The Study of Copper Powder Sintering for Porous Wick Structures with High Capillary Force
Abstract
1. Introduction
2. Materials and Methods
3. Results
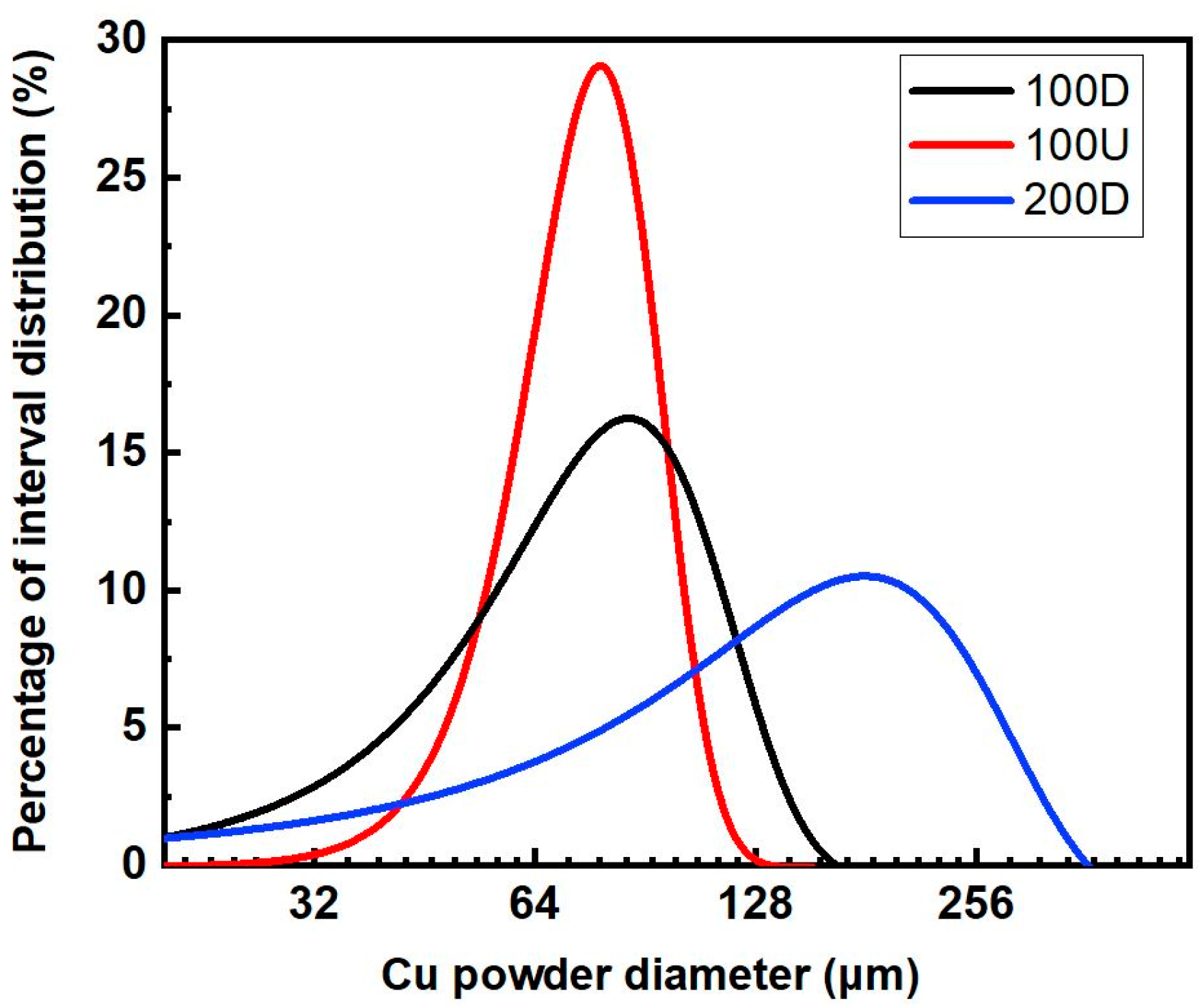
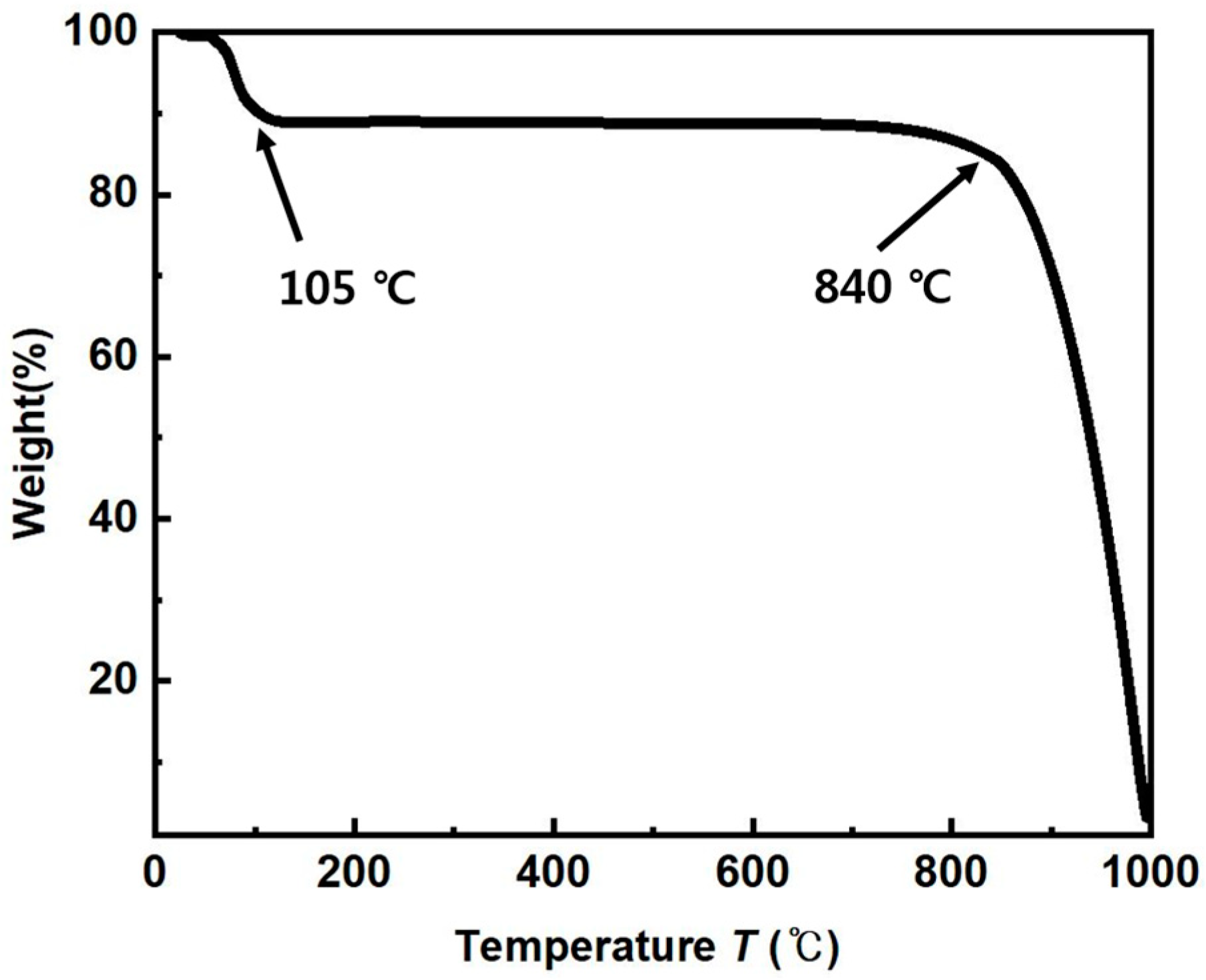
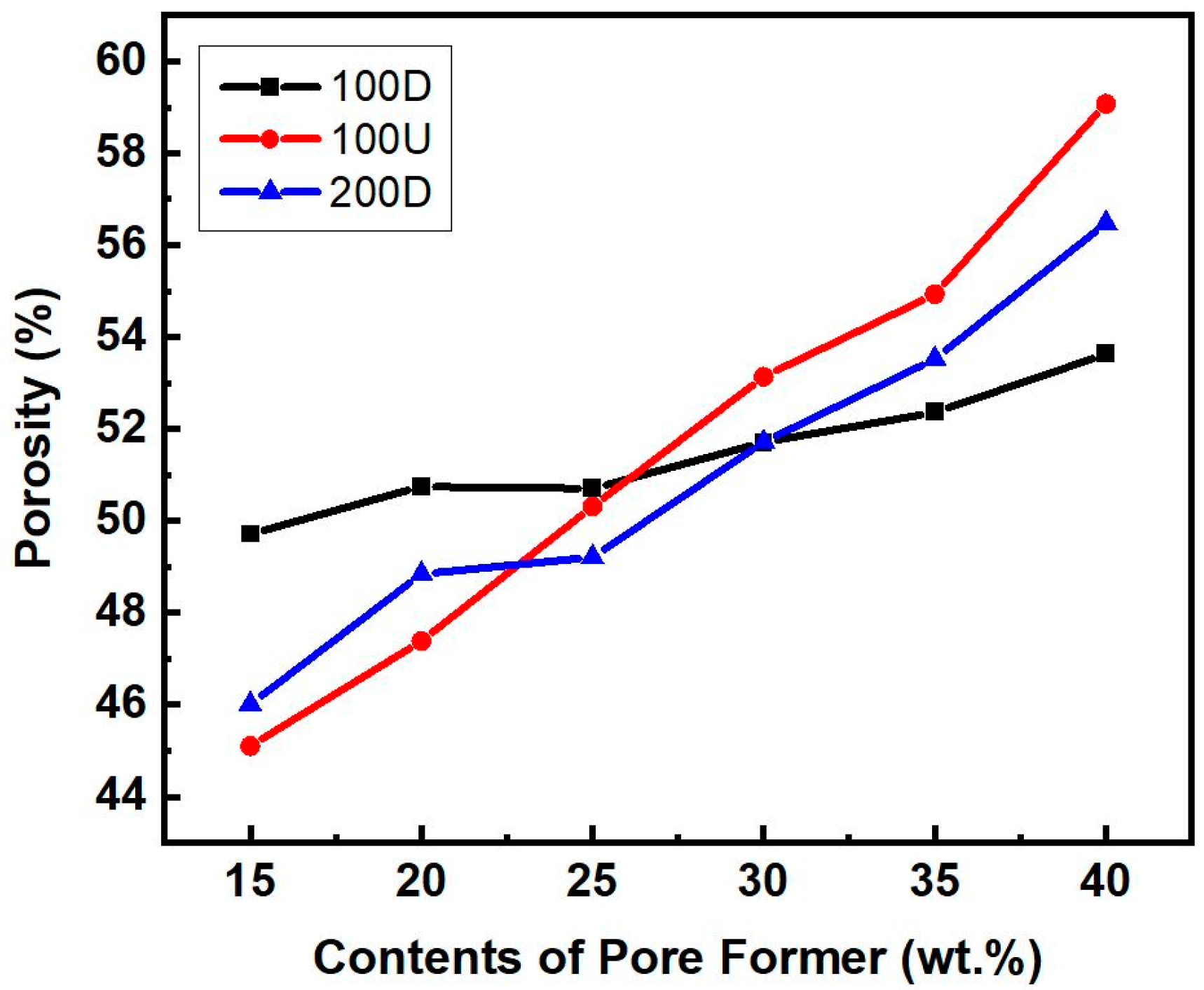
3.1. Powder Sintering and Porosity
3.2. Permeability
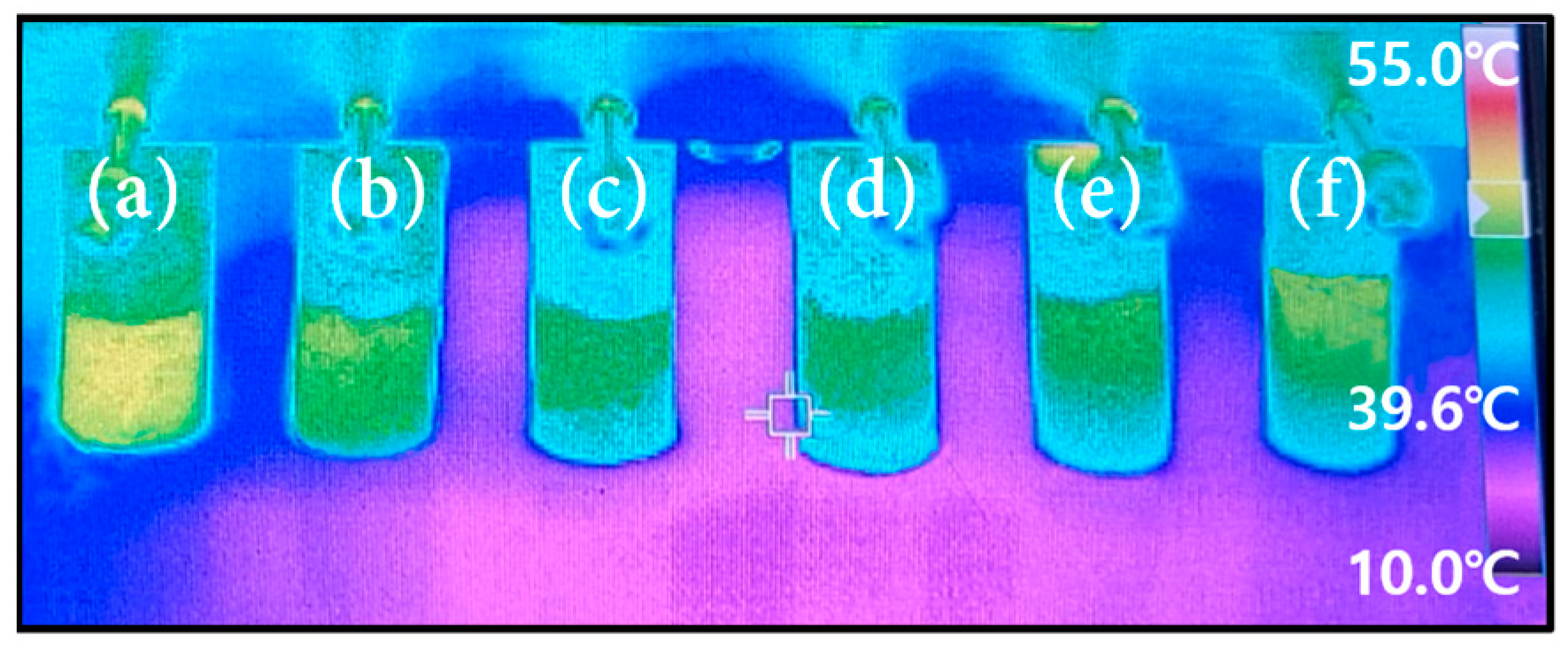
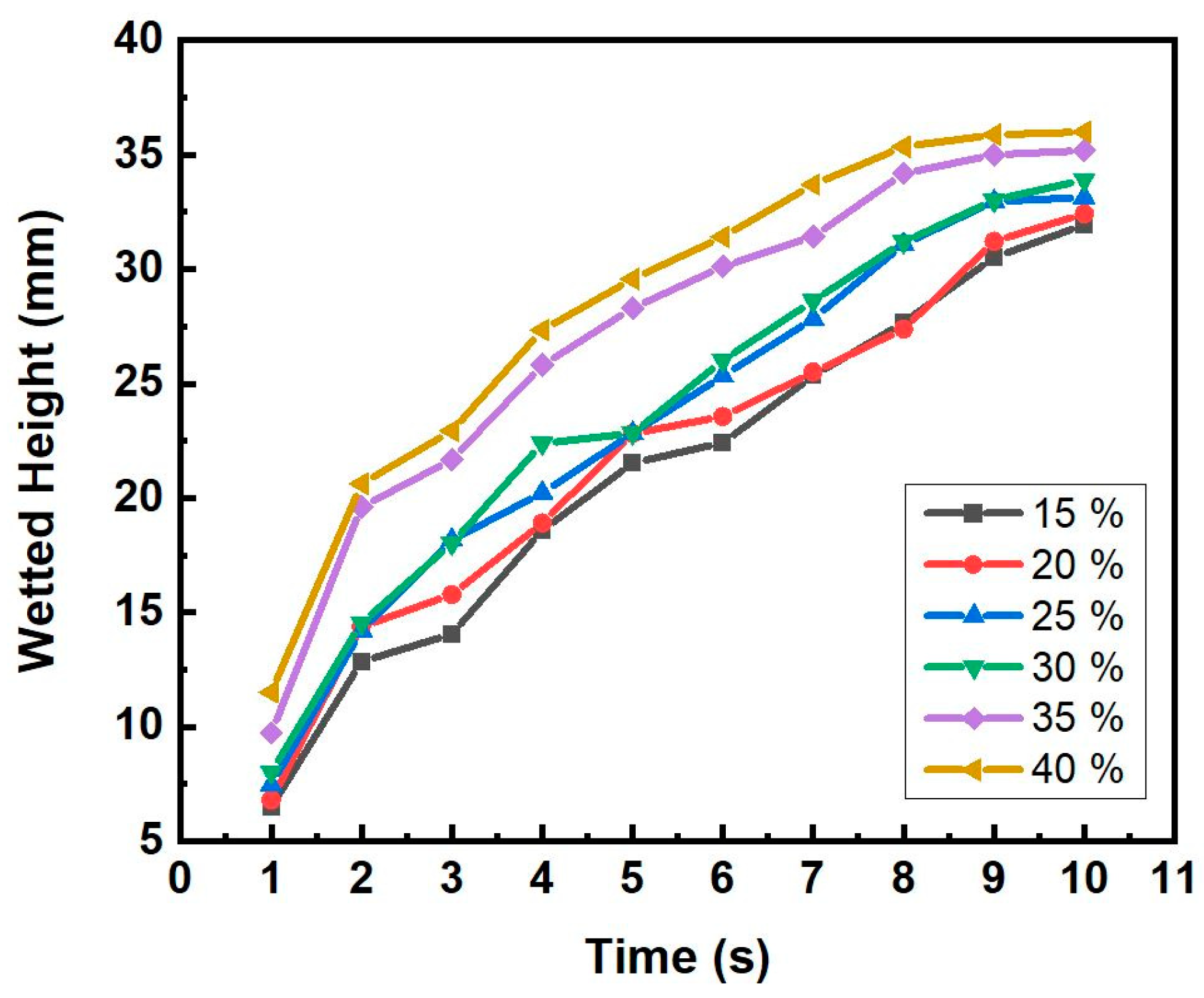
3.3. Capillary Force
4. Conclusions
- The necks between copper powder were formed at a sintering temperature higher than 900 °C and a hydrogen-reducing environment was also found to be necessary for the sintering of copper powder;
- The porosity, permeability, and capillary force increased with increasing pore-former contents ranging from 15 wt.% to 40 wt.%;
- The 100U sample with pore-former contents ranging from 30 wt.% to 40 wt.%, which has an average particle size of 100 μm and has higher porosity and permeability than the 100D and 200D samples, which should be due to the 100U sample’s better size uniformity;
- The 200D sample, which has an average particle size of 200 μm, has lower porosity and permeability than 100U. Yet, it has the highest capillary force, which should be due to the small effective pore radius;
- The porosity, permeability, and capillary force were significantly influenced by the powder’s particle size uniformity. When coarse and fine particles were mixed, the sintering density increased and the porosity and permeability were lowered. However, when it comes to capillary force, the mixture of variously sized particles inside reduced the effective pore radius, which resulted in an increase in the capillary force.
Author Contributions
Funding
Conflicts of Interest
References
- Lee, W.H.; Lee, K.W.; Park, K.H.; Lee, K.J.; Noh, S.Y. Study on a operating characteristics of loop heat pipe using a brass sintered metal wick-water. In Proceedings of the KSME Conference, Seoul, Republic of Korea, 28–30 April 2004; The Korean Society of Mechanical Engineers: Korea, 2004; pp. 1528–1533. [Google Scholar]
- Yun, H.G.; Moon, S.H.; Ko, S.C.; Hwang, G.; Choy, T.G. Manufacturing and operating performance of the heat pipe with sintered wick. Trans. Korean Soc. Mech. Eng. B 2002, 26, 1260–1266. [Google Scholar] [CrossRef]
- Zhao, Y.; Fung, T.; Zhang, L.; Zhang, F. Lost carbonate sintering process for manufacturing metal foams. Scr. Mater. 2005, 52, 295–298. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y. Fabrication of high melting-point porous metals by lost carbonate sintering process via decomposition route. Proc. Inst. Mech. Eng. 2008, 222, 267–271. [Google Scholar] [CrossRef]
- Noorsyakirah, A.; Mazlan, M.; Afian, O.M.; Aswad, M.A.; Jabir, S.M.; Nurazilah, M.; Afiq, N.M.; Bakar, M.; Nizam, A.M.; Zahid, O.A. Application of potassium carbonate as space holder for metal injection molding process of open pore copper foam. Procedia Chem. 2016, 19, 552–557. [Google Scholar] [CrossRef]
- Masoud Parvanian, A.; Panjepour, M. Development of Open Pore Copper Foams to Use as Bipolar Plates in Polymer Electrolyte Membrane Fuel Cell Stacks. Iran. J. Energy Environ. 2013, 4, 99–103. [Google Scholar] [CrossRef]
- Dunn, P.; Reay, D. Heat Pipes; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Kang, H.K.; Chung, H.S.; Kim, S.D.; Kim, C.J. A Study on the Operation Characteristics of the Sintered Metal. Soc. Air-Cond. Refrig. Eng. Korea 2002, 121–127. [Google Scholar]
- Jiang, L.-l.; Yong, T.; Wei, Z.; Jiang, L.-z.; Tan, X.; Yan, L.; Gao, J.-w. Design and fabrication of sintered wick for miniature cylindrical heat pipe. Trans. Nonferrous Met. Soc. China 2014, 24, 292–301. [Google Scholar] [CrossRef]
- Mahaidin, A.; Abdullah, N.; Mohammad, M.; Omar, M.; Suleiman, M.; Zainon, N.; Hadi, M.; Abd Jalil, M.; Omar, A.; Hijazi, M.M. Effect of sintering cycle on physical and mechanical properties of open pore cell copper foam. Procedia Chem. 2016, 19, 546–551. [Google Scholar] [CrossRef]
- Deng, D.; Tang, Y.; Huang, G.; Lu, L.; Yuan, D. Characterization of capillary performance of composite wicks for two-phase heat transfer devices. Int. J. Heat Mass Transf. 2013, 56, 283–293. [Google Scholar] [CrossRef]
- Holley, B.M.; Faghri, A. Permeability and effective pore radius measurements for heat pipe and fuel cell applications. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Anaheim, CA, USA, 13–19 November 2004; Volume 4711, pp. 353–371. [Google Scholar]
- Lu, X.; Zhao, Y.; Wang, G.; Zhu, X. Effects of structure characteristics and fluid on the effective thermal conductivity of sintered copper foam. Results Phys. 2020, 19, 103655. [Google Scholar] [CrossRef]
- Afazov, S.; Roberts, A.; Wright, L.; Jadhav, P.; Holloway, A.; Basoalto, H.; Milne, K.; Brierley, N. Metal powder bed fusion process chains: An overview of modelling techniques. Prog. Addit. Manuf. 2022, 7, 289–314. [Google Scholar] [CrossRef]
- Mostafaei, A.; De Vecchis, P.R.; Nettleship, I.; Chmielus, M. Design, Effect of powder size distribution on densification and microstructural evolution of binder-jet 3D-printed alloy 625. Materials 2019, 162, 375–383. [Google Scholar]
- Leong, K.; Liu, C.; Lu, G. Characterization of sintered copper wicks used in heat pipes. J. Porous Mater. 1997, 4, 303–308. [Google Scholar] [CrossRef]
- Byon, C.; Kim, S.J. Experimental and numerical study on the capillary performance of non-homogeneous micro-post arrays. Trans. Korean Soc. Mech. Eng. B 2013, 37, 1091–1097. [Google Scholar] [CrossRef]
- Meyers, M.A.; Olevsky, E.A. Performance, Sintering Theory and Practice. J. Mater. Eng. 1997, 6, 278. [Google Scholar]
- Tang, Y.; Deng, D.; Lu, L.; Pan, M.; Wang, Q. Experimental investigation on capillary force of composite wick structure by IR thermal imaging camera. Exp. Therm. Fluid Sci. 2010, 34, 190–196. [Google Scholar] [CrossRef]
- Zou, Y.; Malzbender, J. Development and optimization of porosity measurement techniques. J. Ceram. Int. 2016, 42, 2861–2870. [Google Scholar] [CrossRef]







| wt.% | 15 | 20 | 25 | 30 | 35 | 40 |
| vol.% | 39 | 48 | 55 | 61 | 67 | 71 |
| Cu Powder Sizes | 100D | 100U | 200D |
|---|---|---|---|
| Average effective pore radius (μm) | 171.54 | 241.31 | 155.73 |
| Porosity (40 wt.%) | 53.62 | 59.07 | 56.47 |
| Permeability ΔP (40 wt.%) | 0.56 | 0.52 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, I.-N.; Ahn, Y.-S. The Study of Copper Powder Sintering for Porous Wick Structures with High Capillary Force. Materials 2023, 16, 4231. https://doi.org/10.3390/ma16124231
Jang I-N, Ahn Y-S. The Study of Copper Powder Sintering for Porous Wick Structures with High Capillary Force. Materials. 2023; 16(12):4231. https://doi.org/10.3390/ma16124231
Chicago/Turabian StyleJang, Im-Nam, and Yong-Sik Ahn. 2023. "The Study of Copper Powder Sintering for Porous Wick Structures with High Capillary Force" Materials 16, no. 12: 4231. https://doi.org/10.3390/ma16124231
APA StyleJang, I.-N., & Ahn, Y.-S. (2023). The Study of Copper Powder Sintering for Porous Wick Structures with High Capillary Force. Materials, 16(12), 4231. https://doi.org/10.3390/ma16124231








