Plasma Electrolytic Oxidation Coatings of a 6061 Al Alloy in an Electrolyte with the Addition of K2ZrF6
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Preparation
2.2. Coating Characterization
2.3. Coatings’ Thermal Control
3. Results
3.1. Voltage–Time Response
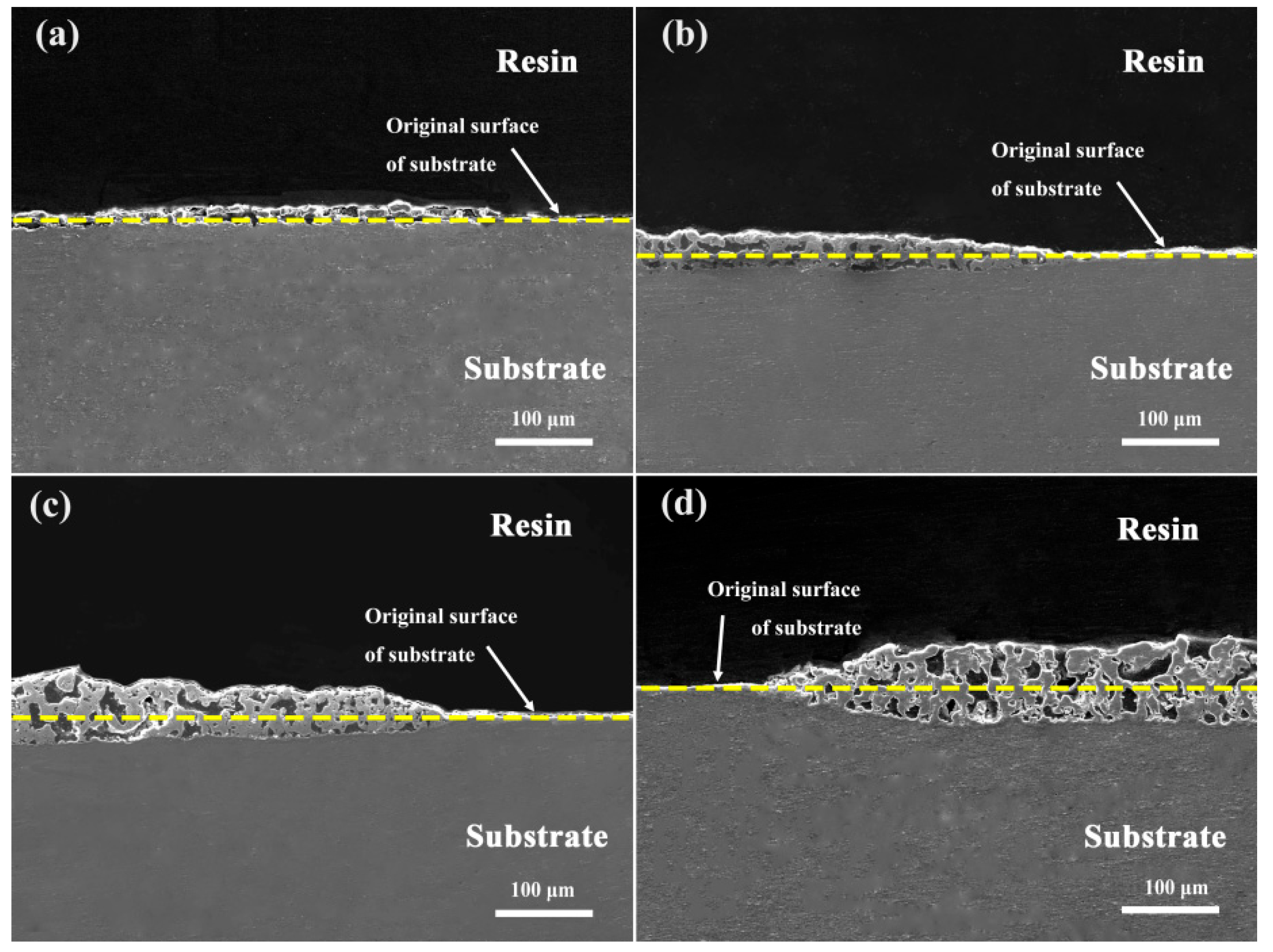
3.2. Effect of K2ZrF6 on Surface Structure and Microstructure
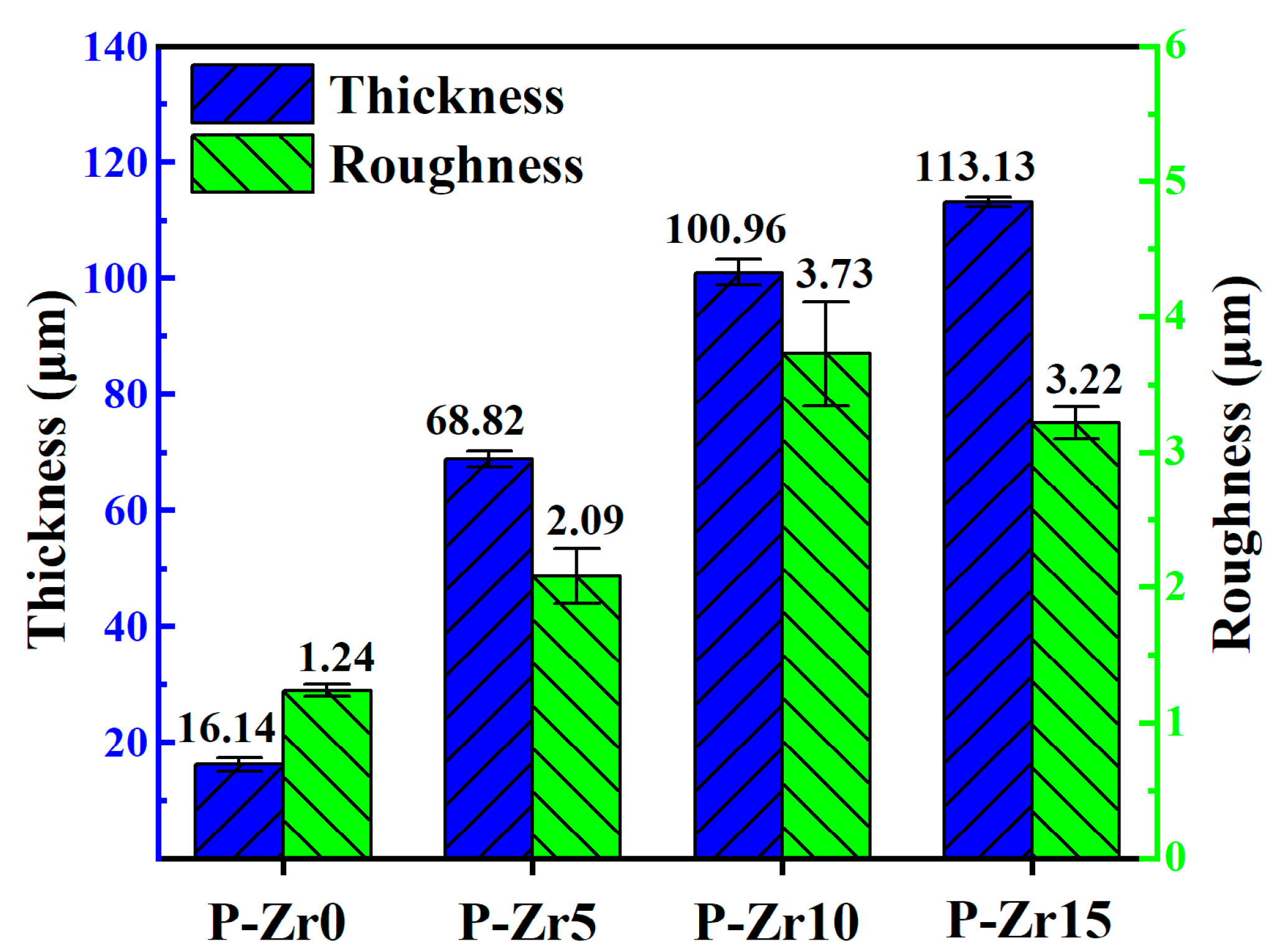
3.3. Effect of K2ZrF6 Addition on the Growth Behavior of Coatings
3.4. Effect of K2ZrF6 on the Thermal Control Properties of Coatings
4. Conclusions
- The addition of K2ZrF6 fostered an accelerated growth rate of the PEO coatings. The roughness tended to stabilize with increasing K2ZrF6 concentration up to a certain value. The best performance of the PEO coating was achieved at a K2ZrF6 concentration of 15 g/L, when the thickness was 113.13 μm, the roughness was 3.22 μm, and the porosity was 17.75%.
- In conventional electrolytes, the presence of trace elements within the substrate matrix influences both the color and absorption characteristics of the coating. This influence is contingent upon whether the trace elements within the matrix contain coloring ions. However, the introduction of K2ZrF6 serves to mitigate the impact of trace elements on the coating. Consequently, the L* value of the coating exhibited a notable increase, rising from 71.69 to 90.53.
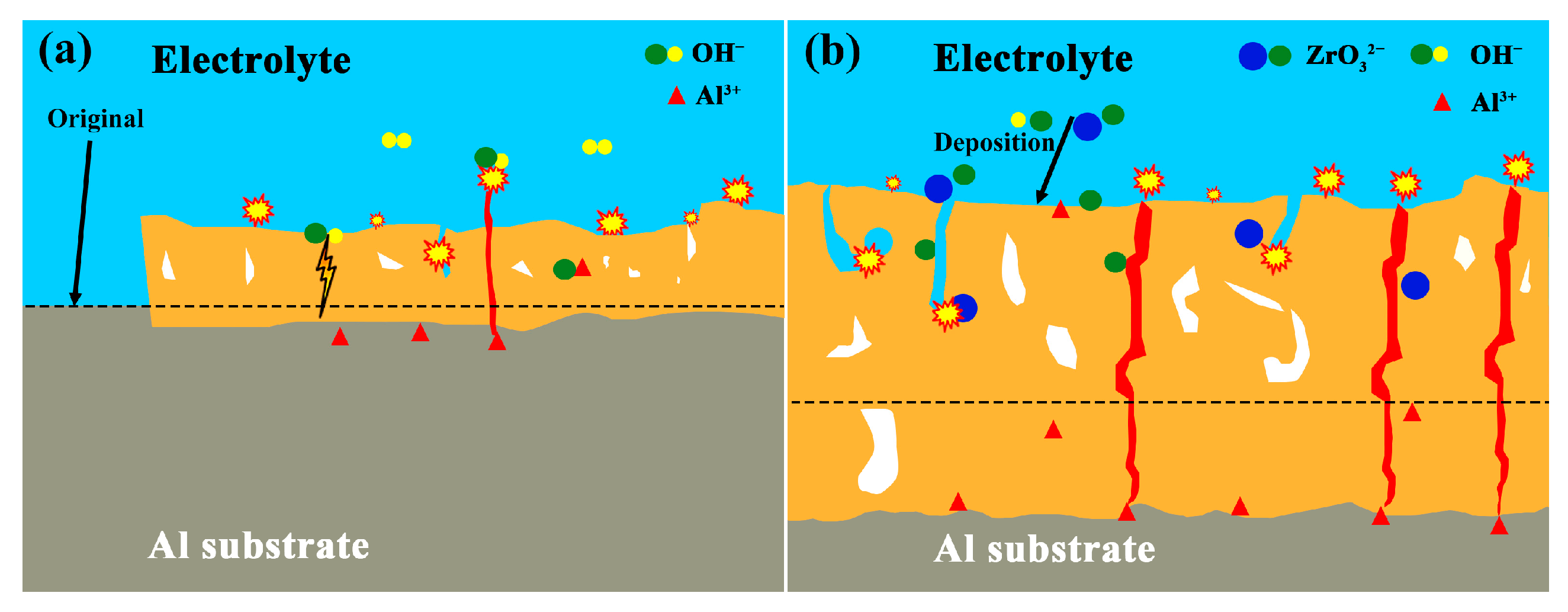
- The introduction of K2ZrF6 resulted in a considerable reduction in the P element content within the coating, approaching near-zero levels. This reduction facilitated the ingress of Na, F, and K elements into the coating matrix. Consequently, the coating predominantly comprised tetragonal zirconia (t-ZrO2) and monoclinic zirconia (m-ZrO2) phases, with their relative proportions increasing proportionally with the concentration of K2ZrF6. Furthermore, amorphous compounds containing F were also present within the coating structure. This phenomenon arises due to the dominant influence of electrolyte deposition in the formation of this particular coating.
- The coating exhibited low absorbance (α ≤ 0.20) and high emissivity (ε ≥ 0.68). As the concentration of K2ZrF6 increased, absorbance α decreased, while emissivity ε increased, which may be due to the combined effect of the phase and structure of the coating. The coating of P-Zr15 had the highest absorbance α (0.16) and the highest emissivity ε (0.71). These PEO coatings have potential applications in solar and infrared heating by optimizing the electrolyte.
- The addition of K2ZrF6 changed the growth mechanism of the coating. In the absence of K2ZrF6 in the electrolyte, the PEO coating on the Al alloy surface predominantly exhibited outward growth. However, with the inclusion of K2ZrF6, the growth pattern of the coating transitioned into a combination of outward and inward growth, with the proportion of inward growth progressively increasing alongside the escalating of K2ZrF6 concentration. The incorporation of K2ZrF6 led to favorable conditions that promoted the inward growth of the coating, consequently enhancing the bonding strength between the coating and the substrate. As a result, the addition of K2ZrF6 led to notable thermal shock resistance in the coating, thus demonstrating improved durability under thermal stress conditions.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, X.Q.; Vassen, R.; Stoever, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Hołyńska, M.; Tighe, A.; Semprimoschnig, C. Coatings and Thin Films for Spacecraft Thermo-Optical and Related Functional Applications. Adv. Mater. Interfaces 2018, 5, 1701644. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ouyang, J.H.; Li, Z.G.; Wang, Y.M.; Zhou, Y. Enhanced infrared emissivity of MAO coatings formed on Ti2AlNb alloy. Surf. Eng. 2014, 30, 460–466. [Google Scholar] [CrossRef]
- Pillai, A.M.; Rajendra, A.; Sharma, A.K.; Bera, P.; Poornima, S.; Sampath, S. Development of vanadium impregnated flat absorber composite PEO coating on AA6061 alloy. Surf. Coat. Technol. 2021, 410, 126891. [Google Scholar] [CrossRef]
- Zhang, H.P.; Wang, C.; Wang, Y.M.; Wang, S.Q.; Chen, G.L.; Zou, Y.C.; Deng, C.M.; Jia, D.C.; Zhou, Y. Ca-Mn co-doping LaCrO3 coating with high emissivity and good mechanical property for enhancing high-temperature radiant heat dissipation. J. Eur. Ceram. Soc. 2022, 42, 7288–7299. [Google Scholar] [CrossRef]
- Zhang, H.P.; Wang, C.; Wang, Y.M.; Wang, S.Q.; Zou, Y.C.; Chen, G.L.; Deng, C.M.; Jia, D.C.; Zhou, Y. Evaluation on the microstructure and thermal infrared emissivity of Ca2+-doped ytterbium chromate coating deposited by saucer-shaped porous feedstocks for high-temperature thermal protection. Appl. Surf. Sci. 2023, 608, 155210. [Google Scholar] [CrossRef]
- Nastic, A.; Jodoin, B. Evaluation of Heat Transfer Transport Coefficient for Cold Spray Through Computational Fluid Dynamics and Particle In-Flight Temperature Measurement Using a High-Speed IR Camera. J. Therm. Spray Technol. 2018, 27, 1491–1517. [Google Scholar] [CrossRef]
- Lu, F.F.; Ma, K.; Li, C.X.; Yasir, M.; Luo, X.T.; Li, C.j. Enhanced corrosion resistance of cold-sprayed and shot-peened aluminum coatings on LA43M magnesium alloy. Surf. Coat. Technol. 2020, 394, 125865. [Google Scholar] [CrossRef]
- Jiao, M.Y.; Wu, J.Y.; Guo, L.L.; Han, P.D.; Du, H.Y.; Hu, X.X. Fiber reinforced scalelike MoSi2-borosilicate glass coating with improved contact damage resistance and thermal shock resistance. Ceram. Int. 2021, 47, 11037–11042. [Google Scholar] [CrossRef]
- Zhang, H.P.; Wang, Y.M.; Hu, Y.L.; Mao, W.; Zou, Y.C.; Chen, G.L.; Wang, S.Q.; Jia, D.C.; Zhou, Y. Preparation, microstructure, and thermophysical properties of Cr3+-modified cordierite high emissivity ceramic for metal thermal protection. J. Alloys Compd. 2022, 892, 162164. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Choi, C.H.; Chung, W. Nanoporous anodic alumina oxide layer and its sealing for the enhancement of radiative heat dissipation of aluminum alloy. Nano Energy 2017, 31, 504–513. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Sridhar, S.; Wei, G.Y.; Yu, Y.D.; Zhang, Z.Q.; Jiang, L.; Yang, Y.M.; Shahzad, M.W.; Chen, X.; Xu, B.B. A highly controlled fabrication of porous anodic aluminium oxide surface with versatile features by spatial thermo-anodization. Surf. Coat. Technol. 2021, 408, 126809. [Google Scholar] [CrossRef]
- Amiri, H.; Mohammadi, I.; Afshar, A. Electrophoretic deposition of nano-zirconia coating on AZ91D magnesium alloy for bio-corrosion control purposes. Surf. Coat. Technol. 2017, 311, 182–190. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.H.; Chen, H.; Hu, C.; Li, L.X.; Hu, B.N.; Song, Z.W.; Yan, D.L.; Yu, G. Electroless deposition and characterization of a double-layered Ni-B/Ni-P coating on AZ91D Mg alloy from eco-friendly fluoride-free baths. Surf. Coat. Technol. 2018, 342, 178–189. [Google Scholar] [CrossRef]
- Jiang, S.X.; Xu, J.T.; Miao, D.G.; Peng, L.H.; Shang, S.M.; Zhu, P. Water-repellency, ultraviolet protection and infrared emissivity properties of AZO film on polyester fabric. Ceram. Int. 2017, 43, 2424–2430. [Google Scholar] [CrossRef]
- Zahoor, A.; Xu, C.; Shahid, T.; Anwar, M.A.; Song, Z.L. Effects of O2 flux on structure, optical properties and hydrophobicity of highly emissive antireflective HfO2 thin films by magnetron sputtering. Vacuum 2022, 197, 110824. [Google Scholar] [CrossRef]
- Wang, Y.M.; Lei, T.Q.; Guo, L.X.; Jiang, B.L. Fretting wear behaviour of microarc oxidation coatings formed on titanium alloy against steel in unlubrication and oil lubrication. Appl. Surf. Sci. 2006, 252, 8113–8120. [Google Scholar] [CrossRef]
- Liu, C.C.; Xu, T.; Shao, Q.Y.; Huang, S.; Jiang, B.L.; Liang, J.; Li, H.T. Effects of beta phase on the growth behavior of plasma electrolytic oxidation coating formed on magnesium alloys. J. Alloys Compd. 2019, 784, 414–421. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Sobolev, A.; Bograchev, D.; Zinigrad, M.; Borodianskiy, K. Evolution of corrosion on microstructure of ceramic coating produced by plasma electrolytic oxidation in molten salt. Ceram. Int. 2022, 48, 10990–10998. [Google Scholar] [CrossRef]
- Sela, S.; Borodianskiy, K. Synthesis of ceramic surface on Zr alloy using plasma electrolytic oxidation in molten salt. Surf. Interfaces 2023, 36, 102533. [Google Scholar] [CrossRef]
- Sobolev, A.; Peretz, T.; Borodianskiy, K. Synthesis and growth mechanism of ceramic coatings on an Al-Cu alloy using plasma electrolytic oxidation in molten salt. J. Alloys Compd. 2021, 869, 159309. [Google Scholar] [CrossRef]
- Shrestha, S.; Dunn, B.D. Advanced plasma electrolytic oxidation treatment for protection of light weight materials and structures in a space environment. Surf. World 2007, 14, 40–44. [Google Scholar]
- Arunnellaiappan, T.; Krishna, L.R.; Anoop, S.; Rani, R.U.; Rameshbabu, N. Fabrication of multifunctional black PEO coatings on AA7075 for spacecraft applications. Surf. Coat. Technol. 2016, 307, 735–746. [Google Scholar]
- Wu, X.H.; Qin, W.; Cui, B.; Jiang, Z.H.; Lu, W.Q. Black Ceramic Thermal Control Coating Prepared by Microarc Oxidation. Int. J. Appl. Ceram. Technol. 2008, 4, 269–275. [Google Scholar] [CrossRef]
- Wang, Y.M.; Tian, H.; Shen, X.E.; Wen, L.; Ouyang, J.H.; Zhou, Y.; Jia, D.C.; Guo, L.X. An elevated temperature infrared emissivity ceramic coating formed on 2024 aluminium alloy by microarc oxidation. Ceram. Int. 2013, 39, 2869–2875. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yang, H.W.; Shin, K.R.; Ko, Y.G.; Shin, D.H. Heat dissipation properties of oxide layers formed on 7075 Al alloy via plasma electrolytic oxidation. Surf. Coat. Technol. 2015, 269, 114–118. [Google Scholar] [CrossRef]
- Kim, D.; Sung, D.; Lee, J.; Kim, Y.; Chung, W. Composite plasma electrolytic oxidation to improve the thermal radiation performance and corrosion resistance on an Al substrate. Appl. Surf. Sci. 2015, 357, 1396–1402. [Google Scholar] [CrossRef]
- Yao, Z.P.; Su, P.B.; Shen, Q.X.; Ju, P.F.; Wu, C.; Zhai, Y.F.; Jiang, Z.H. Preparation of thermal control coatings on Ti alloy by plasma electrolytic oxidation in K2ZrF6 solution. Surf. Coat. Technol. 2015, 269, 273–278. [Google Scholar] [CrossRef]
- Yao, Z.P.; Xia, Q.X.; Shen, Q.X.; Ju, P.F.; Su, P.B.; Hu, B.; Jiang, Z.H. A facile preparation of ceramic coatings on Ti alloys for thermal protection systems. Sol. Energy Mater. Sol. Cells 2015, 143, 236–241. [Google Scholar] [CrossRef]
- Xia, Q.X.; Wang, J.K.; Liu, G.J.; Wei, H.; Li, D.Q.; Yao, Z.P.; Jiang, Z.H. Effects of electric parameters on structure and thermal control property of PEO ceramic coatings on Ti alloys. Surf. Coat. Technol. 2016, 307, 1284–1290. [Google Scholar] [CrossRef]
- Al Bosta, M.M.S.; Ma, K.J.; Chien, H.H. The effect of MAO processing time on surface properties and low temperature infrared emissivity of ceramic coating on aluminium 6061 alloy. Infrared Phys. Technol. 2013, 60, 323–334. [Google Scholar] [CrossRef]
- Al Bosta, M.M.S.; Ma, K.J. Influence of electrolyte temperature on properties and infrared emissivity of MAO ceramic coating on 6061 aluminum alloy. Infrared Phys. Technol. 2014, 67, 63–72. [Google Scholar] [CrossRef]
- Ge, Y.L.; Wang, Y.M.; Zhang, Y.F.; Guo, L.X.; Jia, D.C.; Ouyang, J.H.; Zhou, Y. The improved thermal radiation property of SiC doped microarc oxidation ceramic coating formed on niobium metal for metal thermal protective system. Surf. Coat. Technol. 2017, 309, 880–886. [Google Scholar] [CrossRef]
- Zou, Y.C.; Wang, Y.M.; Zhang, H.J.; Wei, D.Q.; Jin, T.; Wang, H.W.; Liao, S.Z.; Jia, D.C.; Zhou, Y. Al2O3/reduced graphene oxide double-layer radiative coating for efficient heat dissipation. Mater. Design. 2018, 157, 130–140. [Google Scholar] [CrossRef]
- Fan, D.S.; Sun, H.; Li, Q. Thermal control properties of radiative cooling foil based on transparent fluorinated polyimide. Sol. Energy Mater. Sol. Cells 2019, 195, 250–257. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wang, Y.M.; Liu, Y.; Xu, J.L.; Guo, L.X.; Zhou, Y.; Ouyang, J.H.; Dai, J.M. Microstructure and infrared emissivity property of coating containing TiO2 formed on titanium alloy by microarc oxidation. Curr. Appl. Phys. 2011, 11, 1405–1409. [Google Scholar] [CrossRef]
- Wu, Z.D.; Yao, Z.P.; Jiang, Z.H. Preparation and structure of microarc oxidation ceramic coatings containing ZrO2 grown on LY12 Al alloy. Rare Met. 2008, 27, 55–58. [Google Scholar] [CrossRef]
- Barati, N.; Meletis, E.I.; Fard, F.G.; Yerokhin, A.; Rastegari, S.; Faghihi-Sani, M.A. Al2O3-ZrO2 nanostructured coatings using DC plasma electrolytic oxidation to improve tribological properties of Al substrates. Appl. Surf. Sci. 2015, 356, 927–934. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Shin, S.H.; Kaseem, M.; Uzair, M.; Koo, B.H. Towards a compact coating formed on A16061 alloy in phosphate based electrolyte via two-step PEO process and K2ZrF6 additives. Surf. Coat. Technol. 2017, 328, 355–360. [Google Scholar] [CrossRef]
- Pintilei, G.L.; Crismaru, V.I.; Abrudeanu, M.; Munteanu, C.; Luca, D.; Istrate, B. The Influence of Zro2/20%Y2O3 and Al2O3 Deposited Coatings to the Behavior of an Aluminum Alloy Subjected to Mechanical Shock. Appl. Surf. Sci. 2015, 352, 169–177. [Google Scholar] [CrossRef]
- Ma, D.L.; Lu, C.H.; Fang, Z.G.; Yan, W.G.; Wei, L.; Ni, Y.R.; Xu, Z.Z. Preparation of high absorbance and high emittance coatings on 6061 aluminum alloy with a pre-deposition method by plasma electrolytic oxidation. Appl. Surf. Sci. 2016, 389, 874–881. [Google Scholar] [CrossRef]
- Li, Q.B.; Yang, W.B.; Liu, C.C.; Wang, D.A.; Liang, J. Correlations between the growth mechanism and properties of micro-arc oxidation coatings on titanium alloy: Effects of electrolytes. Surf. Coat. Technol. 2017, 316, 162–170. [Google Scholar] [CrossRef]
- Xia, Q.X.; Li, X.; Yao, Z.P.; Jiang, Z.H. Investigations on the thermal control properties and corrosion resistance of MAO coatings prepared on Mg-5Y-7Gd-1Nd-0.5Zr alloy. Surf. Coat. Technol. 2021, 409, 126874. [Google Scholar] [CrossRef]
- Bayati, M.R.; Zargar, H.R.; Talimian, A.; Ziaee, A.; Molaei, R. Characterization of Al2O3–TiO2 nano porous solar absorbers derived via MAO/sol-gel hybrid process. Surf. Coat. Technol. 2011, 205, 2483–2489. [Google Scholar] [CrossRef]


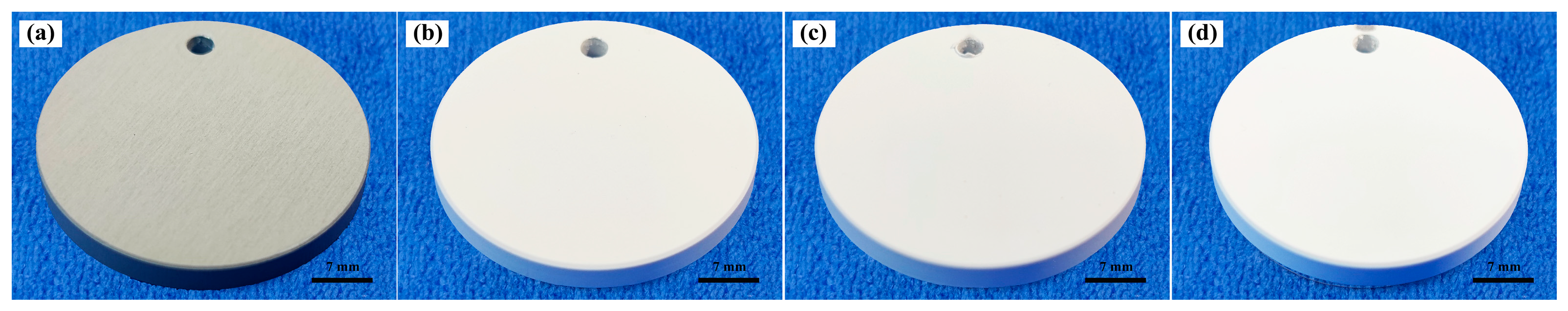


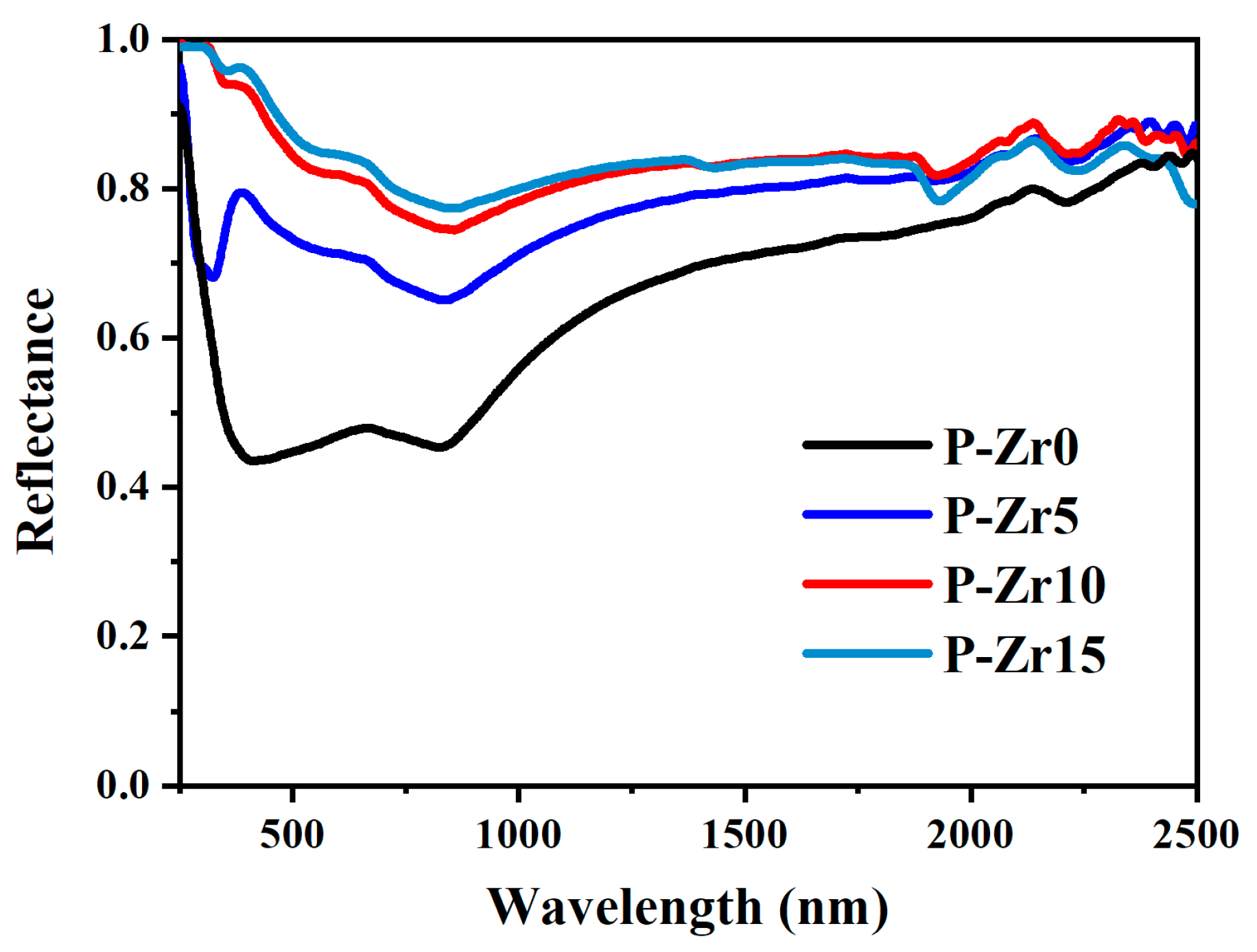
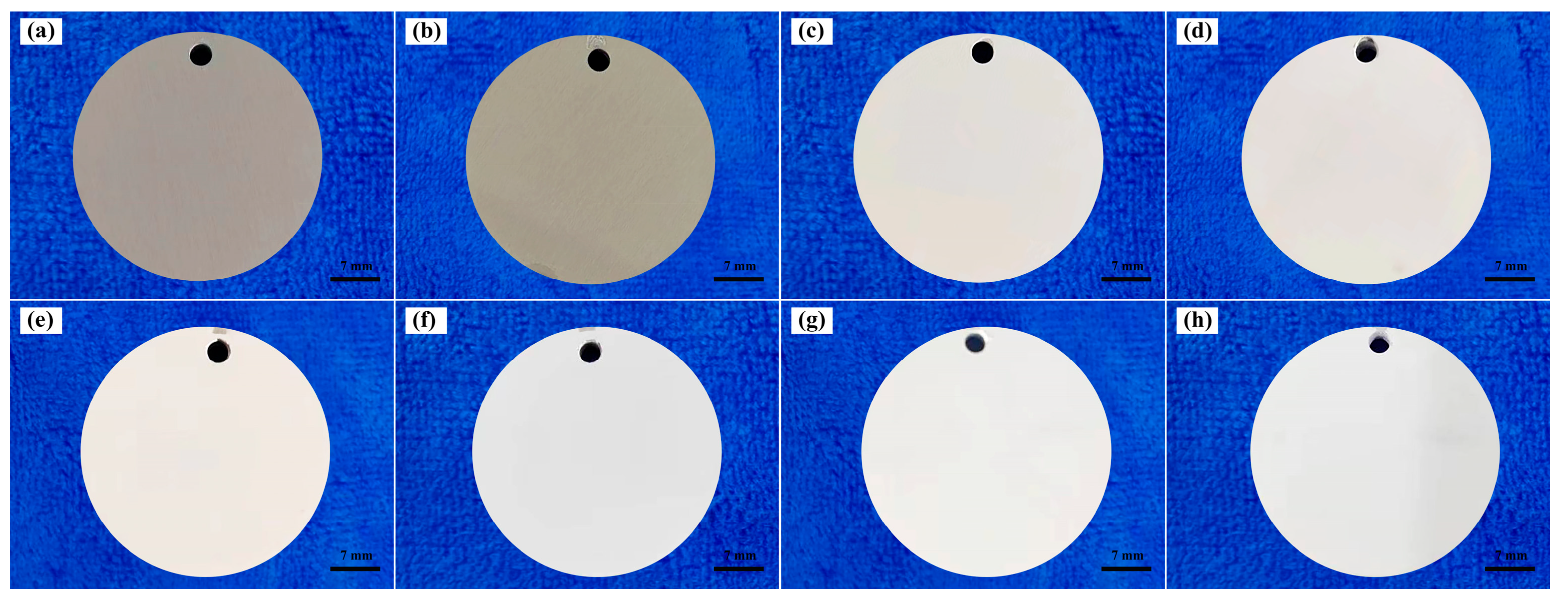
| Specimens | Porosity (%) | L* | a* | b* |
|---|---|---|---|---|
| P-Zr0 | 0.53 | 71.69 | 1.44 | 5.57 |
| P-Zr5 | 7.07 | 84.4 | 0.36 | 1.85 |
| P-Zr10 | 11.95 | 89.33 | 0.61 | 1.06 |
| P-Zr15 | 17.75 | 90.53 | 0.98 | 0.48 |
| Specimens | Content of Elements (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| O | Al | P | Si | Na | Zr | K | F | |
| P-Zr0 | 40.78 | 56.44 | 1.13 | 1.20 | 0.45 | 0 | 0 | 0 |
| P-Zr5 | 21.64 | 19.66 | 0 | 0.31 | 3.58 | 39.87 | 4.51 | 10.43 |
| P-Zr10 | 19.97 | 10.31 | 0.88 | 0.99 | 5.69 | 38.09 | 7.84 | 16.23 |
| P-Zr15 | 20.62 | 10.35 | 0.01 | 1.04 | 5.19 | 42.11 | 7.52 | 13.16 |
| Specimens | α | ε |
|---|---|---|
| P-Zr0 | 0.48 | 0.49 |
| P-Zr5 | 0.27 | 0.68 |
| P-Zr10 | 0.18 | 0.69 |
| P-Zr15 | 0.16 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, C.; Chen, X.; Liu, C.; Li, D. Plasma Electrolytic Oxidation Coatings of a 6061 Al Alloy in an Electrolyte with the Addition of K2ZrF6. Materials 2023, 16, 4142. https://doi.org/10.3390/ma16114142
Tu C, Chen X, Liu C, Li D. Plasma Electrolytic Oxidation Coatings of a 6061 Al Alloy in an Electrolyte with the Addition of K2ZrF6. Materials. 2023; 16(11):4142. https://doi.org/10.3390/ma16114142
Chicago/Turabian StyleTu, Chaohui, Xuanyu Chen, Cancan Liu, and Deye Li. 2023. "Plasma Electrolytic Oxidation Coatings of a 6061 Al Alloy in an Electrolyte with the Addition of K2ZrF6" Materials 16, no. 11: 4142. https://doi.org/10.3390/ma16114142
APA StyleTu, C., Chen, X., Liu, C., & Li, D. (2023). Plasma Electrolytic Oxidation Coatings of a 6061 Al Alloy in an Electrolyte with the Addition of K2ZrF6. Materials, 16(11), 4142. https://doi.org/10.3390/ma16114142





