Copper Phosphide Nanowires as High-Performance Catalysts for Urea-Assisted Hydrogen Evolution in Alkaline Medium
Abstract
1. Introduction
2. Experimental Section
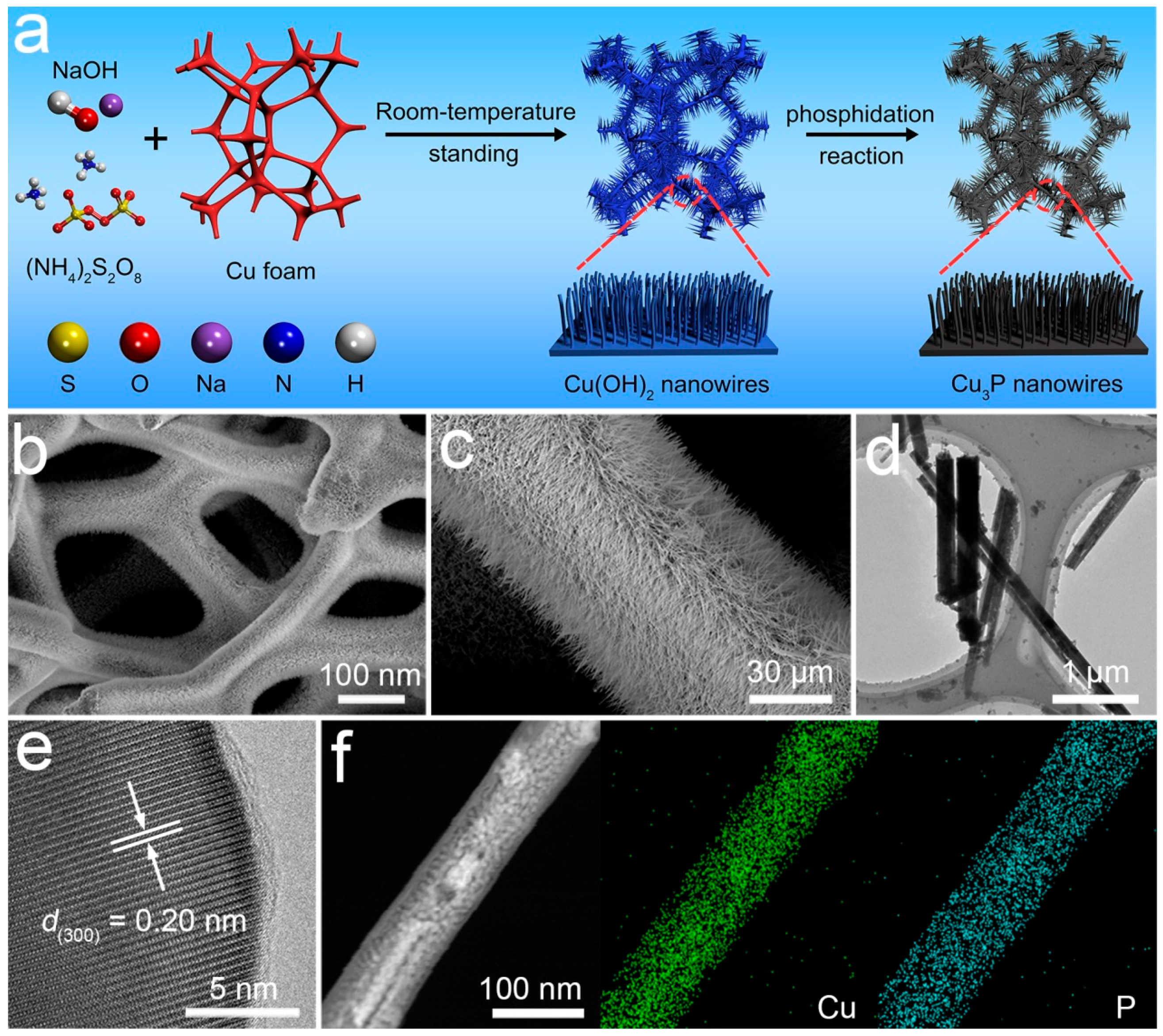
2.1. Materials and Synthesis
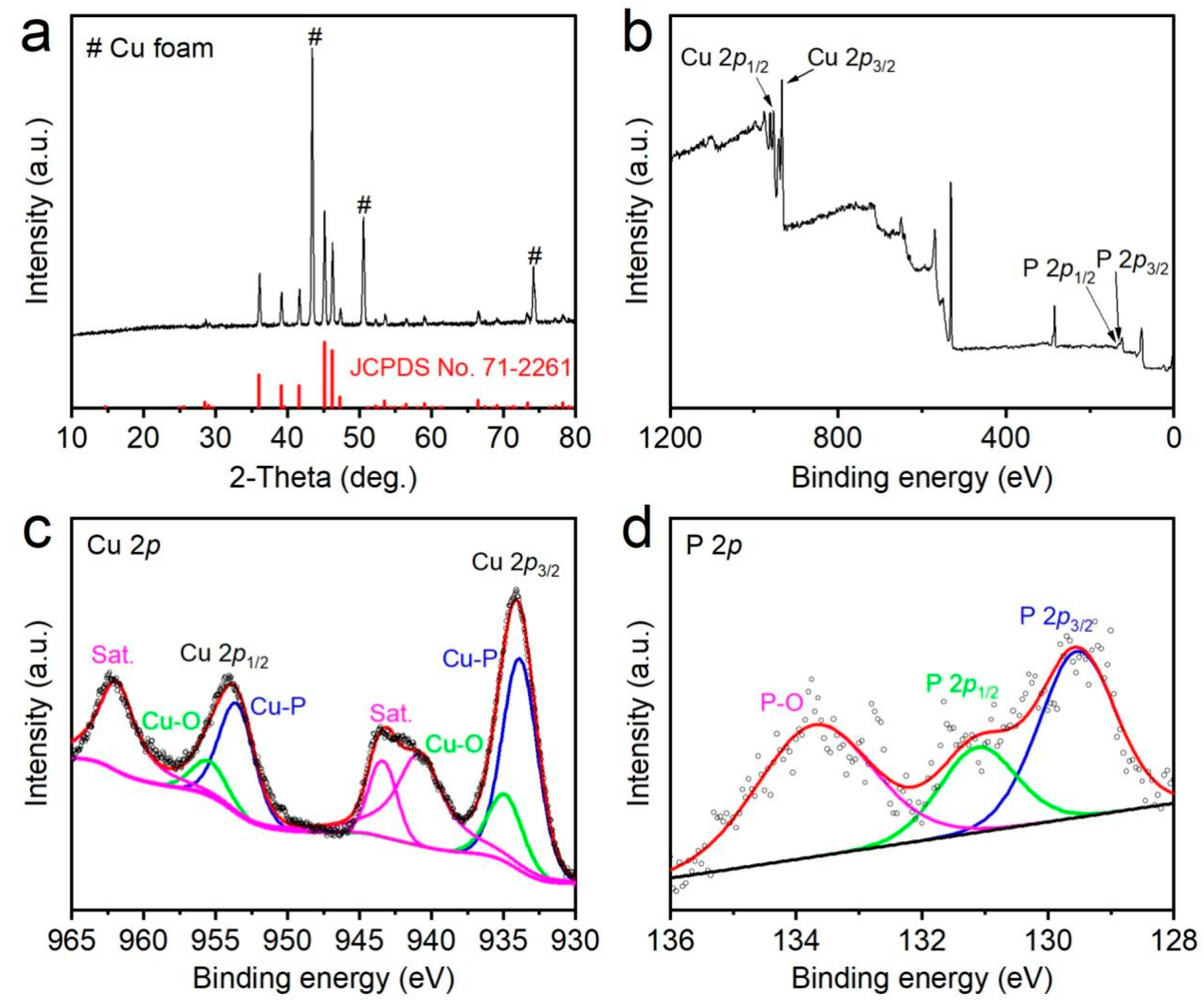
2.2. Characterizations
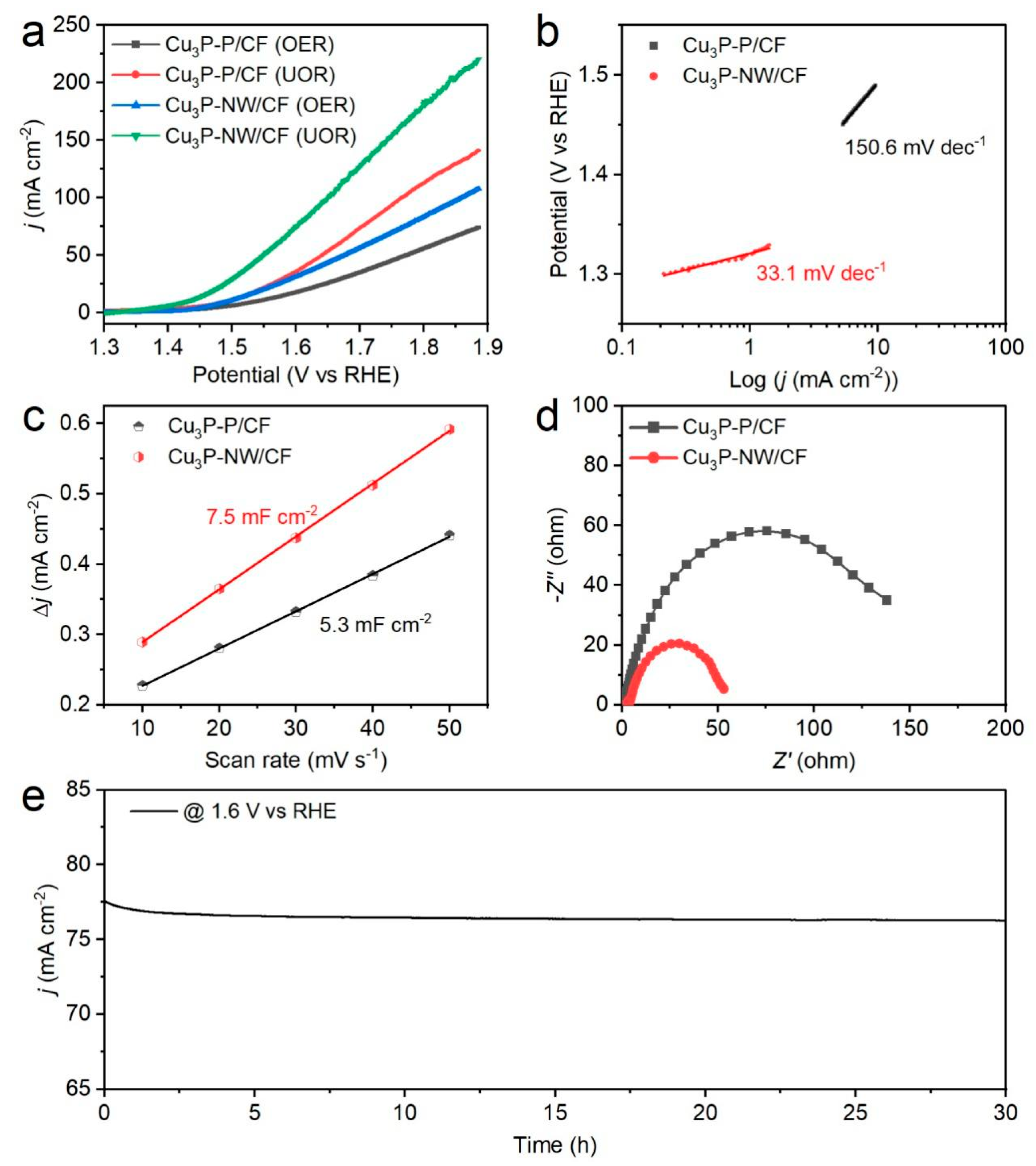
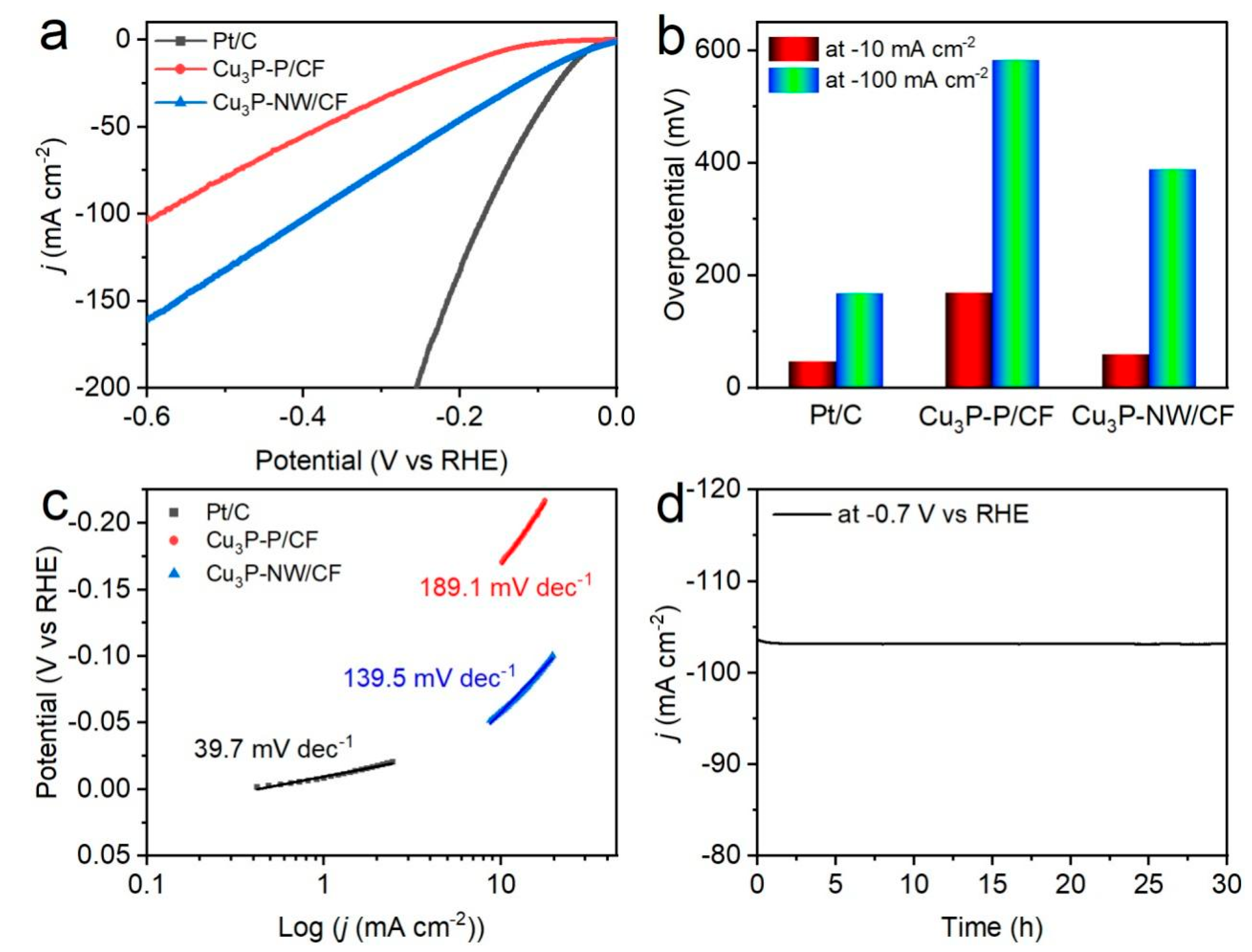
2.3. Electrochemical Tests
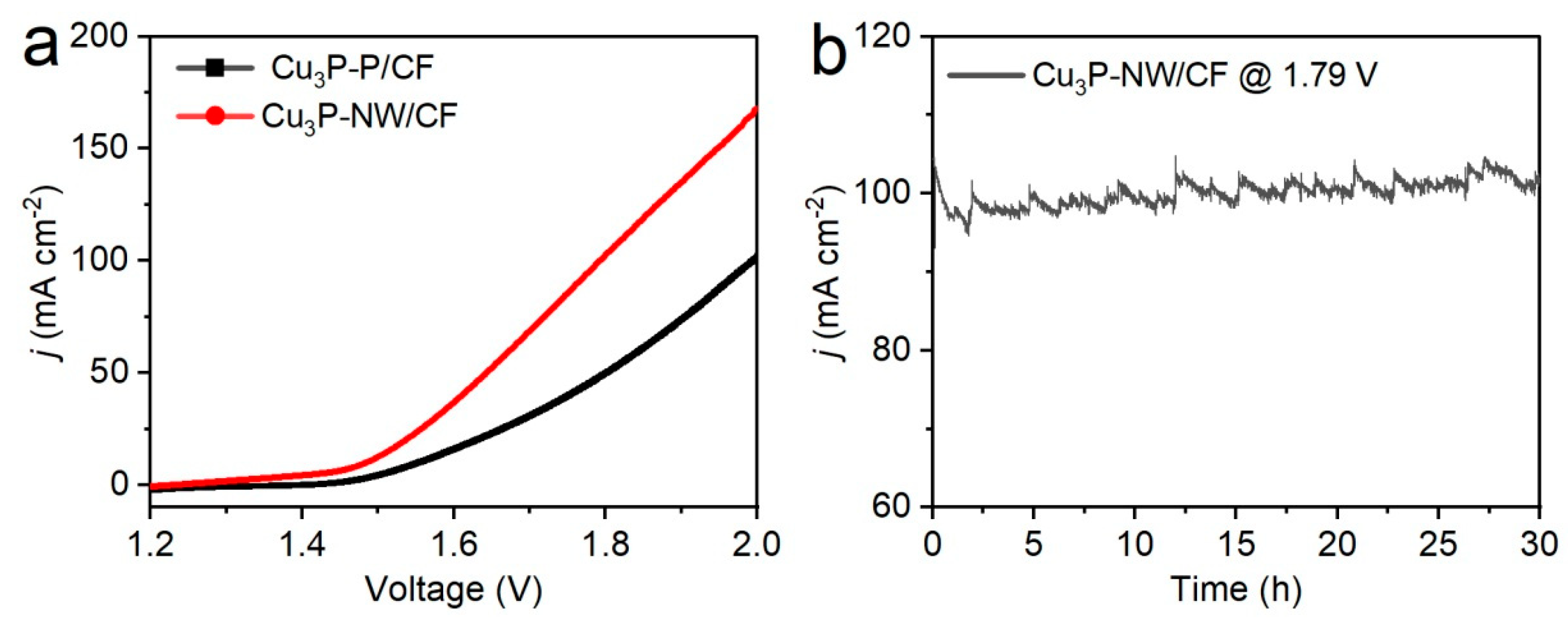
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| OER | Oxygen evolution reaction |
| UOR | Urea oxidation reaction |
| HER | Hydrogen evolution reaction |
| Cu3P-NW/CF | Cu3P nanowires on Cu foam |
| vs. RHE | Versus the reversible hydrogen electrode |
| Cu(OH)2-NA/CF | Cu(OH)2 nanowires on Cu foam |
| Cu3P-P/CF | Cu3P powder on Cu foam |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EDX | Energy dispersive X-ray |
| HRTEM | High-resolution TEM |
| XPS | X-ray photoelectron spectroscopy |
| CV | Cyclic voltammetry |
| LSV | Linear sweep voltammetry |
| EIS | Electrochemical impedance spectra |
| Cdl | double layer capacitance |
| ECSA | electrochemical active surface area |
| SAED | selected area electron diffraction |
References
- Boretti, A. Concentrated Solar Energy with Thermal Energy Storage for Hydrogen Production by Three-Step Thermochemical Water-Splitting Cycles. Energy Fuels 2021, 35, 10832–10840. [Google Scholar] [CrossRef]
- Wang, K.; Xu, W.; Zhang, W.; Wang, X.; Yang, X.; Li, J.; Zhang, H.; Li, J.; Wang, Z. Bio-inspired water-driven electricity generators: From fundamental mechanisms to practical applications. Nano Res. Energy 2023, 2, e9120042. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Yue, L.; Dong, K.; Li, J.; Zhao, D.; Li, Z.; Sun, S.; Luo, Y.; Liu, Q.; et al. Benzoate anions-intercalated NiFe-layered double hydroxide nanosheet array with enhanced stability for electrochemical seawater oxidation. Nano Res. Energy 2022, 1, e9120028. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Liu, Q.; Yu, P.; Luo, J.; Hu, G.; Liu, X. CoSe2 nanocrystals embedded into carbon framework as efficient bifunctional catalyst for alkaline seawater splitting. Inorg. Chem. Commun. 2022, 146, 110170. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Chu, P.K.; Liu, Q.; Liu, X.; Zhang, S.; Luo, J.; Wang, X.; Hu, G. Recent advances in non-noble metal-based bifunctional electrocatalysts for overall seawater splitting. J. Alloys Compd. 2022, 922, 166113. [Google Scholar] [CrossRef]
- Liu, X.; He, J.; Zhao, S.; Liu, Y.; Zhao, Z.; Luo, J.; Hu, G.; Sun, X.; Ding, Y. Self-powered H2 production with bifunctional hydrazine as sole consumable. Nat. Commun. 2018, 9, 4365. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Luo, Y.; Liu, Q.; Luo, J.; Chu, P.K.; Liu, X. Preparation of high entropy alloys and application to catalytical water electrolysis. APL Mater. 2022, 10, 070701. [Google Scholar] [CrossRef]
- Wang, J.; Kim, J.; Choi, S.; Wang, H.; Lim, J. A Review of Carbon-Supported Nonprecious Metals as Energy-Related Electrocatalysts. Small Methods 2020, 4, 2000621. [Google Scholar] [CrossRef]
- Meng, L.; Li, L. Recent research progress on operational stability of metal oxide/sulfide photoanodes in photoelectrochemical cells. Nano Res. Energy 2022, 1, e9120020. [Google Scholar] [CrossRef]
- Ding, J.; Yang, H.; Zhang, S.; Liu, Q.; Cao, H.; Luo, J.; Liu, X. Advances in the Electrocatalytic Hydrogen Evolution Reaction by Metal Nanoclusters-based Materials. Small 2022, 18, 2204524. [Google Scholar] [CrossRef]
- Paygozar, S.; Aghdam, A.S.R.; Hassanizadeh, E.; Andaveh, R.; Darband, G.B. Recent progress in non-noble metal-based electrocatalysts for urea-assisted electrochemical hydrogen production. Int. J. Hydrogen Energy 2023, 48, 7219–7259. [Google Scholar] [CrossRef]
- Rajpure, M.M.; Bandal, H.A.; Jadhav, H.S.; Kim, H. Systematic development of bimetallic MOF and its phosphide derivative as an efficient multifunctional electrocatalyst for urea-assisted water splitting in alkaline medium. J. Electroanal. Chem. 2022, 923, 116825. [Google Scholar] [CrossRef]
- Ding, J.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. A Mini Review: Recent Advances in Asymmetrically Coordinated Atom Sites for High-Efficiency Hydrogen Evolution Reaction. Energies 2023, 16, 2664. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Chang, J.; Feng, L. A review of Ni based powder catalyst for urea oxidation in assisting water splitting reaction. Adv. Powder Mater. 2022, 1, 100030. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, G.; Liu, W.; Zhang, S.; Liu, Q.; Luo, J.; Liu, X. Bimetallic phosphoselenide nanosheets as bifunctional catalysts for 5-hydroxymethylfurfural oxidation and hydrogen evolution. Inorg. Chem. Front. 2023, 10, 2423–2429. [Google Scholar] [CrossRef]
- Sathe, B.R.; Balan, B.K.; Pillai, V.K. Enhanced electrocatalytic performance of interconnected Rh nano-chains towards formic acid oxidation. Energy Environ. Sci. 2011, 4, 1029–1036. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Jaroń, A.; Simka, W. Electrocatalytic oxidation of urea on a sintered Ni–Pt electrode. J. Appl. Electrochem. 2017, 47, 133–138. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Huang, Y.; Jiang, L.-W.; Meng, C.; Yin, Z.-H.; Liu, H.; Wang, J.-J. Modulating the electronic structure of CoS2 by Sn doping boosting urea oxidation for efficient alkaline hydrogen production. J. Colloid Interface Sci. 2023, 642, 574–583. [Google Scholar] [CrossRef]
- Fang, Y.; Li, M.; Guo, X.; Duan, Z.; Safikhani, A. Pulse-reverse electrodeposition of Ni–Mo–S nanosheets for energy saving electrochemical hydrogen production assisted by urea oxidation. Int. J. Hydrogen Energy 2023, 48, 19087–19102. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Graham, N.; Zhang, G.; Yu, W. Modulation of dual centers on cobalt-molybdenum oxides featuring synergistic effect of intermediate activation and radical mediator for electrocatalytic urea splitting. Nano Energy 2021, 87, 106217. [Google Scholar] [CrossRef]
- Ding, R.; Qi, L.; Jia, M.; Wang, H. Facile synthesis of mesoporous spinel NiCo2O4 nanostructures as highly efficient electrocatalysts for urea electro-oxidation. Nanoscale 2014, 6, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Liu, Y.; Hu, G.; Zhao, Y.; Qian, Y.; Wang, H.; Shi, Y.; Li, Z. NiFe nanosheets as urea oxidation reaction electrocatalysts for urea removal and energy-saving hydrogen production. Biosens. Bioelectron. 2022, 211, 114380. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Lian, J. Mesoporous Cu(OH)2 nanowire arrays for urea electrooxidation in alkaline medium. Mater. Chem. Phys. 2020, 242, 122517. [Google Scholar] [CrossRef]
- Yang, J.-H.; Chen, M.; Xu, X.; Jiang, S.; Zhang, Y.; Wang, Y.; Li, Y.; Zhang, J.; Yang, D. CuO-Ni(OH)2 nanosheets as effective electro-catalysts for urea oxidation. Appl. Surf. Sci. 2021, 560, 150009. [Google Scholar] [CrossRef]
- Han, A.; Zhang, H.; Yuan, R.; Ji, H.; Du, P. Crystalline Copper Phosphide Nanosheets as an Efficient Janus Catalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Wei, B.; Li, Y.; Ma, X.; Liang, S.; Wang, S.; Xu, L. Self-supported Hierarchical Fe(PO3)2@Cu3P nanotube arrays for efficient hydrogen evolution in alkaline media. J. Alloys Compd. 2020, 820, 153185. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, T.; Li, P.; Cao, Z.; Xi, W.; Zhao, Y.; Ding, Y. Self-Supported Hierarchical Nanostructured NiFe-LDH and Cu3P Weaving Mesh Electrodes for Efficient Water Splitting. ACS Sustain. Chem. Eng. 2018, 6, 380–388. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.; Song, D.; Kwon, Y.; Kim, E.-J.; Cho, E. Cu3P/PAN derived N-doped carbon catalyst with non-toxic synthesis for alkaline hydrogen evolution reaction. Sustain. Energy Fuels 2020, 4, 5247–5253. [Google Scholar] [CrossRef]
- Liu, W.; Feng, J.; Wei, T.; Liu, Q.; Zhang, S.; Luo, Y.; Luo, J.; Liu, X. Active-site and interface engineering of cathode materials for aqueous Zn—Gas batteries. Nano Res. 2023, 16, 2325–2346. [Google Scholar] [CrossRef]
- Guo, J.; Zhan, Z.; Lei, T.; Yin, P. Electrochemical tuning of a Cu3P/Ni2P hybrid for a promoted hydrogen evolution reaction. Dalton Trans. 2022, 51, 14329–14337. [Google Scholar] [CrossRef]
- Rauf, A.; Ma, M.; Kim, S.; Shah, M.S.A.S.; Chung, C.-H.; Park, J.H.; Yoo, P.J. Mediator- and co-catalyst-free direct Z-scheme composites of Bi2WO6–Cu3P for solar-water splitting. Nanoscale 2018, 10, 3026–3036. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z. Theoretically guiding the construction of a novel Cu2O@Cu97P3@Cu3P heterojunction with a 3D hierarchical structure for efficient photocatalytic hydrogen evolution. Nanoscale 2021, 13, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, S.; Liu, Q.; Qiu, Y.; Luo, J.; Liu, X. Oxygen Vacancy-Rich Amorphous Copper Oxide Enables Highly Selective Electroreduction of Carbon Dioxide to Ethylene. Acta Phys. Chim. Sin. 2023, 39, 2207026. [Google Scholar] [CrossRef]
- Gang, C.; Chen, J.; Li, X.; Ma, B.; Zhao, X.; Chen, Y. Cu3P@CoO core–shell heterostructure with synergistic effect for highly efficient hydrogen evolution. Nanoscale 2021, 13, 19430–19437. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Hou, X.; Qiu, Y.; Zhang, S.; Liu, Q.; Luo, J.; Liu, X. Iron-doping strategy promotes electroreduction of nitrate to ammonia on MoS2 nanosheets. Inorg. Chem. Commun. 2023, 151, 110621. [Google Scholar] [CrossRef]
- Wei, T.; Liu, W.; Zhang, S.; Liu, Q.; Luo, J.; Liu, X. A dual-functional Bi-doped Co3O4 nanosheet array towards high efficiency 5-hydroxymethylfurfural oxidation and hydrogen production. Chem. Commun. 2023, 59, 442–445. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating metal–organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. Susmat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Q.; Alshehri, A.A.; Sun, X. Recent advances in nanostructured heterogeneous catalysts for N-cycle electrocatalysis. Nano Res. Energy 2022, 1, e9120010. [Google Scholar] [CrossRef]
- Qi, D.; Lv, F.; Wei, T.; Jin, M.; Meng, G.; Zhang, S.; Liu, Q.; Liu, W.; Ma, D.; Hamdy, M.S.; et al. High-efficiency electrocatalytic NO reduction to NH3 by nanoporous VN. Nano Res. Energy 2022, 1, e9120022. [Google Scholar] [CrossRef]
- Shen, H.; Wei, T.; Liu, Q.; Zhang, S.; Luo, J.; Liu, X. Heterogeneous Ni-MoN nanosheet-assembled microspheres for urea-assisted hydrogen production. J. Colloid Interface Sci. 2023, 634, 730–736. [Google Scholar] [CrossRef]
- Wang, T.; Gao, S.; Wei, T.; Qin, Y.; Zhang, S.; Ding, J.; Liu, Q.; Luo, J.; Liu, X. Co Nanoparticles Confined in Mesoporous Mo/N Co-Doped Polyhedral Carbon Frameworks towards High-Efficiency Oxygen Reduction. Chem. Eur. J. 2023, 29, e202204034. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Cao, H.; Wei, T.; Liu, Q.; Fu, J.; Zhang, S.; Luo, J.; Liu, X. Highly dispersed Ru clusters toward an efficient and durable hydrogen oxidation reaction. Chem. Commun. 2022, 58, 11839–11842. [Google Scholar] [CrossRef]
- Lu, X.; Qiao, K.; Shaik, F.; Zheng, Y.; Chu, Z.; Qian, H.; Liu, X.; Zhang, W. Evoking robust immunogenic cell death by synergistic sonodynamic therapy and glucose depletion using Au clusters/single atoms modified TiO2 nanosheets. Nano Res. 2023. [Google Scholar] [CrossRef]
- Liang, H.; Gandi, A.N.; Anjum, D.H.; Wang, X.; Schwingenschlögl, U.; Alshareef, H.N. Plasma-Assisted Synthesis of NiCoP for Efficient Overall Water Splitting. Nano Lett. 2016, 16, 7718–7725. [Google Scholar] [CrossRef] [PubMed]
- Maarisetty, D.; Hang, D.-R.; Chou, M.M.C.; Parida, S. Tuning the Ni/Co Ratios and Surface Concentration of Reduced Molybdenum States for Enhanced Electrocatalytic Performance in Trimetallic Molybdates: OER, HER, and MOR Activity. ACS Appl. Energy Mater. 2022, 5, 14059–14070. [Google Scholar] [CrossRef]
- Singh, K.P.; Shin, C.-H.; Lee, H.-Y.; Razmjooei, F.; Sinhamahapatra, A.; Kang, J.; Yu, J.-S. TiO2/ZrO2 Nanoparticle Composites for Electrochemical Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 3634–3645. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, L.; Hou, J.; Liu, S.; Qin, Y.; Liu, Q.; Cai, X.; Sun, Z.; Yang, M.; Luo, J.; et al. Cu2O-Derived PtCu Nanoalloy toward Energy-Efficient Hydrogen Production via Hydrazine Electrolysis under Large Current Density. ACS Appl. Energy Mater. 2022, 5, 9487–9494. [Google Scholar] [CrossRef]
- Solati, N.; Karakaya, C.; Kaya, S. Advancing the Understanding of the Structure–Activity–Durability Relation of 2D MoS2 for the Hydrogen Evolution Reaction. ACS Catal. 2023, 13, 342–354. [Google Scholar] [CrossRef]
- Chen, S.; Lian, K.; Liu, W.; Liu, Q.; Qi, G.; Luo, J.; Liu, X. Engineering active sites of cathodic materials for high-performance Zn-nitrogen batteries. Nano Res. 2023. [Google Scholar] [CrossRef]
- Liu, W.; Que, W.; Yin, R.; Dai, J.; Zheng, D.; Feng, J.; Xu, X.; Wu, F.; Shi, W.; Liu, X.; et al. Ferrum-molybdenum dual incorporated cobalt oxides as efficient bifunctional anti-corrosion electrocatalyst for seawater splitting. Appl. Catal. B Environ. 2023, 328, 122488. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Z.; Tian, Y.; Yu, C.; Liu, K.; Jiang, L. Engineering electrode wettability to enhance mass transfer in hydrogen evolution reaction. Nano Res. Energy 2023, 2, e9120063. [Google Scholar] [CrossRef]
- Yan, J.; Ye, F.; Dai, Q.; Ma, X.; Fang, Z.; Dai, L.; Hu, C. Recent progress in carbon-based electrochemical catalysts: From structure design to potential applications. Nano Res. Energy 2023, 2, e9120047. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Wei, T.; Ding, J.; Liu, X. Copper Phosphide Nanowires as High-Performance Catalysts for Urea-Assisted Hydrogen Evolution in Alkaline Medium. Materials 2023, 16, 4169. https://doi.org/10.3390/ma16114169
Shen H, Wei T, Ding J, Liu X. Copper Phosphide Nanowires as High-Performance Catalysts for Urea-Assisted Hydrogen Evolution in Alkaline Medium. Materials. 2023; 16(11):4169. https://doi.org/10.3390/ma16114169
Chicago/Turabian StyleShen, Hui, Tianran Wei, Junyang Ding, and Xijun Liu. 2023. "Copper Phosphide Nanowires as High-Performance Catalysts for Urea-Assisted Hydrogen Evolution in Alkaline Medium" Materials 16, no. 11: 4169. https://doi.org/10.3390/ma16114169
APA StyleShen, H., Wei, T., Ding, J., & Liu, X. (2023). Copper Phosphide Nanowires as High-Performance Catalysts for Urea-Assisted Hydrogen Evolution in Alkaline Medium. Materials, 16(11), 4169. https://doi.org/10.3390/ma16114169






