Preparation and Characterization of Thermal-Insulating Microporous Breathable Al/LLDPE/CaCO3 Composite Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
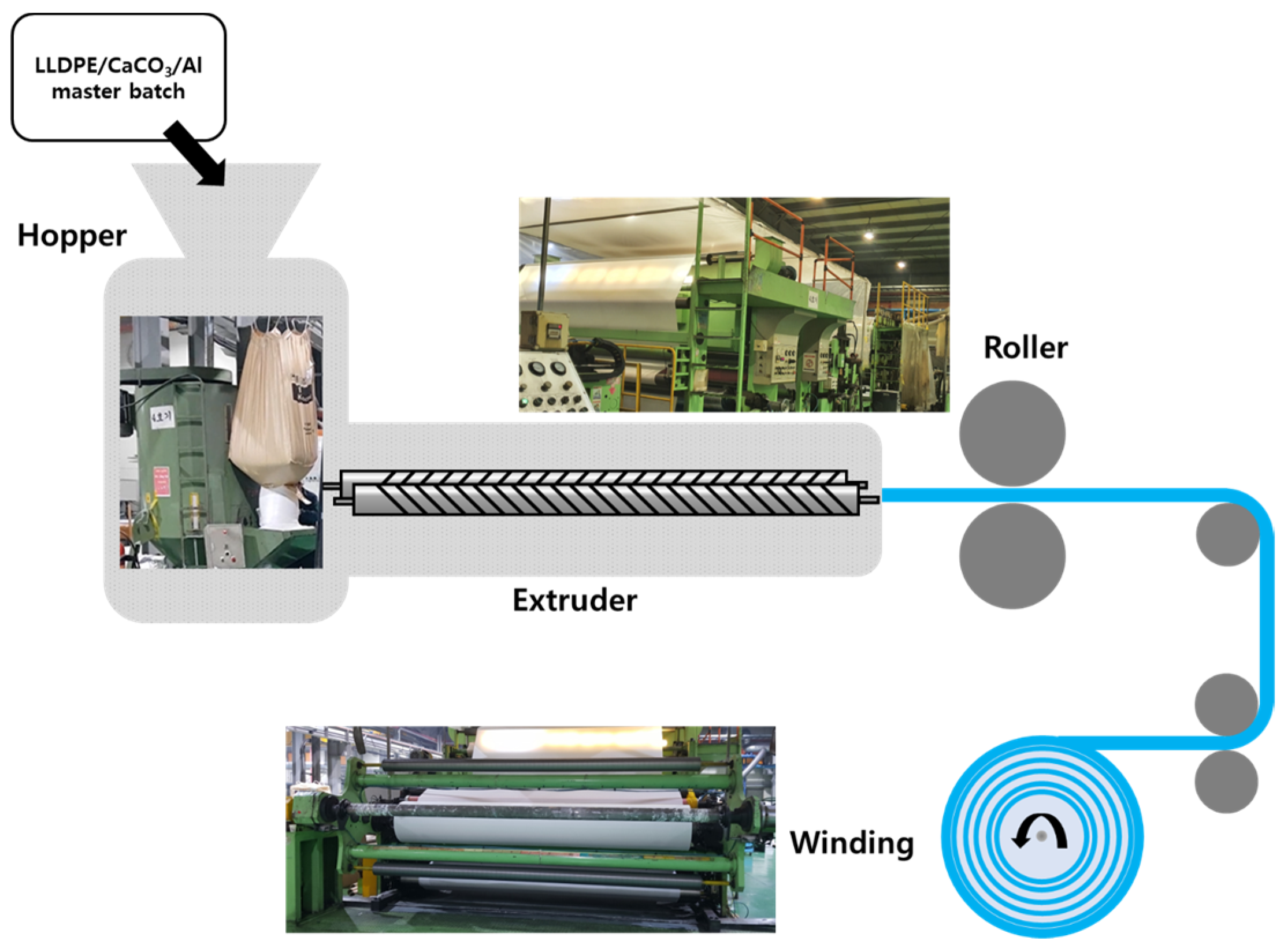
2.2. Preparation of the Al/LLDPE/CaCO3 Extruded Composite Films
2.3. Instrumental Analysis
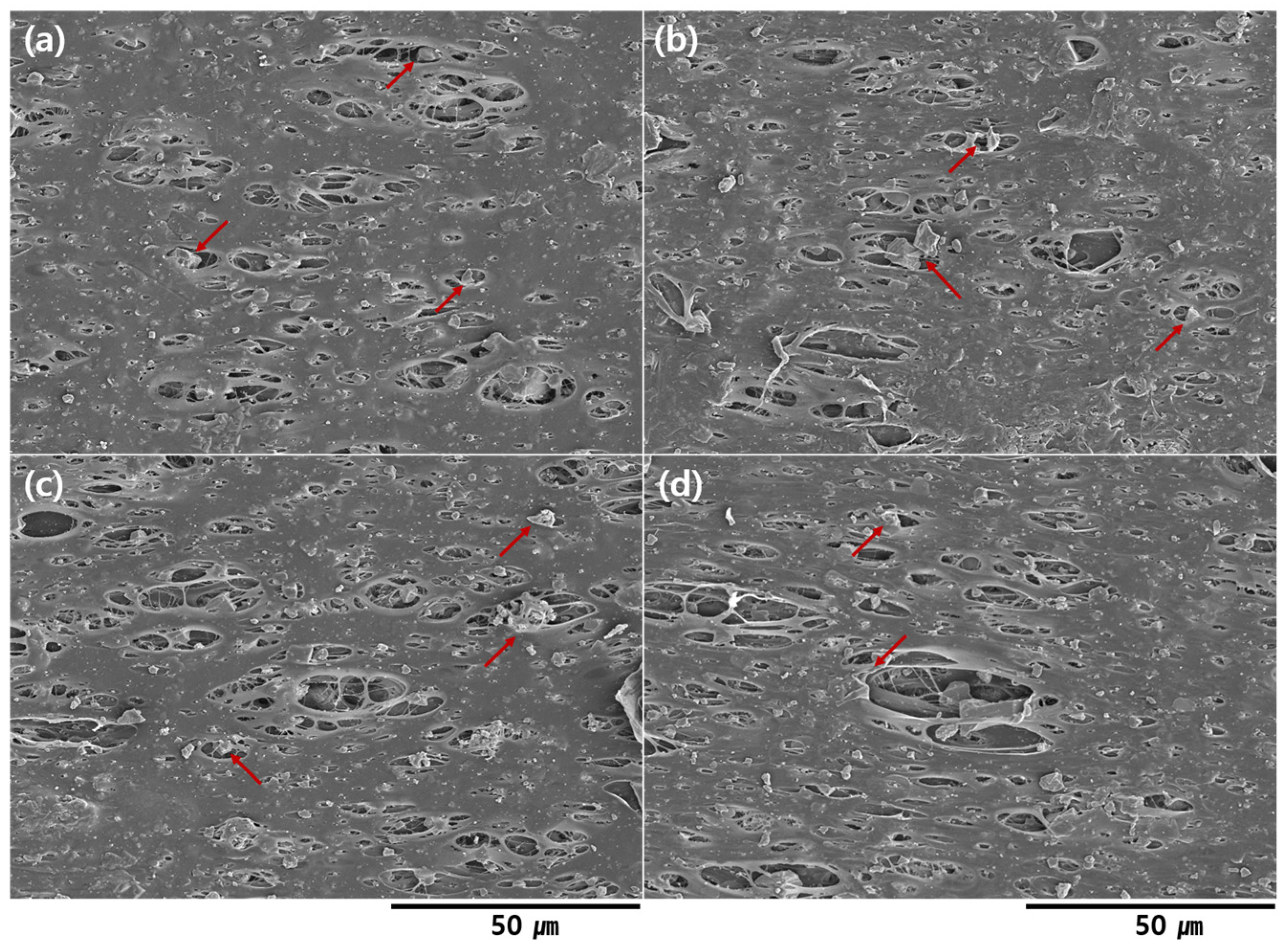
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kontou, E.; Niaounakis, M. Thermo-mechanical properties of LLDPE/SiO2 nanocomposites. Polymer 2006, 47, 1267–1280. [Google Scholar] [CrossRef]
- Eiras, D.; Pessan, L.A. Influence of calcium carbonate nanoparticles on the crystallization of polypropylene. Mater. Res. 2009, 12, 523–527. [Google Scholar] [CrossRef]
- Jhansi, K.; Babu, K.N.; Rao, L.B. The effect of moisture on the properties of glass fiber polymer matrix composites with MoS2 and CaCO3. Int. J. Eng. Adv. Technol. 2019, 8, 4800–4806. [Google Scholar] [CrossRef]
- Nagō, S.; Mizutani, Y. Preparation of microporous polypropylene sheets containing CaCO3 filler: Effect of draft ratio. J. Appl. Polym. Sci. 1996, 61, 31–35. [Google Scholar] [CrossRef]
- Nagō, S.; Mizutani, Y. Microporous polypropylene sheets containing CaCO3 filler: Effects of stretching ratio and removing CaCO3 filler. J. Appl. Polym. Sci. 1998, 68, 1543–1553. [Google Scholar] [CrossRef]
- Nagō, S.; Nakamura, S.; Mizutani, Y. Structure of microporous polypropylene sheets containing CaCO3 filler. J. Appl. Polym. Sci. 1992, 45, 1527–1535. [Google Scholar] [CrossRef]
- Nakamura, S.; Kaneko, S.; Mizutani, Y. Microporous polypropylene sheets containing CaCO3 filler. J. Appl. Polym. Sci. 1993, 49, 143–150. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.M.; Jung, J.H.; Kim, T.Y.; Han, M.D.; Seo, J.M.; Seo, M.J.; Yang, S.B.; Yeum, J.H. Characterization of LLDPE/CaCO 3 Composite Drawn Film. Text. Colorat. Finish. 2022, 34, 68–75. [Google Scholar]
- Mihindukulasuriya, S.; Lim, L.T. Effects of liquid contaminants on heat seal strength of low-density polyethylene film. Packag. Technol. Sci. 2012, 25, 271–284. [Google Scholar] [CrossRef]
- Planes, E.; Marouani, S.; Flandin, L. Optimizing the heat sealing parameters of multilayers polymeric films. J. Mater. Sci. 2011, 46, 5948–5958. [Google Scholar] [CrossRef]
- Su, J.F.; Wang, X.Y.; Huang, Z.; Yuan, X.Y.; Li, M.; Xia, W.L.; Feng, C.L. Heat-sealing properties of soy protein isolate/poly(vinyl alcohol) blend films: Effect of the heat-sealing temperature. J. Appl. Polym. Sci. 2010, 115, 1901–1911. [Google Scholar] [CrossRef]
- Marouani, S. Investigation of the resistance welding of multilayers aluminum-coated polymer complexes used as envelopes of vacuum insulation panels. Mater. Des. 2012, 36, 546–556. [Google Scholar] [CrossRef]
- Guo, Z.; Fan, Y. Heat seal properties of polymer–aluminum–polymer composite films for application in pouch lithium-ion battery. RSC Adv. 2016, 6, 8971–8979. [Google Scholar] [CrossRef]
- Kundu, P.P.; Biswas, J.; Kim, H.; Choe, S. Influence of film preparation procedures on the crystallinity, morphology and mechanical properties of LLDPE films. Eur. Polym. J. 2003, 39, 1585–1593. [Google Scholar] [CrossRef]
- ASTM D638; The Definitive Guide to Plastic Tensile Testing. ASTM: Philadelphia, PA, USA, 2010.
- ASTM E96/E96M-12; Standard Test Methods for Water Vapor Transmission of Materials. ASTM: Philadelphia, PA, USA, 2022.
- Abd Al-Ghani, M.M.; Azzam, R.A.; Madkour, T.M. Design and development of enhanced antimicrobial breathable biodegradable polymeric films for food packaging applications. Polymers 2021, 13, 3527. [Google Scholar] [CrossRef]
- Chanunpanich, N.; Ulman, A.; Malagon, A.; Strzhemechny, Y.; Schwarz, S.; Janke, A.; Kratzmueller, T.; Braun, H. Surface modification of polyethylene films via bromination: Reactions of brominated polyethylene with aromatic thiolate compounds. Langmuir 2000, 16, 3557–3560. [Google Scholar] [CrossRef]
- Stark, N.M.; Matuana, L.M. Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy. Polym. Degradat. Stab. 2004, 86, 1–9. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Cai, G.-B.; Chen, S.-F.; Liu, L.; Jiang, J.; Yao, H.-B.; Xu, A.-W.; Yu, S.-H. 1, 3-Diamino-2-hydroxypropane-N, N, N′, N′-tetraacetic acid stabilized amorphous calcium carbonate: Nucleation, transformation and crystal growth. CrystEngComm 2010, 12, 234–241. [Google Scholar] [CrossRef]
- Kim, H.; Jung, D.-H.; Jung, I.; Cifuentes, J.; Rhee, K.; Hui, D. Enhancement of mechanical properties of aluminium/epoxy composites with silane functionalization of aluminium powder. Compos. Part B Eng. 2012, 43, 1743–1748. [Google Scholar] [CrossRef]
- De Moura, A.P.; da Silva, E.H.; dos Santos, V.S.; Galera, M.F.; Sales, F.C.; Elizario, S.; de Moura, M.R.; Rigo, V.A.; da Costa, R.R. Structural and mechanical characterization of polyurethane-CaCO3 composites synthesized at high calcium carbonate loading: An experimental and theoretical study. J. Compos. Mater. 2021, 55, 2857–2866. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; Zhang, B.; Chen, H.; Zhang, J.; Wang, T.; Zhang, K.; Zhang, J.; Huang, P. Effect of Mn content on microstructure and properties of 6000 series aluminum alloy. Appl. Phys. A 2019, 125, 1–9. [Google Scholar] [CrossRef]
- Zain, A.H.M.; bin Ab Wahab, M.K.; Ismail, H. Effect of calcium carbonate incorporation on the properties of low linear density polyethylene/thermoplastic starch blends. J. Eng. Sci. 2019, 15, 97–108. [Google Scholar]
- Mysiukiewicz, O.; Kosmela, P.; Barczewski, M.; Hejna, A. Mechanical, thermal and rheological properties of polyethylene-based composites filled with micrometric aluminum powder. Materials 2020, 13, 1242. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, H.; Lei, F.; Li, D.; Leng, J.; Chen, L.; Huang, Y.; Sun, D. High performance linear low density polyethylene nanocomposites reinforced by two-dimensional layered nanomaterials. Polymer 2019, 172, 142–151. [Google Scholar] [CrossRef]
- Zeng, T.; Jiang, H.; Hao, F. Study on the effect of aluminium foil on packaging thermal insulation performance in cold chain logistics. Packag. Technol. Sci. 2022, 35, 395–403. [Google Scholar] [CrossRef]
- Özen, İ.; Şimşek, S. Effect of stretching temperature on breathability and waterproofness properties of polyethylene films containing different calcium carbonates. J. Plast. Film Sheet. 2016, 32, 380–401. [Google Scholar] [CrossRef]
- Duan, Z.; Thomas, N. Water vapour permeability of poly (lactic acid): Crystallinity and the tortuous path model. J. Appl. Phys. 2014, 115, 064903. [Google Scholar] [CrossRef]
- Anjum, A.S.; Son, E.J.; Yu, J.H.; Ryu, I.; Park, M.S.; Hwang, C.S.; Ahn, J.W.; Choi, J.Y.; Jeong, S.H. Fabrication of durable hydrophobic porous polyurethane membrane via water droplet induced phase separation for protective textiles. Text. Res. J. 2020, 90, 1245–1261. [Google Scholar] [CrossRef]
- Yang, S.B.; Lee, J.; Yeasmin, S.; Park, J.M.; Han, M.D.; Kwon, D.-J.; Yeum, J.H. Blown Composite Films of Low-Density/Linear-Low-Density Polyethylene and Silica Aerogel for Transparent Heat Retention Films and Influence of Silica Aerogel on Biaxial Properties. Materials 2022, 15, 5314. [Google Scholar] [CrossRef]
- Shi, S.-C.; Zeng, X.-X. Effect of the strengthening mechanism of SiO2 reinforced poly (methyl methacrylate) on ductility performance. J. Polym. Res. 2022, 29, 408. [Google Scholar] [CrossRef]
- Liu, X.; Zou, L.; Chang, B.; Shi, H.; Yang, Q.; Cheng, K.; Li, T.; Schneider, K.; Heinrich, G.; Liu, C. Strain dependent crystallization of isotactic polypropylene during solid-state stretching. Polym. Test. 2021, 104, 107404. [Google Scholar] [CrossRef]
- Botta, L.; Teresi, R.; Titone, V.; Salvaggio, G.; La Mantia, F.P.; Lopresti, F. Use of biochar as filler for biocomposite blown films: Structure-processing-properties relationships. Polymers 2021, 13, 3953. [Google Scholar] [CrossRef] [PubMed]
- Alghdeir, M.; Mayya, K.; Dib, M. Nanosilica Composite for Greenhouse Application. In Composite and Nanocomposite Materials—From Knowledge to Industrial Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Chawla, N.; Deng, X. Microstructure and mechanical behavior of porous sintered steels. Mater. Sci. Eng. A 2005, 390, 98–112. [Google Scholar] [CrossRef]




| Name | Grade | Melt Flow Index | Density | Melting Point | Haze (%) | Gloss |
|---|---|---|---|---|---|---|
| LLDPE | CEFOR 1221P | 2.0 g/10 min | 0.918 g/cm3 | 116 °C | 0.56 | 151 |
| Contents | Subsection |
|---|---|
| Extrusion temperature (°C) | 120 |
| Extrusion speed (rpm) | 200 |
| Stretching temperature (°C) | 70 |
| Stretching ratio | 2.5 |
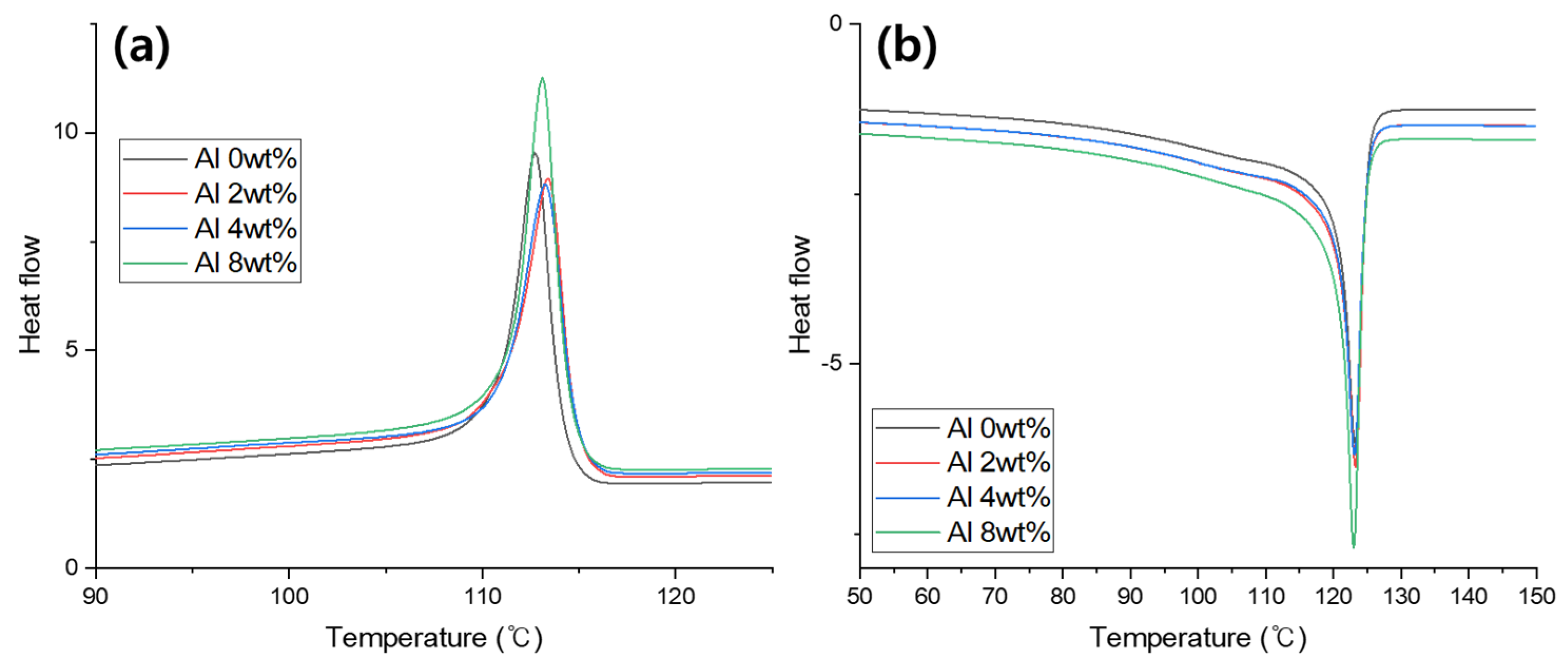
| Sample Type | Tonset (°C) | Tendset (°C) | Tpeak (°C) | ∆T (°C) | Tm (°C) | ∆Hc (J/g) |
|---|---|---|---|---|---|---|
| Al 0 wt.% | 116.00 | 76.24 | 112.71 | 3.69 | 123.24 | 57.20 |
| Al 2 wt.% | 116.92 | 72.93 | 113.45 | 3.47 | 123.28 | 58.82 |
| Al 4 wt.% | 116.31 | 72.42 | 113.19 | 3.12 | 123.08 | 58.31 |
| Al 8 wt.% | 116.31 | 75.57 | 113.10 | 3.21 | 123.03 | 63.54 |
| Sample Type | Thermal Insulation (%) |
|---|---|
| Al 0 wt.% | 22.9 ± 0.2 |
| Al 2 wt.% | 34.2 ± 0.3 |
| Al 4 wt.% | 34.3 ± 0.3 |
| Al 8 wt.% | 34.6 ± 0.3 |
| Sample Type | WVTR (g/m2/24 h) | Water Pressure (mm H2O) |
|---|---|---|
| Al 0 wt.% | 6653.93 ± 45 | 983.5 ± 2.5 |
| Al 2 wt.% | 6564.76 ± 42 | 1030 ± 3.0 |
| Al 4 wt.% | 6603.52 ± 39 | 1076 ± 20.5 |
| Al 8 wt.% | 6713.38 ± 43 | 807 ± 53.0 |
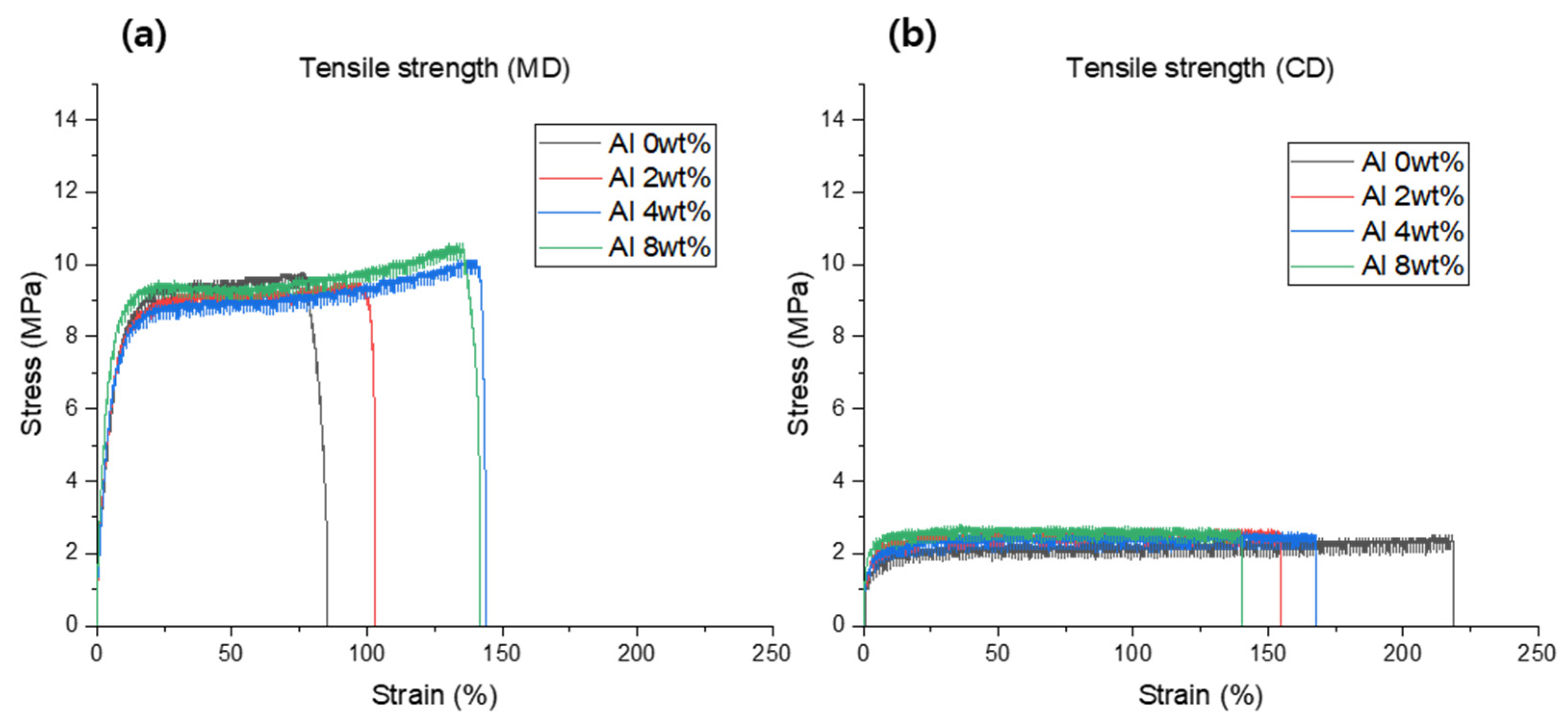
| Sample Type | Thickness (mm) | Stress-At-Break (MPa) | Strain-At-Break (%) | ||
|---|---|---|---|---|---|
| MD | CD | MD | CD | ||
| Al 0 wt.% | 0.05 | 9.75 | 2.41 | 76.15 | 217.94 |
| Al 2 wt.% | 0.05 | 9.36 | 2.62 | 97.28 | 154.18 |
| Al 4 wt.% | 0.05 | 10.02 | 2.52 | 138.03 | 167.03 |
| Al 8 wt.% | 0.05 | 10.41 | 2.67 | 133.81 | 139.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yeasmin, S.; Jung, J.H.; Kim, T.Y.; Kwon, T.Y.; Kwon, D.Y.; Yeum, J.H. Preparation and Characterization of Thermal-Insulating Microporous Breathable Al/LLDPE/CaCO3 Composite Films. Materials 2023, 16, 4230. https://doi.org/10.3390/ma16124230
Lee J, Yeasmin S, Jung JH, Kim TY, Kwon TY, Kwon DY, Yeum JH. Preparation and Characterization of Thermal-Insulating Microporous Breathable Al/LLDPE/CaCO3 Composite Films. Materials. 2023; 16(12):4230. https://doi.org/10.3390/ma16124230
Chicago/Turabian StyleLee, Jungeon, Sabina Yeasmin, Jae Hoon Jung, Tae Young Kim, Tae Yeong Kwon, Da Yeong Kwon, and Jeong Hyun Yeum. 2023. "Preparation and Characterization of Thermal-Insulating Microporous Breathable Al/LLDPE/CaCO3 Composite Films" Materials 16, no. 12: 4230. https://doi.org/10.3390/ma16124230
APA StyleLee, J., Yeasmin, S., Jung, J. H., Kim, T. Y., Kwon, T. Y., Kwon, D. Y., & Yeum, J. H. (2023). Preparation and Characterization of Thermal-Insulating Microporous Breathable Al/LLDPE/CaCO3 Composite Films. Materials, 16(12), 4230. https://doi.org/10.3390/ma16124230





