Engineering of Silica Mesoporous Materials for CO2 Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of KIT-6, SBA-15, and SBA-16
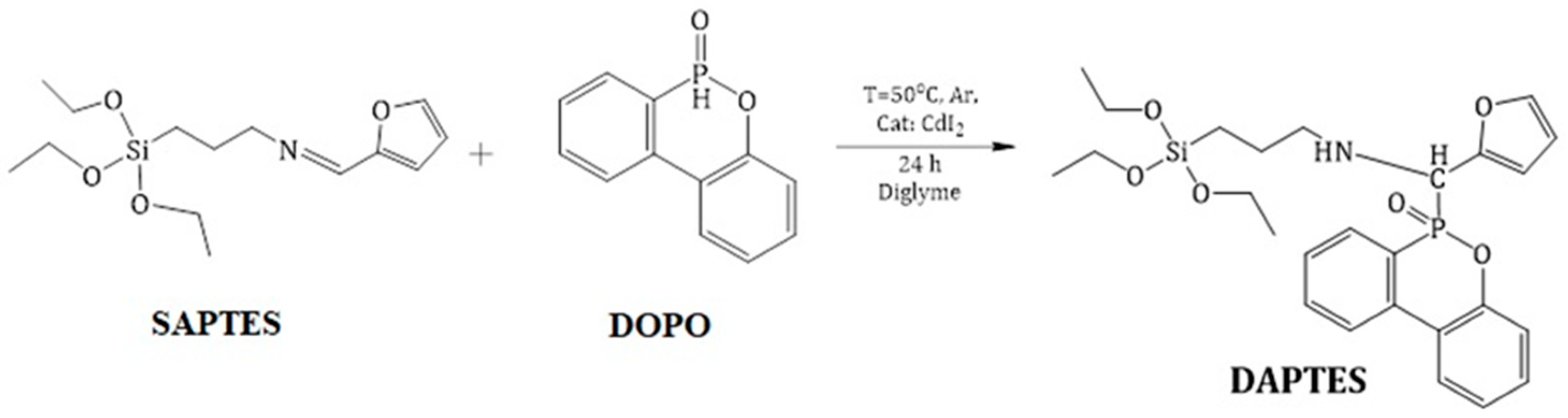
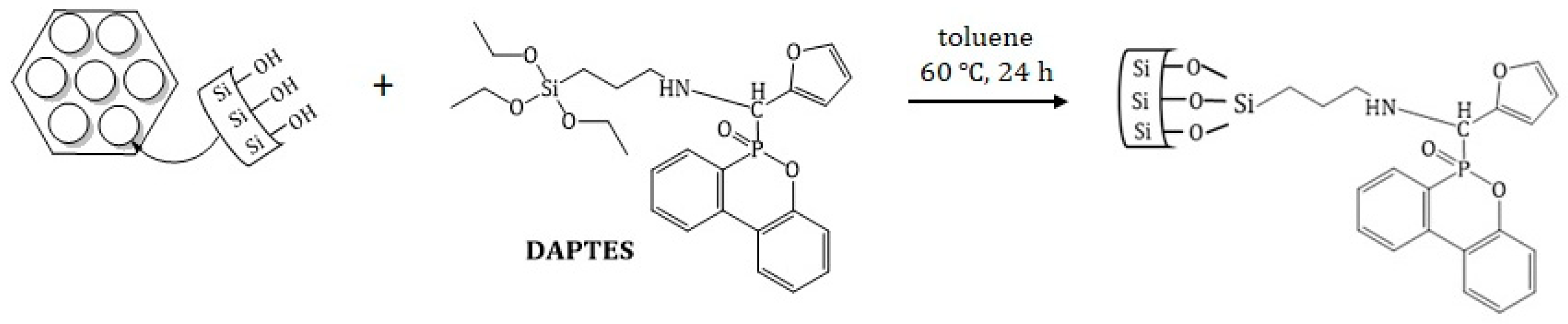
2.3. Preparation of DOPO Derivative of APTES
2.4. Modification of Mesoporous Silicas with DAPTES
2.5. Methods
2.5.1. Material Characterization
2.5.2. CO2 Adsorption
3. Results and Discussion
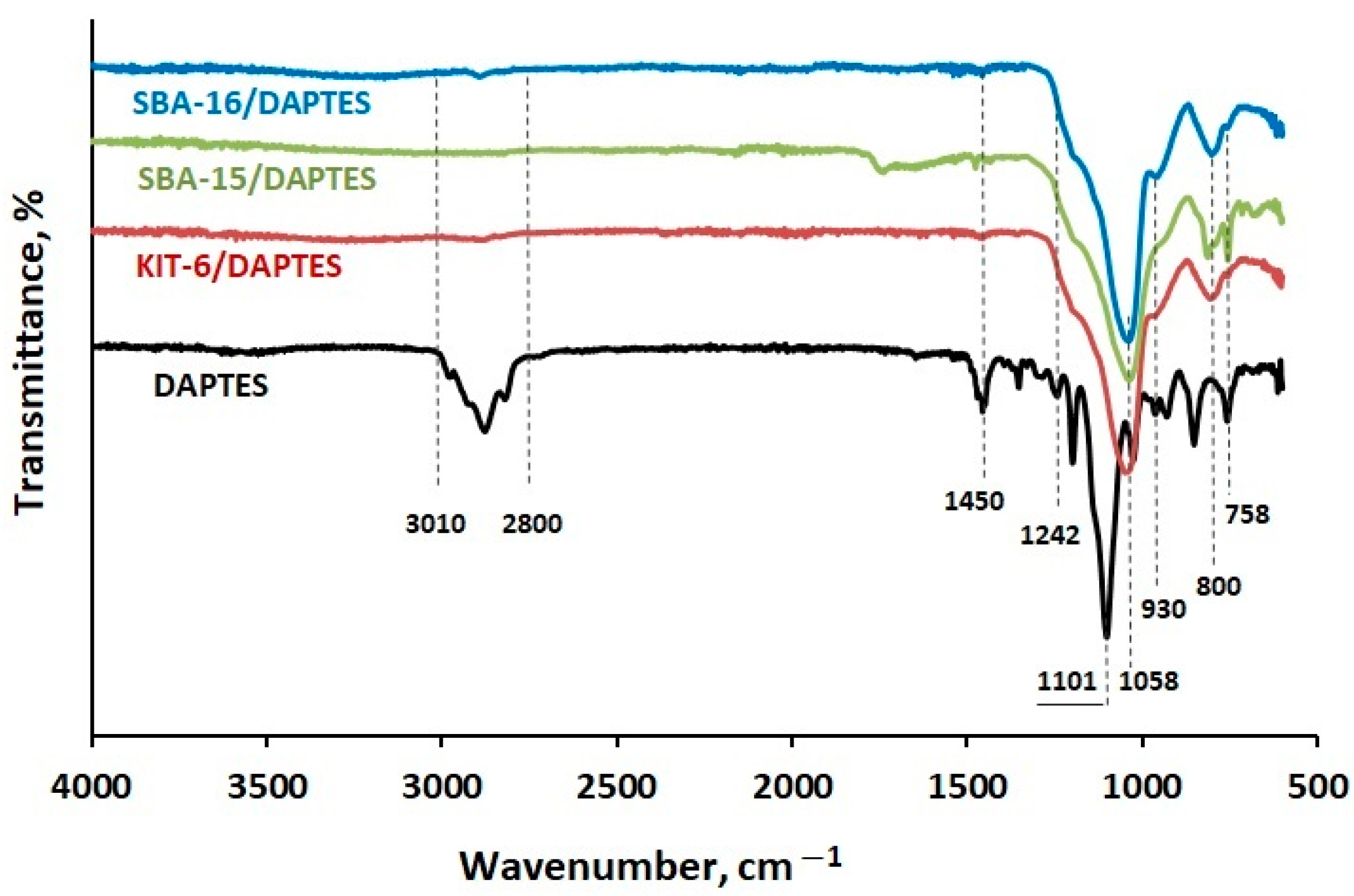
3.1. Synthesis and Characterization of DAPTES
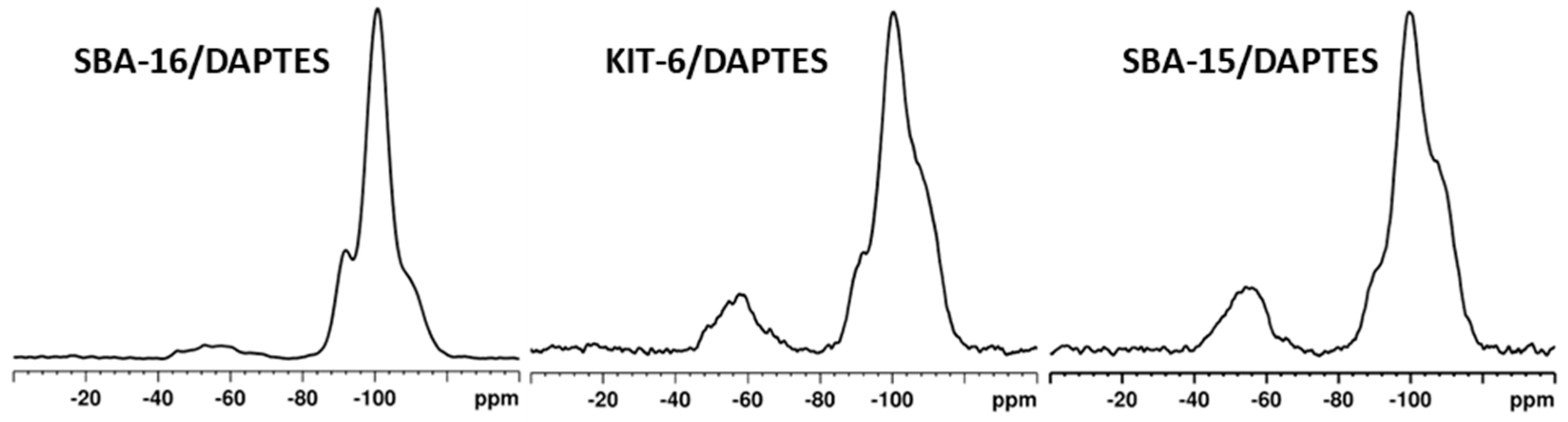
3.2. Modification of KIT-6, SBA-15, and SBA-16 Silica Materials
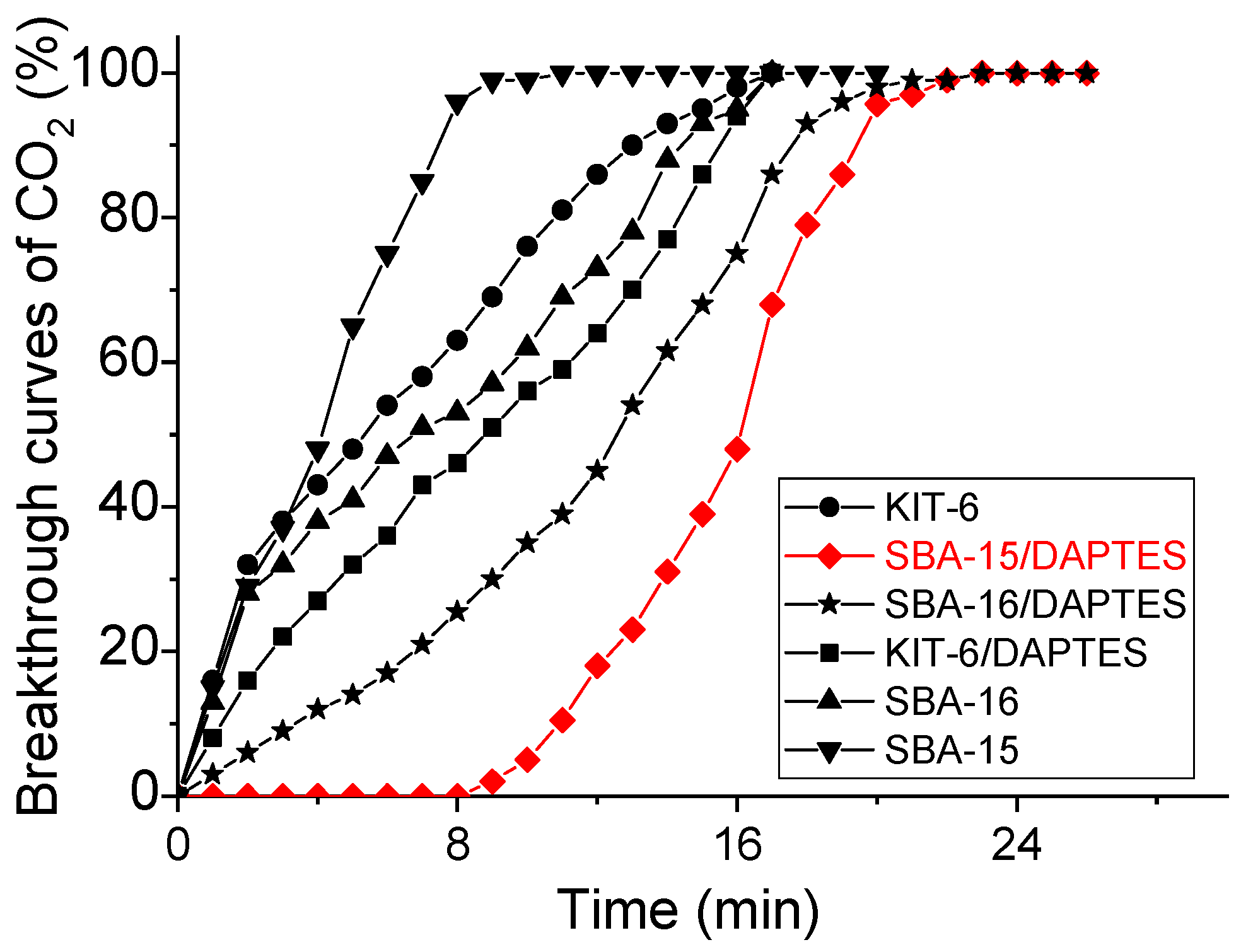
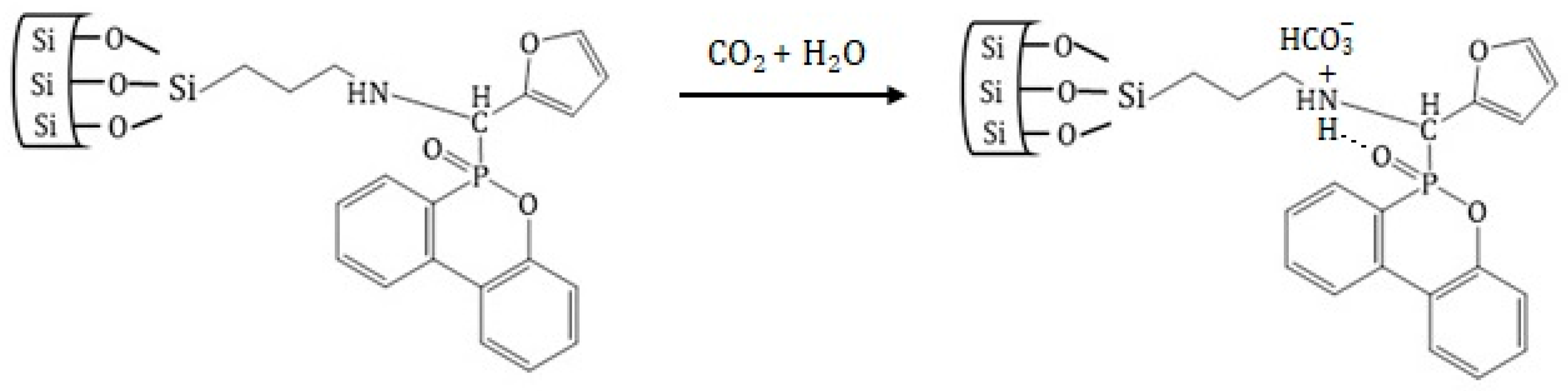
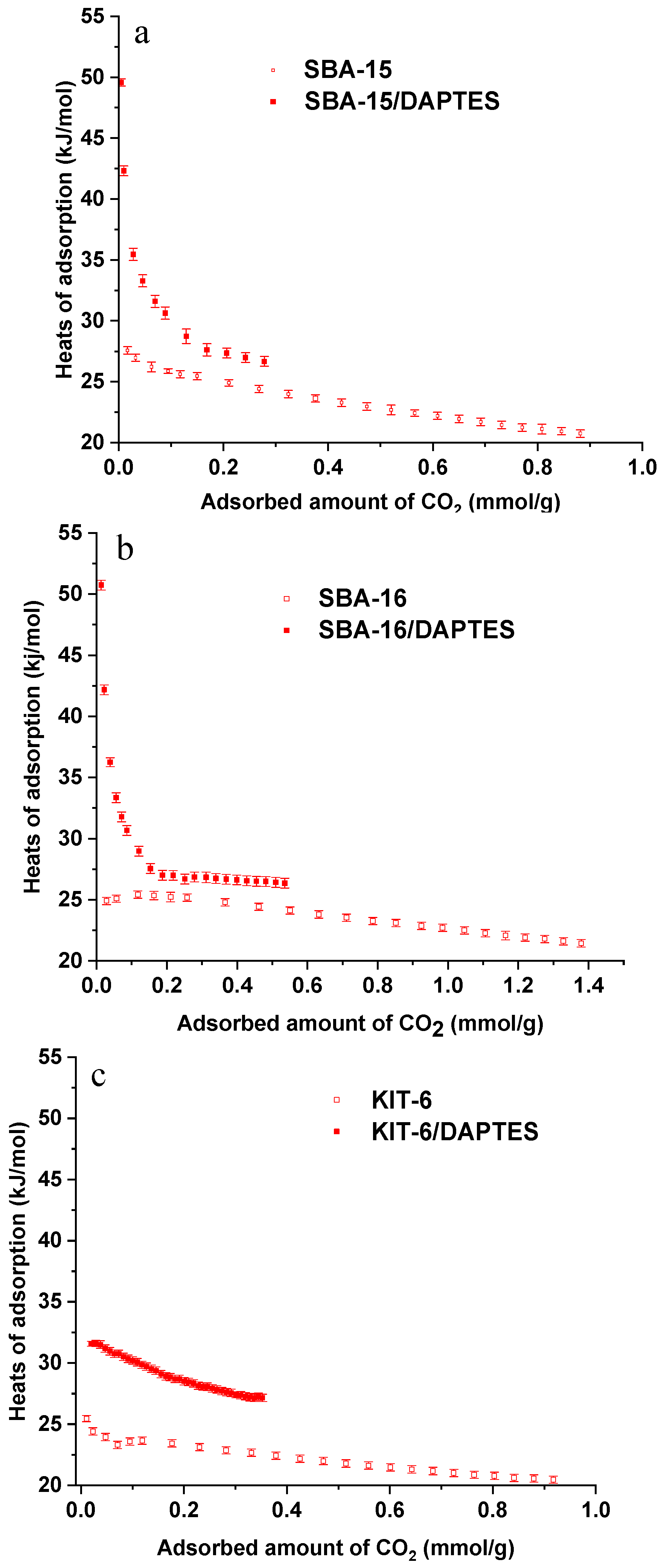
3.3. CO2 Adsorption Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balas, M.; Beaudoin, S.; Proust, A.; Launay, F.; Villanneau, R. Advantages of Covalent Immobilization of Metal-Salophen on Amino-Functionalized Mesoporous Silica in Terms of Recycling and Catalytic Activity for CO2 Cycloaddition onto Epoxides. Eur. J. Inorg. Chem. 2021, 2021, 1581–1591. [Google Scholar] [CrossRef]
- Le Treut, H.; Somerville, R.; Cubasch, U.; Ding, Y.; Mauritzen, C.; Mokssit, A.; Peterson, T.; Prather, M.; Widmann, M. Historical overview of climate change science. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Lee, S.-Y.; Park, S.-J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Liu, H.; Hou, X. AS-synthesized mesoporous silica MSU-1 modified with tetraethylenepentamine for CO2 adsorption. Microporous Mesoporous Mater. 2011, 142, 564–569. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Chester, A.W.; Derouane, E.G. Zeolite Chemistry and Catalysis; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Chang, F.-Y.; Chao, K.-J.; Cheng, H.-H.; Tan, C.-S. Adsorption of CO2 onto amine-grafted mesoporous silicas. Sep. Purif. Technol. 2009, 70, 87–95. [Google Scholar] [CrossRef]
- Kato, M.; Yoshikawa, S.; Nakagawa, K. Carbon dioxide absorption by lithium orthosilicate in a wide range of temperature and carbon dioxide concentrations. J. Mater. Sci. Lett. 2002, 21, 485–487. [Google Scholar] [CrossRef]
- Gupta, H.; Fan, L.-S. Carbonation−Calcination Cycle Using High Reactivity Calcium Oxide for Carbon Dioxide Separation from Flue Gas. Ind. Eng. Chem. Res. 2002, 41, 4035–4042. [Google Scholar] [CrossRef]
- Pevida, C.; Plaza, M.G.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. Surface modification of activated carbons for CO2 capture. Appl. Surf. Sci. 2008, 254, 7165–7172. [Google Scholar] [CrossRef]
- Pevida, C.; Drage, T.C.; Snape, C.E. Silica-templated melamine–formaldehyde resin derived adsorbents for CO2 capture. Carbon 2008, 46, 1464–1474. [Google Scholar] [CrossRef]
- Chen, J.; Loo, L.S.; Wang, K. High-Pressure CO2 Adsorption on a Polymer-Derived Carbon Molecular Sieve. J. Chem. Eng. Data 2008, 53, 2–4. [Google Scholar] [CrossRef]
- Konduru, N.; Lindner, P.; Assaf-Anid, N.M. Curbing the greenhouse effect by carbon dioxide adsorption with Zeolite 13X. AIChE J. 2007, 53, 3137–3143. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Adsorption Equilibrium of Methane, Carbon Dioxide, and Nitrogen on Zeolite 13X at High Pressures. J. Chem. Eng. Data 2004, 49, 1095–1101. [Google Scholar] [CrossRef]
- Das, D.D.; Harlick, P.J.E.; Sayari, A. Applications of pore-expanded MCM-41 silica: 4. Synthesis of a highly active base catalyst. Catal. Commun. 2007, 8, 829–833. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T.; Chinn, D.; Munson, C.L. Amine-Grafted MCM-48 and Silica Xerogel as Superior Sorbents for Acidic Gas Removal from Natural Gas. Ind. Eng. Chem. Res. 2003, 42, 2427–2433. [Google Scholar] [CrossRef]
- Kim, S.-N.; Son, W.-J.; Choi, J.-S.; Ahn, W.-S. CO2 adsorption using amine-functionalized mesoporous silica prepared via anionic surfactant-mediated synthesis. Microporous Mesoporous Mater. 2008, 115, 497–503. [Google Scholar] [CrossRef]
- Kim, M.-I.; Choi, S.-J.; Kim, D.-W.; Park, D.-W. Catalytic performance of zinc containing ionic liquids immobilized on silica for the synthesis of cyclic carbonates. J. Ind. Eng. Chem. 2014, 20, 3102–3107. [Google Scholar] [CrossRef]
- Alkhabbaz, M.A.; Khunsupat, R.; Jones, C.W. Guanidinylated poly(allylamine) supported on mesoporous silica for CO2 capture from flue gas. Fuel 2014, 121, 79–85. [Google Scholar] [CrossRef]
- Liu, F.; Chen, S.; Gao, Y. Synthesis of porous polymer based solid amine adsorbent: Effect of pore size and amine loading on CO2 adsorption. J. Colloid Interface Sci. 2017, 506, 236–244. [Google Scholar] [CrossRef]
- Khatri, R.A.; Chuang, S.S.C.; Soong, Y.; Gray, M. Thermal and Chemical Stability of Regenerable Solid Amine Sorbent for CO2 Capture. Energy Fuels 2006, 20, 1514–1520. [Google Scholar] [CrossRef]
- Mebane, D.S.; Kress, J.D.; Storlie, C.B.; Fauth, D.J.; Gray, M.L.; Li, K. Transport, Zwitterions, and the Role of Water for CO2 Adsorption in Mesoporous Silica-Supported Amine Sorbents. J. Phys. Chem. C 2013, 117, 26617–26627. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. Amino functionalized mesostructured SBA-15 silica for CO2 capture: Exploring the relation between the adsorption capacity and the distribution of amino groups by TEM. Microporous Mesoporous Mater. 2012, 158, 309–317. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Han, Y.; Hwang, G.; Kim, H.; Haznedaroglu, B.Z.; Lee, B. Amine-impregnated millimeter-sized spherical silica foams with hierarchical mesoporous–macroporous structure for CO2 capture. Chem. Eng. J. 2015, 259, 653–662. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. CO2 capture with pore-expanded MCM-41 silica modified with amino groups by double functionalization. Microporous Mesoporous Mater. 2015, 209, 165–171. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Chen, J.; Ye, Q.; Pan, H.; Shao, Z.; Shi, Y. Dynamic performance of CO2 adsorption with tetraethylenepentamine-loaded KIT-6. Microporous Mesoporous Mater. 2010, 134, 16–21. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Serna-Guerrero, R.; Sayari, A. Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2: Kinetics and breakthrough curves. Chem. Eng. J. 2010, 161, 182–190. [Google Scholar] [CrossRef]
- Wei, J.; Liao, L.; Xiao, Y.; Zhang, P.; Shi, Y. Capture of carbon dioxide by amine-impregnated as-synthesized MCM-41. J. Environ. Sci. 2010, 22, 1558–1563. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Yogo, K.; Yashima, T. Adsorption characteristics of carbon dioxide on organically functionalized SBA-15. Microporous Mesoporous Mater. 2005, 84, 357–365. [Google Scholar] [CrossRef]
- Hicks, J.C.; Drese, J.H.; Fauth, D.J.; Gray, M.L.; Qi, G.; Jones, C.W. Designing adsorbents for CO2 capture from flue gas-hyperbranched aminosilicas capable of capturing CO2 reversibly. J. Am. Chem. Soc. 2008, 130, 2902–2903. [Google Scholar] [CrossRef]
- Wei, J.; Shi, J.; Pan, H.; Su, Q.; Zhu, J.; Shi, Y. Thermal and hydrothermal stability of amino-functionalized SBA-16 and promotion of hydrophobicity by silylation. Microporous Mesoporous Mater. 2009, 117, 596–602. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W.-S. CO2 capture by amine-functionalized nanoporous materials: A review. Korean J. Chem. Eng. 2014, 31, 1919–1934. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arias, B.; Fermoso, J.; Arenillas, A.; Rubiera, F.; Pis, J.J. Application of thermogravimetric analysis to the evaluation of aminated solid sorbents for CO2 capture. J. Therm. Anal. Calorim. 2008, 92, 601–606. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Kuo, S.-C.; Zeng, W. Adsorption of CO2 on Amine-Functionalized Y-Type Zeolites. Energy Fuels 2010, 24, 1441–1448. [Google Scholar] [CrossRef]
- Ii, J.C.F.; Tanthana, J.; Chuang, S.S. Oxide-supported tetraethylenepentamine for CO2 capture. Environ. Prog. Sustain. Energy 2009, 28, 589–598. [Google Scholar] [CrossRef]
- He, Y.; Zhu, X.; Li, Y.; Peng, C.; Hu, J.; Liu, H. Efficient CO2 capture by triptycene-based microporous organic polymer with functionalized modification. Microporous Mesoporous Mater. 2015, 214, 181–187. [Google Scholar] [CrossRef]
- Tumurbaatar, O.; Lazarova, H.; Popova, M.; Mitova, V.; Shestakova, P.; Koseva, N. CO2 Adsorption on the N- and P-Modified Mesoporous Silicas. Nanomaterials 2022, 12, 1224. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, M.; Hammershøj, P.; Zhou, D.; Han, Y.; Laursen, B.W.; Yan, C.-G.; Han, B.-H. Microporous polycarbazole with high specific surface area for gas storage and separation. J. Am. Chem. Soc. 2012, 134, 6084–6087. [Google Scholar] [CrossRef] [PubMed]
- Iugaia, I.A.; Steksova, Y.P.; Vedyagin, A.A.; Mishakov, I.V.; Bauman, Y.I.; Belyy, V.A.; Danilovich, D.P.; Krivoshapkina, E.F.; Krivoshapkin, P.V. MgO/carbon nanofibers composite coatings on porous ceramic surface for CO2 capture. Surf. Coat. Technol. 2020, 400, 126208. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Z.; Fang, Z. Fabrication of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-decorated fullerene to improve the anti-oxidative and flame-retardant properties of polypropylene. Compos. Part B Eng. 2020, 183, 107672. [Google Scholar] [CrossRef]
- Kim, T.-W.; Kleitz, F.; Paul, B.; Ryoo, R. MCM-48-like large mesoporous silicas with tailored pore structure: Facile synthesis domain in a ternary triblock copolymer-butanol-water system. J. Am. Chem. Soc. 2005, 127, 7601–7610. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Heo, W.; Kleitz, F.; Ryoo, R. Facile synthesis of high quality mesoporous SBA-15 with enhanced control of the porous network connectivity and wall thickness. Chem. Commun. 2003, 12, 1340–1341. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhi, Z.; Zhao, Q.; Wu, C.; Zhao, P.; Jiang, H.; Jiang, T.; Wang, S. 3D cubic mesoporous silica microsphere as a carrier for poorly soluble drug carvedilol. Microporous Mesoporous Mater. 2012, 147, 94–101. [Google Scholar] [CrossRef]
- Troev, K. Chemistry and Application of H-Phosphonates; Elsevier Science: Amsterdam, The Netherlands, 2006; p. 291. [Google Scholar] [CrossRef]
- Atherton, F.R.; Openshaw, H.T.; Todd, A.R. 174. Studies on phosphorylation. Part II. The reaction of dialkyl phosphites with polyhalogen compounds in presence of bases. A new method for the phosphorylation of amines. J. Chem. Soc. 1945, 660–663. [Google Scholar] [CrossRef]
- Ma, Y.; Gong, X.; Liao, C.; Geng, X.; Wang, C.; Chu, F. Preparation and Characterization of DOPO-ITA Modified Ethyl Cellulose and Its Application in Phenolic Foams. Polymers 2018, 10, 1049. [Google Scholar] [CrossRef]
- Kwiatkowski, J.S.; Leszczyński, J.; Teca, I. Molecular structure and infrared spectra of furan, thiophene, selenophene and their 2,5-N and 3,4-N derivatives: Density functional theory and conventional post-Hartree-Fock MP2 studies. J. Mol. Struct. 1997, 436–437, 451–480. [Google Scholar] [CrossRef]
- Shan, G.; Jia, L.; Zhao, T.; Jin, C.; Liu, R.; Xiao, Y. A novel DDPSi-FR flame retardant treatment and its effects on the properties of wool fabrics. Fibers Polym. 2017, 18, 2196–2203. [Google Scholar] [CrossRef]
- Bhagiyalakshmi, M.; Park, S.D.; Cha, W.S.; Jang, H.T. Development of TREN dendrimers over mesoporous SBA-15 for CO2 adsorption. Appl. Surf. Sci. 2010, 256, 6660–6666. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wei, J.; Shi, J.; Pan, H.; Zhao, W.; Ye, Q.; Shi, Y. Adsorption of carbon dioxide on organically functionalized SBA-16. Microporous Mesoporous Mater. 2008, 116, 394–399. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hakiki, A.; Bouazizi, N.; Beltrao-Nunes, A.-P.; Launay, F.; Pailleret, A.; Pillier, F.; Bengueddach, A.; Hamacha, R.; Azzouz, A. Mesoporous silica supported amine and amine-copper complex for CO2 adsorption: Detailed reaction mechanism of hydrophilic character and CO2 retention. J. Mol. Struct. 2019, 1191, 175–182. [Google Scholar] [CrossRef]
- Bhatta, L.K.G.; Subramanyam, S.; Chengala, M.D.; Bhatta, U.M.; Pandit, N.; Venkatesh, K. Investigation of CO2 adsorption on carbon material derived from Mesua ferrea L. seed cake. J. Environ. Chem. Eng. 2015, 3, 2957–2965. [Google Scholar] [CrossRef]

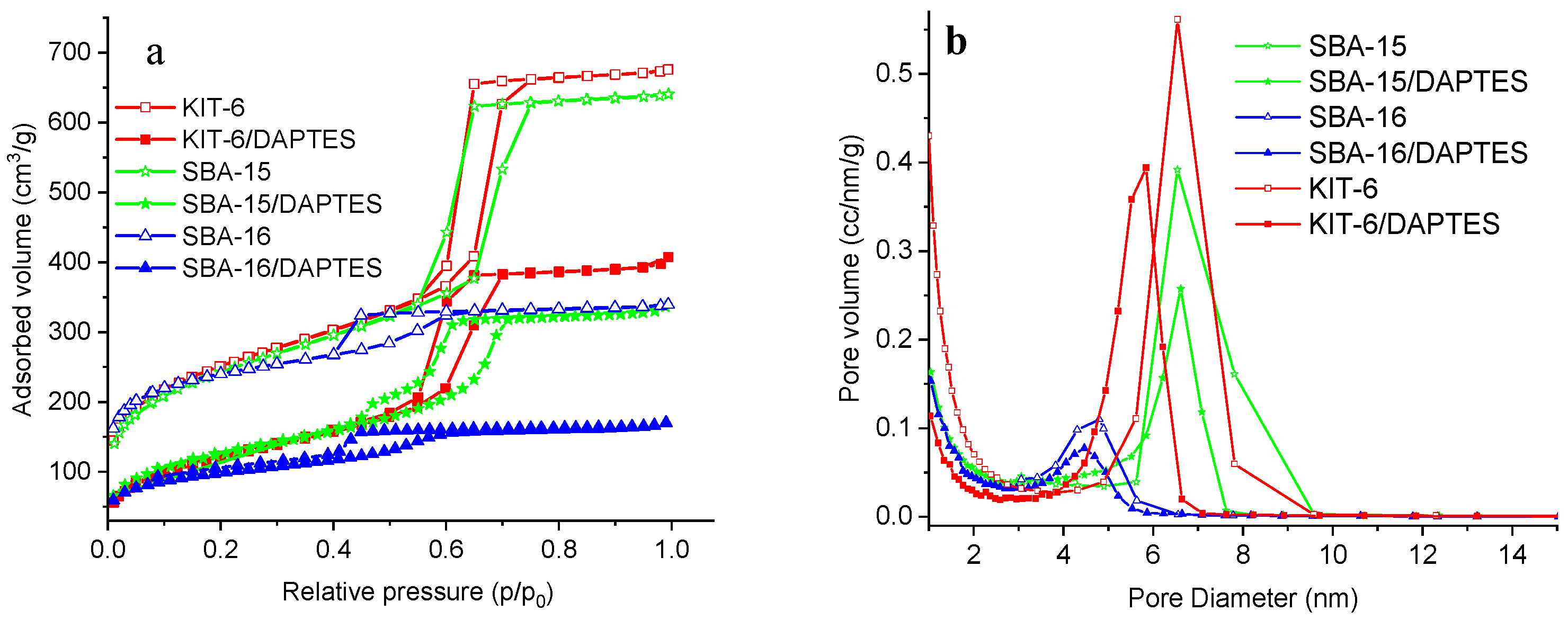
| Sample Name | SBET (m2/g) | Pore Volume 1 (cm3/g) | Pore Diameter 2 (nm) | DAPTES Content 3, wt% |
|---|---|---|---|---|
| KIT-6 | 906 | 1.11 | 6.5 | - |
| KIT-6/DAPTES | 441 | 0.71 | 5.9 | 6.3 |
| SBA-15 | 880 | 1.00 | 6.5 | - |
| SBA-15/DAPTES | 451 | 0.57 | 6.5 | 9.2 |
| SBA-16 | 890 | 0.53 | 4.8 | - |
| SBA-16/DAPTES | 357 | 0.27 | 4.5 | 9.4 |
| Samples | CO2 Adsorption from CO2/N2 mmol/g | Adsorption of CO2 from CO2/H2O/N2 1 mmol/g | Adsorption of CO2 from CO2/H2O/N2 2 mmol/g |
|---|---|---|---|
| SBA-15 | 1.5 | 1.4 | 1.3 |
| SBA-16 | 2.1 | 2.0 | 1.8 |
| KIT-6 | 2.0 | 1.9 | 1.9 |
| SBA-15/DAPTES | 3.9 | 4.2 | 4.1 |
| SBA-16/DAPTES | 3.0 | 3.1 | 3.0 |
| KIT-6/DAPTES | 2.4 | 2.5 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumurbaatar, O.; Popova, M.; Mitova, V.; Shestakova, P.; Koseva, N. Engineering of Silica Mesoporous Materials for CO2 Adsorption. Materials 2023, 16, 4179. https://doi.org/10.3390/ma16114179
Tumurbaatar O, Popova M, Mitova V, Shestakova P, Koseva N. Engineering of Silica Mesoporous Materials for CO2 Adsorption. Materials. 2023; 16(11):4179. https://doi.org/10.3390/ma16114179
Chicago/Turabian StyleTumurbaatar, Oyundari, Margarita Popova, Violeta Mitova, Pavletta Shestakova, and Neli Koseva. 2023. "Engineering of Silica Mesoporous Materials for CO2 Adsorption" Materials 16, no. 11: 4179. https://doi.org/10.3390/ma16114179
APA StyleTumurbaatar, O., Popova, M., Mitova, V., Shestakova, P., & Koseva, N. (2023). Engineering of Silica Mesoporous Materials for CO2 Adsorption. Materials, 16(11), 4179. https://doi.org/10.3390/ma16114179





