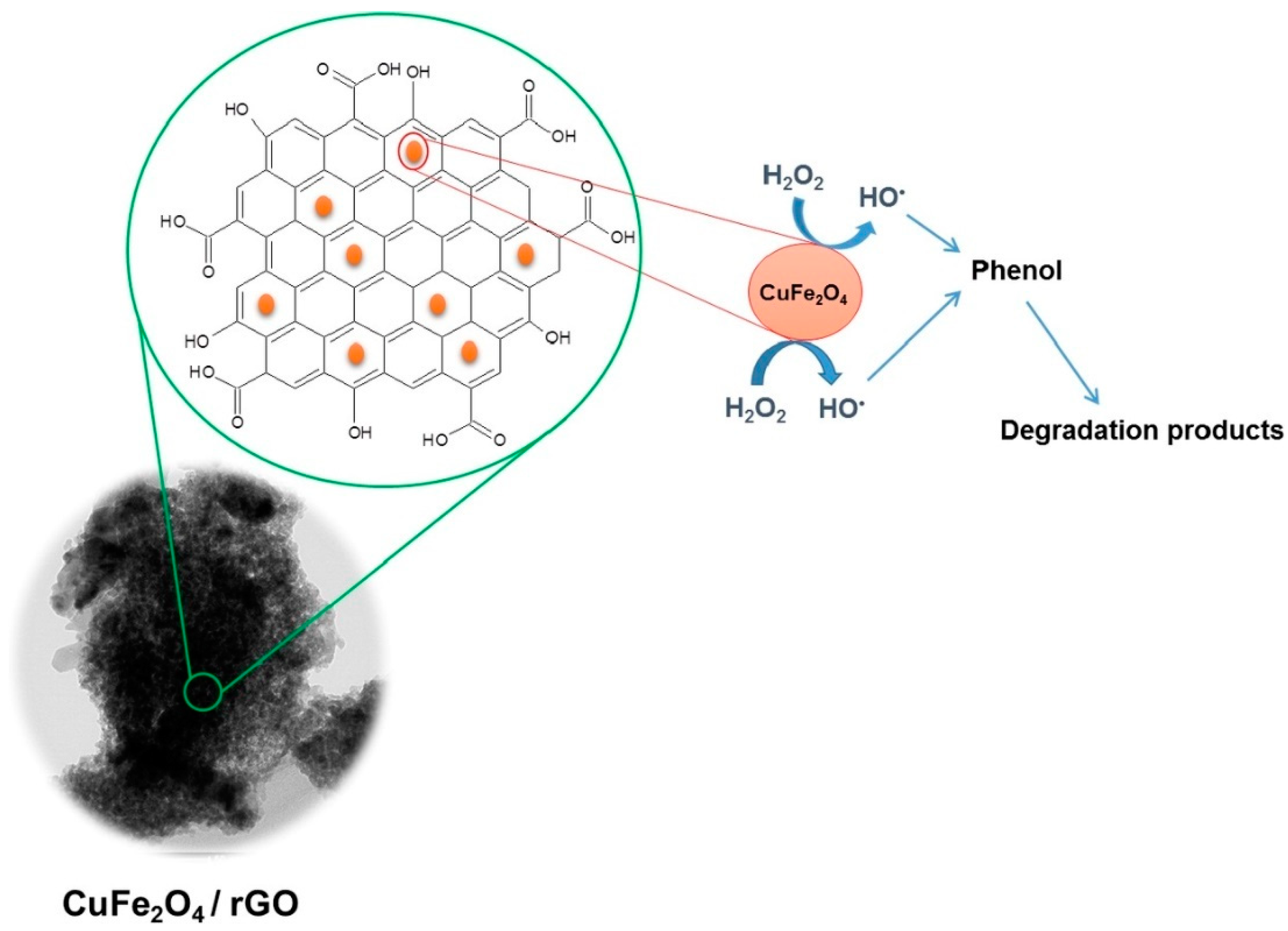
- Othman, I.; Abu Haija, M.; Ismail, I.; Zain, J.H.; Banat, F. Preparation and catalytic performance of CuFe2O4 nanoparticles supported on reduced graphene oxide (CuFe2O4/rGO) for phenol degradation. Mater. Chem. Phys. 2019, 238, 121931. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.D.; Purohit, L.P. Novel ZnO tetrapod-reduced graphene oxide nanocomposites for enhanced photocatalytic degradation of phenolic compounds and MB dye. J. Mol. Liq. 2021, 327, 114814. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, N.; Yuen, G.; de Lannoy, C.-F. Cross-linked iron nanoparticle-doped reduced graphene oxide membranes for micropollutant removal from water. Chem. Eng. J. 2023, 455, 140624. [Google Scholar] [CrossRef]
- Priyadharshini, S.D.; Manikandan, S.; Kiruthiga, R.; Rednam, U.; Babu, P.S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Graphene oxide-based nanomaterials for the treatment of pollutants in the aquatic environment: Recent trends and perspectives—A review. Environ. Pollut. 2022, 306, 119377. [Google Scholar] [CrossRef]
- Kokkinos, P.; Mantzavinos, D.; Venieri, D. Current trends in the application of nanomaterials for the removal of emerging micropollutants and pathogens from water. Molecules 2020, 25, 2016. [Google Scholar] [CrossRef] [PubMed]
- Paschke, A.; Popp, P. Solid-phase microextraction fibre–water distribution constants of more hydrophobic organic compounds and their correlations with octanol–water partition coefficients. J. Chromatogr. A 2003, 999, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of Metal and Metal Oxide Nanoparticles. ChemBioEng Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Boonyuen, S.; Andou, Y. Porous Graphene-Based Materials for Enhanced Adsorption Towards Emerging Micropollutants (EMs). In Handbook of Porous Carbon Materials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 547–570. [Google Scholar]
- Mudhoo, A.; Gautam, R.K.; Ncibi, M.C.; Zhao, F.; Garg, V.K.; Sillanpää, M. Green synthesis, activation and functionalization of adsorbents for dye sequestration. Environ. Chem. Lett. 2019, 17, 157–193. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Yin, Z.; Cui, C.; Chen, H.; Yu, X.; Qian, W. The application of carbon nanotube/graphene-based nanomaterials in wastewater treatment. Small 2020, 16, 1902301. [Google Scholar] [CrossRef] [PubMed]
- Mishakov, I.V.; Bauman, Y.I.; Brzhezinskaya, M.; Netskina, O.V.; Shubin, Y.V.; Kibis, L.S.; Stoyanovskii, V.O.; Larionov, K.B.; Serkova, A.N.; Vedyagin, A.A. Water purification from chlorobenzenes using heteroatom-functionalized carbon nanofibers produced on self-organizing Ni-Pd catalyst. J. Environ. Chem. Eng. 2022, 10, 107873. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Perspectives on green fabrication and sustainable utilization of adsorption materials for wastewater treatment. Chem. Eng. Res. Des. 2022, 187, 541–548. [Google Scholar] [CrossRef]
- Onyancha, R.B.; Aigbe, U.O.; Ukhurebor, K.E.; Muchiri, P.W. Facile synthesis and applications of carbon nanotubes in heavy-metal remediation and biomedical fields: A comprehensive review. J. Mol. Struct. 2021, 1238, 130462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obayomi, K.S.; Lau, S.Y.; Danquah, M.K.; Zhang, J.; Chiong, T.; Takeo, M.; Jeevanandam, J. Novel Concepts for Graphene-Based Nanomaterials Synthesis for Phenol Removal from Palm Oil Mill Effluent (POME). Materials 2023, 16, 4379. https://doi.org/10.3390/ma16124379
Obayomi KS, Lau SY, Danquah MK, Zhang J, Chiong T, Takeo M, Jeevanandam J. Novel Concepts for Graphene-Based Nanomaterials Synthesis for Phenol Removal from Palm Oil Mill Effluent (POME). Materials. 2023; 16(12):4379. https://doi.org/10.3390/ma16124379
Chicago/Turabian StyleObayomi, Kehinde Shola, Sie Yon Lau, Michael K. Danquah, Jianhua Zhang, Tung Chiong, Masahiro Takeo, and Jaison Jeevanandam. 2023. "Novel Concepts for Graphene-Based Nanomaterials Synthesis for Phenol Removal from Palm Oil Mill Effluent (POME)" Materials 16, no. 12: 4379. https://doi.org/10.3390/ma16124379
APA StyleObayomi, K. S., Lau, S. Y., Danquah, M. K., Zhang, J., Chiong, T., Takeo, M., & Jeevanandam, J. (2023). Novel Concepts for Graphene-Based Nanomaterials Synthesis for Phenol Removal from Palm Oil Mill Effluent (POME). Materials, 16(12), 4379. https://doi.org/10.3390/ma16124379









