Improved Yield and Electrical Properties of Poly(Lactic Acid)/Carbon Nanotube Composites by Shear and Anneal
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Sample Preparation
2.2. Sample Testing
2.2.1. Measurement of Mechanical Properties
2.2.2. Measurement of Electrical Conductivity
2.2.3. X-ray Measurements
2.2.4. Optical Microscopy (OM)
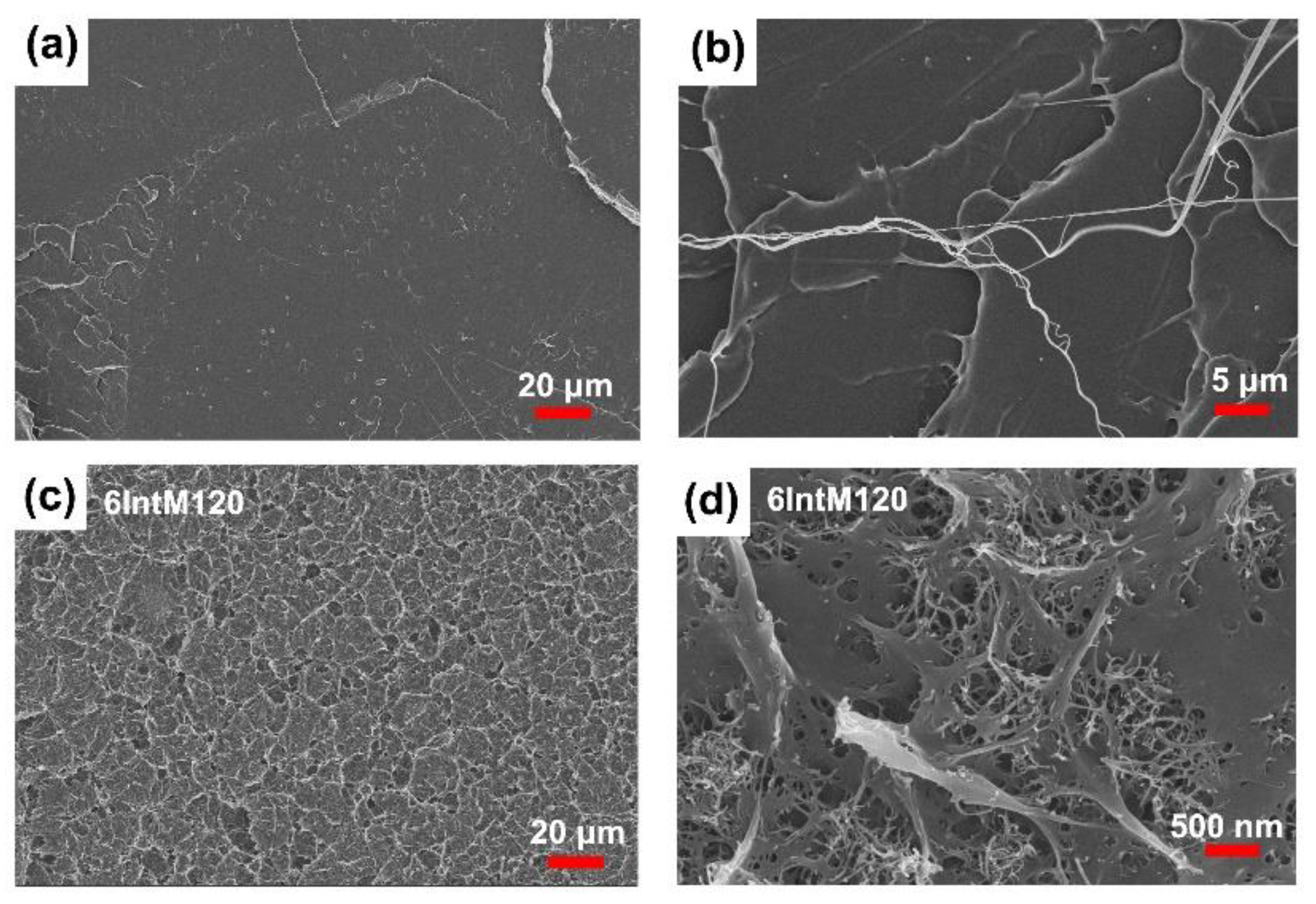
2.2.5. Scanning Electron Microscopy (SEM)
2.2.6. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
3.1. Sample Structure
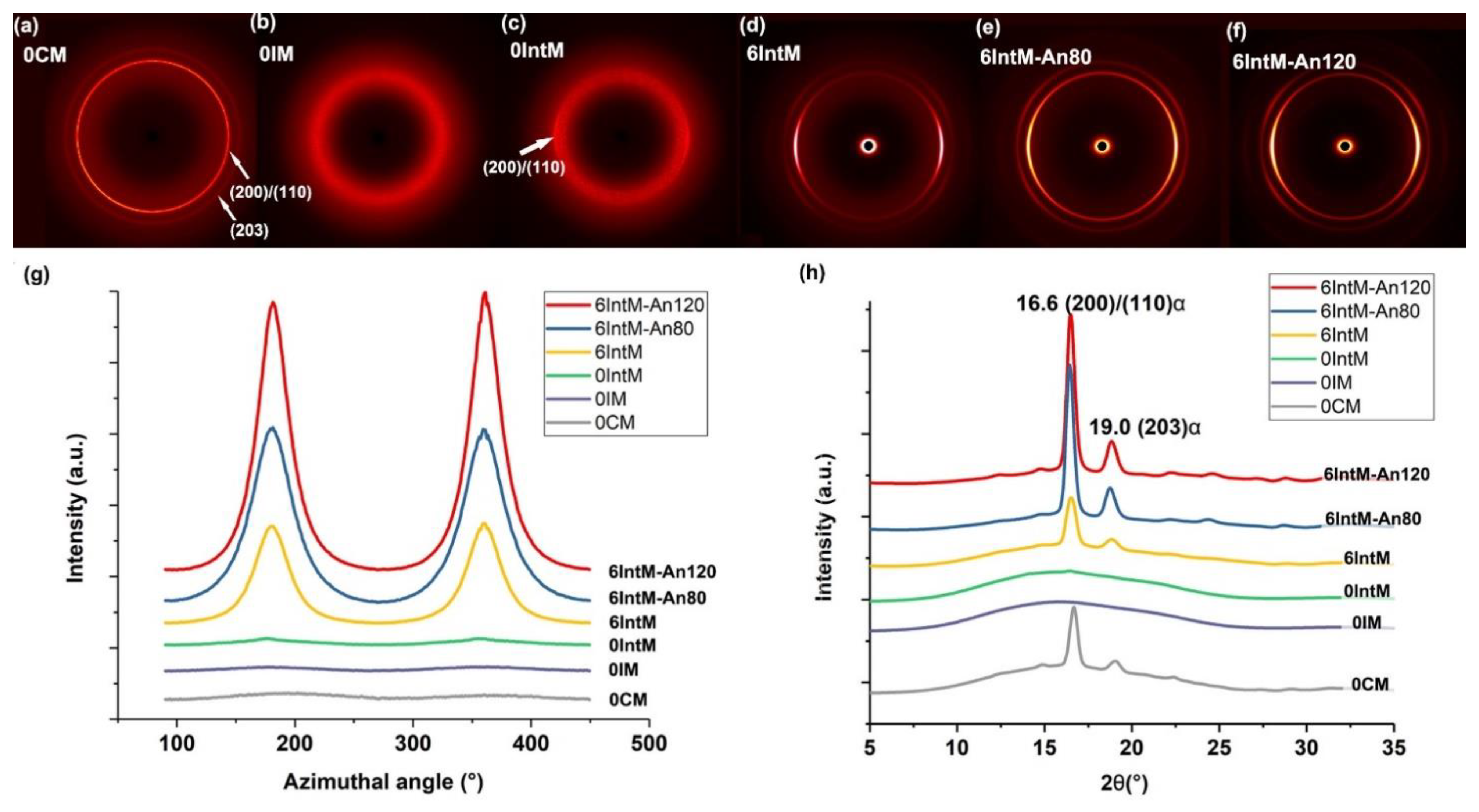
3.1.1. Crystallite and Orientation
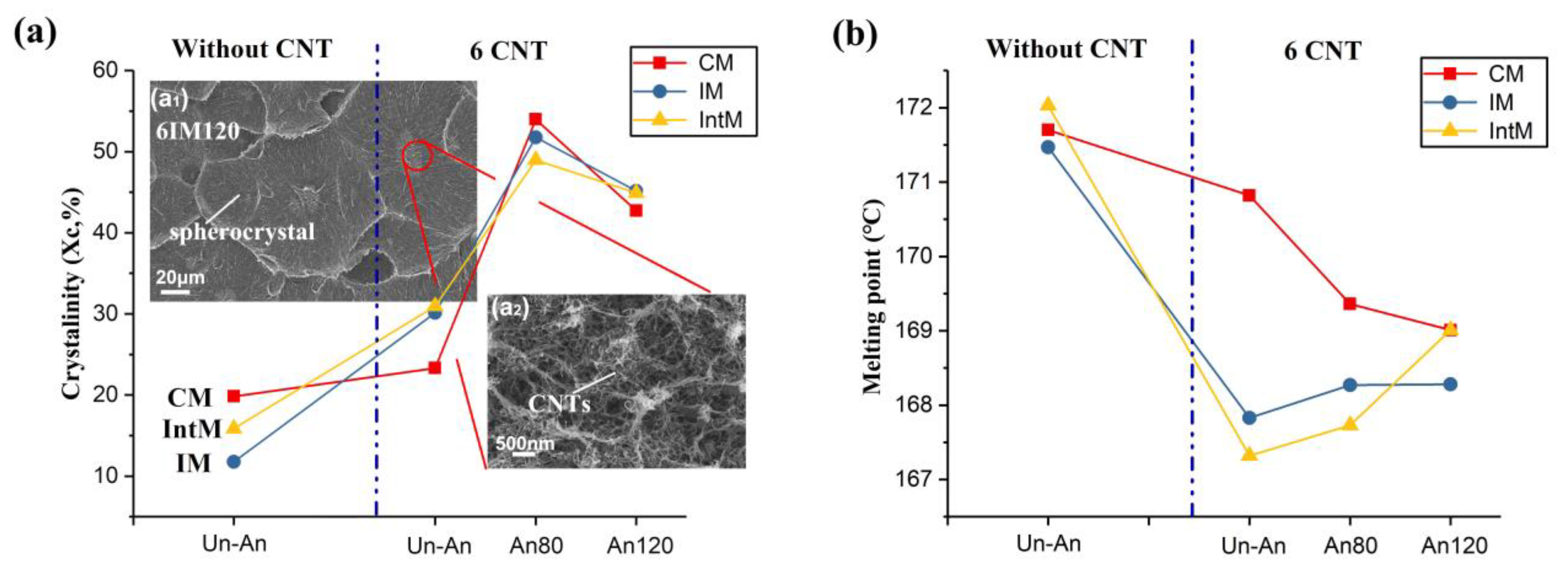
3.1.2. Crystallinity and Thermal Properties
3.1.3. CNT Agglomerates Orientation
3.2. Yield and Electrical Properties
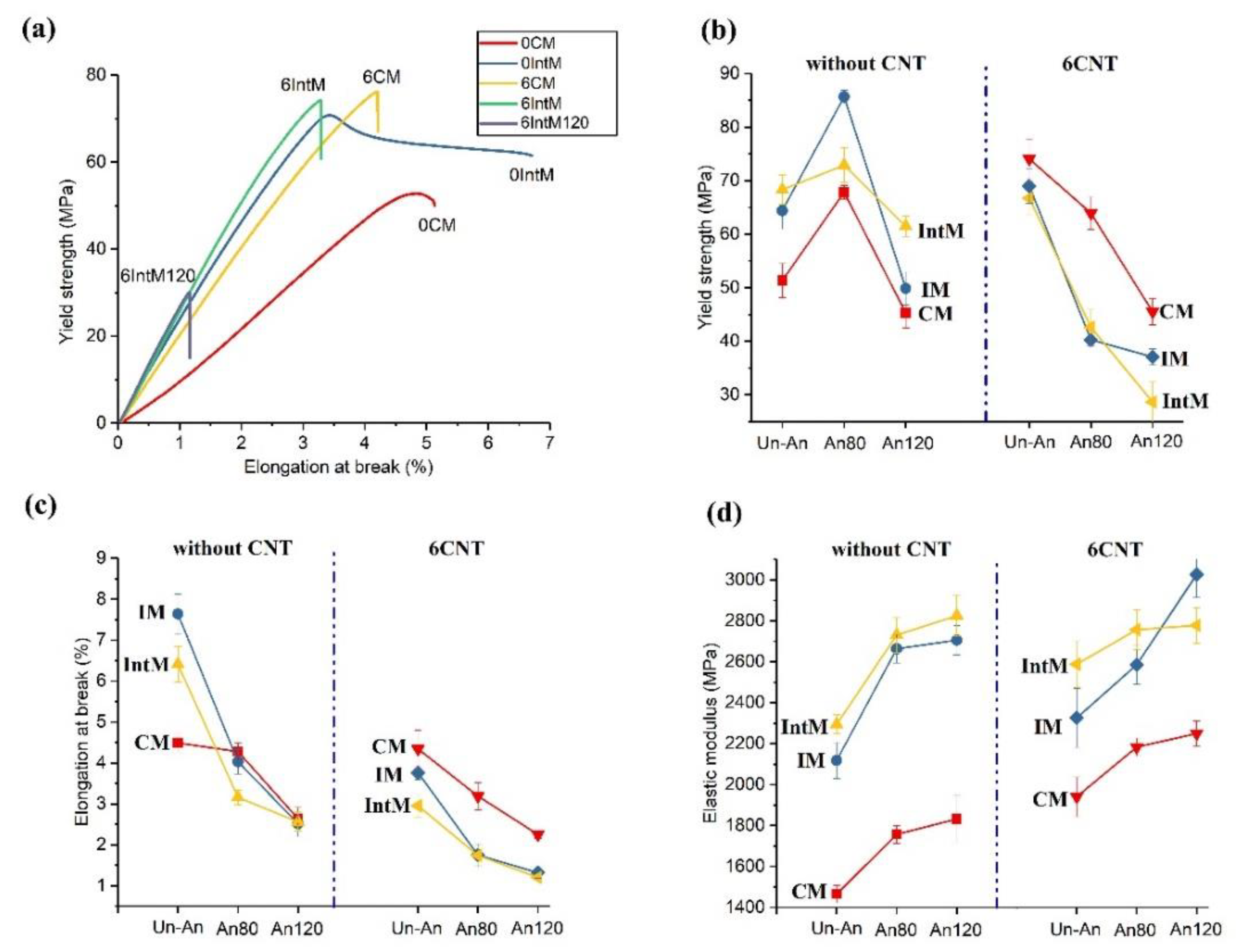
3.2.1. Yield Properties
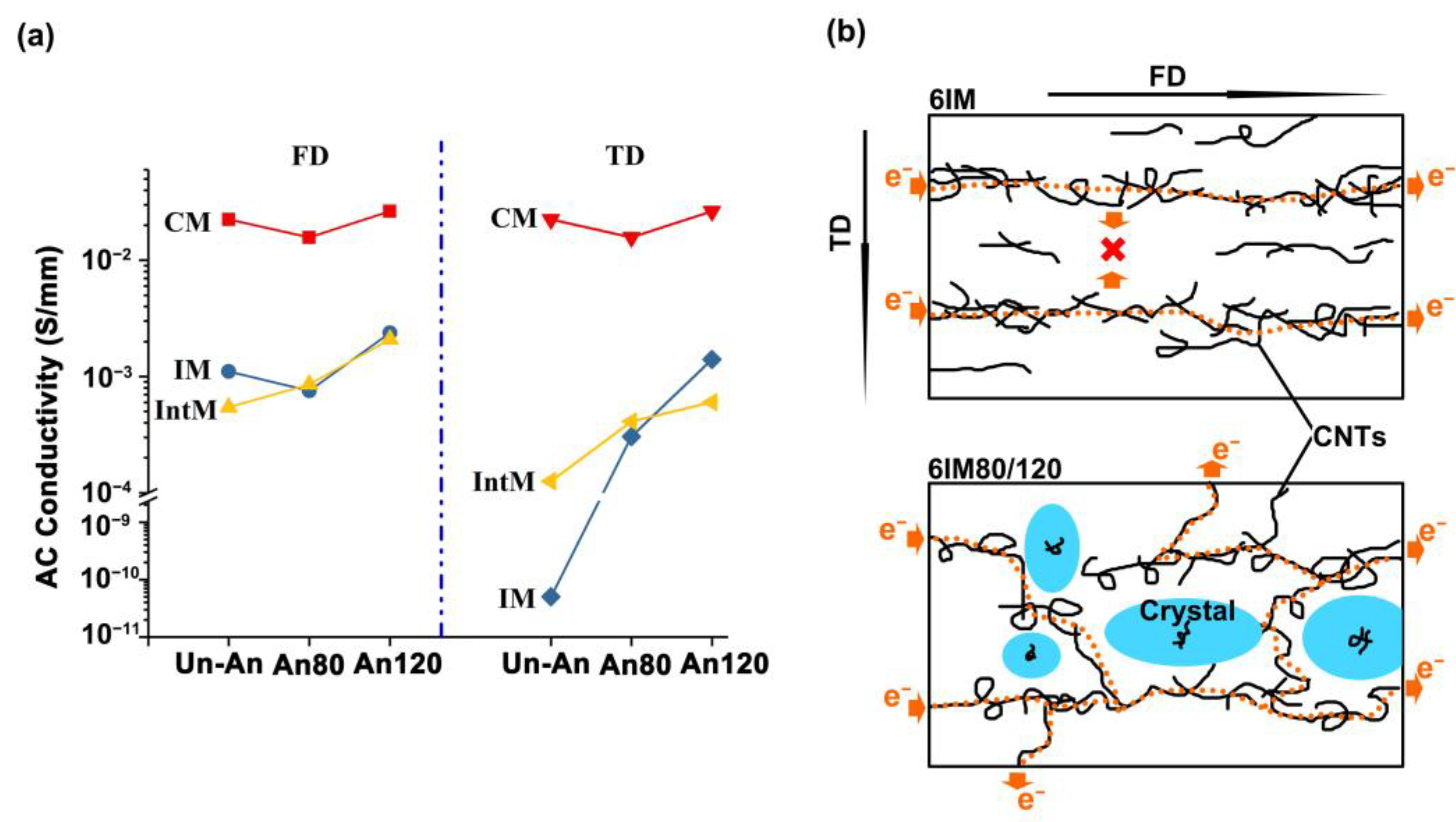
3.2.2. Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chai, J.; Wang, G.; Zhao, J.; Zhang, A.; Shi, Z.; Wei, C.; Zhao, G. Microcellular PLA/PMMA foam fabricated by CO2 foaming with outstanding shape-memory performance. J. CO2 Util. 2021, 49, 101553. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Li, B.; Wan, G.; Zhao, G.; Zhang, A. Strong and thermal-resistance glass fiber-reinforced polylactic acid (PLA) composites enabled by heat treatment. Int. J. Biol. Macromol. 2019, 129, 448–459. [Google Scholar] [CrossRef]
- Komal, U.K.; Lila, M.K.; Singh, I. PLA/banana fiber based sustainable biocomposites: A manufacturing perspective. Compos. Part B Eng. 2020, 180, 107535. [Google Scholar] [CrossRef]
- Gonçalves, C.; Gonçalves, I.C.; Magalhães, F.D.; Pinto, A.M. Poly (lactic acid) composites containing carbon-based nanomaterials: A review. Polymers 2017, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, J.; Jiang, T.; Zhang, X.; Xia, L.; Wu, H.; Guo, S.; Zhang, X. Extensional flow-induced hybrid crystalline fibrils (shish) in CNT/PLA nanocomposite. Carbon 2018, 129, 720–729. [Google Scholar] [CrossRef]
- Refaa, Z.; Boutaous, M.H.; Xin, S.; Fulchiron, R. Synergistic effects of shear flow and nucleating agents on the crystallization mechanisms of Poly (Lactic Acid). J. Polym. Res. 2017, 24, 18. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Zhan, H.; Wang, J.N. New processing method to fabricate high-performance carbon-nanotube/polyvinyl alcohol composite films. Carbon 2016, 110, 490–496. [Google Scholar] [CrossRef]
- Song, K.; Zhang, Y.; Meng, J.; Green, E.C.; Tajaddod, N.; Li, H.; Minus, M.L.J.M. Structural polymer-based carbon nanotube composite fibers: Understanding the processing–structure–performance relationship. Materials 2013, 6, 2543–2577. [Google Scholar] [CrossRef]
- Munir, M.U.; Mayer-Gall, T.; Gutmann, J.S.; Ali, W.; Etemad-Parishanzadeh, O.; Khanzada, H.; Mikučioniene, D. Development of Carbon-Nanodot-Loaded PLA Nanofibers and Study of Their Barrier Performance for Medical Applications. Nanomaterials 2023, 13, 1195. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H. Synergistic effect of clay platelets and carbon nanotubes in styrene–butadiene rubber nanocomposites. Macromol. Chem. Phys. 2016, 217, 2617–2625. [Google Scholar] [CrossRef]
- Szatkowski, P.; Czechowski, L.; Gralewski, J.; Szatkowska, M. Mechanical Properties of Polylactide Admixed with Carbon Nanotubes or Graphene Nanopowder. Materials 2021, 14, 5955. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, O.M.; Benelfellah, A.; Bikiaris, D.N.; Ait Hocine, N. Effect of rigid nanoparticles and preparation techniques on the performances of poly (lactic acid) nanocomposites: A review. Polym. Adv. Technol. 2021, 32, 444–460. [Google Scholar] [CrossRef]
- Minus, M.L.; Chae, H.G.; Kumar, S. Single wall carbon nanotube templated oriented crystallization of poly (vinyl alcohol). Polymer 2006, 47, 3705–3710. [Google Scholar] [CrossRef]
- Jerschow, P.; Janeschitz-Kriegl, H. The role of long molecules and nucleating agents in shear induced crystallization of isotactic polypropylenes. Int. Polym. Process. 1997, 12, 72–77. [Google Scholar] [CrossRef]
- Mei, J.; Lei, X.; Liang, M.; Wu, H.; Zhou, S.; Zou, H.; Chen, Y. Comparative study on the electrical, thermal, and mechanical properties of multiwalled carbon nanotubes filled polypropylene and polyamide 6 micromoldings. J. Appl. Polym. Sci. 2021, 138, 49984. [Google Scholar] [CrossRef]
- Naudy, S.; David, L.; Rochas, C.; Fulchiron, R. Shear induced crystallization of poly (m-xylylene adipamide) with and without nucleating additives. Polymer 2007, 48, 3273–3285. [Google Scholar] [CrossRef]
- Tang, H.; Chen, J.-B.; Wang, Y.; Xu, J.-Z.; Hsiao, B.S.; Zhong, G.-J.; Li, Z.-M. Shear flow and carbon nanotubes synergistically induced nonisothermal crystallization of poly (lactic acid) and its application in injection molding. Biomacromolecules 2012, 13, 3858–3867. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, S.J.; Qiao, J.L.; Zhang, L.Y. The electrical properties and crystallization of stereocomplex poly (lactic acid) filled with carbon nanotubes. Polymer 2012, 53, 4547–4552. [Google Scholar] [CrossRef]
- Lu, K.; Grossiord, N.; Koning, C.E.; Miltner, H.E.; Mele, B.V.; Loos, J. Carbon nanotube/isotactic polypropylene composites prepared by latex technology: Morphology analysis of CNT-induced nucleation. Macromolecules 2008, 41, 8081–8085. [Google Scholar] [CrossRef]
- Laird, E.D.; Li, C.Y. Structure and morphology control in crystalline polymer–carbon nanotube nanocomposites. Macromolecules 2013, 46, 2877–2891. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Petrova-Doycheva, I.; Menseidov, D.; Ivanov, E.; Paddubskaya, A.; Kuzhir, P. Exploring thermal annealing and graphene-carbon nanotube additives to enhance crystallinity, thermal, electrical and tensile properties of aged poly (lactic) acid-based filament for 3D printing. Compos. Sci. Technol. 2019, 181, 107712. [Google Scholar] [CrossRef]
- Tábi, T.; Sajó, I.; Szabó, F.; Luyt, A.; Kovács, J. Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym. Lett. 2010, 4, 659–668. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.; Maspoch, M.L. Processing of poly (lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Mi, D.; Zhao, Z.; Jia, S. Further improved mechanical properties of polypropylene by shish-kebab structure and high-temperature annealing. Polym. Eng. Sci. 2022, 62, 3349–3362. [Google Scholar] [CrossRef]
- Mi, D.; Zhou, M.; Hou, F.; Zhang, J. Effect of high-temperature annealing on the microstructure and mechanical properties of polypropylene with shish kebab or spherulite structure. J. Appl. Polym. Sci. 2018, 135, 46465. [Google Scholar] [CrossRef]
- Chen, B.; Yang, H.; Shen, K.; Fu, Q.; Gao, X. The effect of high-temperature annealing on thermal properties and morphology of polyethylene pipes prepared by rotational shear. Polymer 2020, 204, 122770. [Google Scholar] [CrossRef]
- Du, H.; Zhang, Y.; Liu, H.; Liu, K.; Jin, M.; Li, X.; Zhang, J. Influence of phase morphology and crystalline structure on the toughness of rubber-toughened isotatic polypropylene blends. Polymer 2014, 55, 5001–5012. [Google Scholar] [CrossRef]
- Ru, J.-F.; Yang, S.-G.; Zhou, D.; Yin, H.-M.; Lei, J.; Li, Z.-M. Dominant β-form of poly (l-lactic acid) obtained directly from melt under shear and pressure fields. Macromolecules 2016, 49, 3826–3837. [Google Scholar] [CrossRef]
- Fischer, E.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Ishibe, T.; Kaneko, T.; Uematsu, Y.; Sato-Akaba, H.; Komura, M.; Iyoda, T.; Nakamura, Y. Tunable Thermal Switch via Order–Order Transition in Liquid Crystalline Block Copolymer. Nano Lett. 2022, 22, 6105–6111. [Google Scholar] [CrossRef] [PubMed]
- Downes, R.D.; Hao, A.; Park, J.G.; Su, Y.-F.; Liang, R.; Jensen, B.D.; Siochi, E.J.; Wise, K.E. Geometrically constrained self-assembly and crystal packing of flattened and aligned carbon nanotubes. Carbon 2015, 93, 953–966. [Google Scholar] [CrossRef]
- Schulz, S.; Faiella, G.; Buschhorn, S.T.; Prado, L.A.S.d.A.; Giordano, M.; Schulte, K.; Bauhofer, W. Combined electrical and rheological properties of shear induced multiwall carbon nanotube agglomerates in epoxy suspensions. Eur. Polym. J. 2011, 47, 2069–2077. [Google Scholar] [CrossRef]
- Ameli, A.; Kazemi, Y.; Wang, S.; Park, C.; Pötschke, P. Manufacturing, Process-microstructure-electrical conductivity relationships in injection-molded polypropylene/carbon nanotube nanocomposite foams. Compos. Part A Appl. Sci. 2017, 96, 28–36. [Google Scholar] [CrossRef]
- Wang, J.; Kazemi, Y.; Wang, S.; Hamidinejad, M.; Mahmud, M.B.; Pötschke, P.; Park, C.B. Enhancing the electrical conductivity of PP/CNT nanocomposites through crystal-induced volume exclusion effect with a slow cooling rate. Compos. Part B Eng. 2020, 183, 107663. [Google Scholar] [CrossRef]
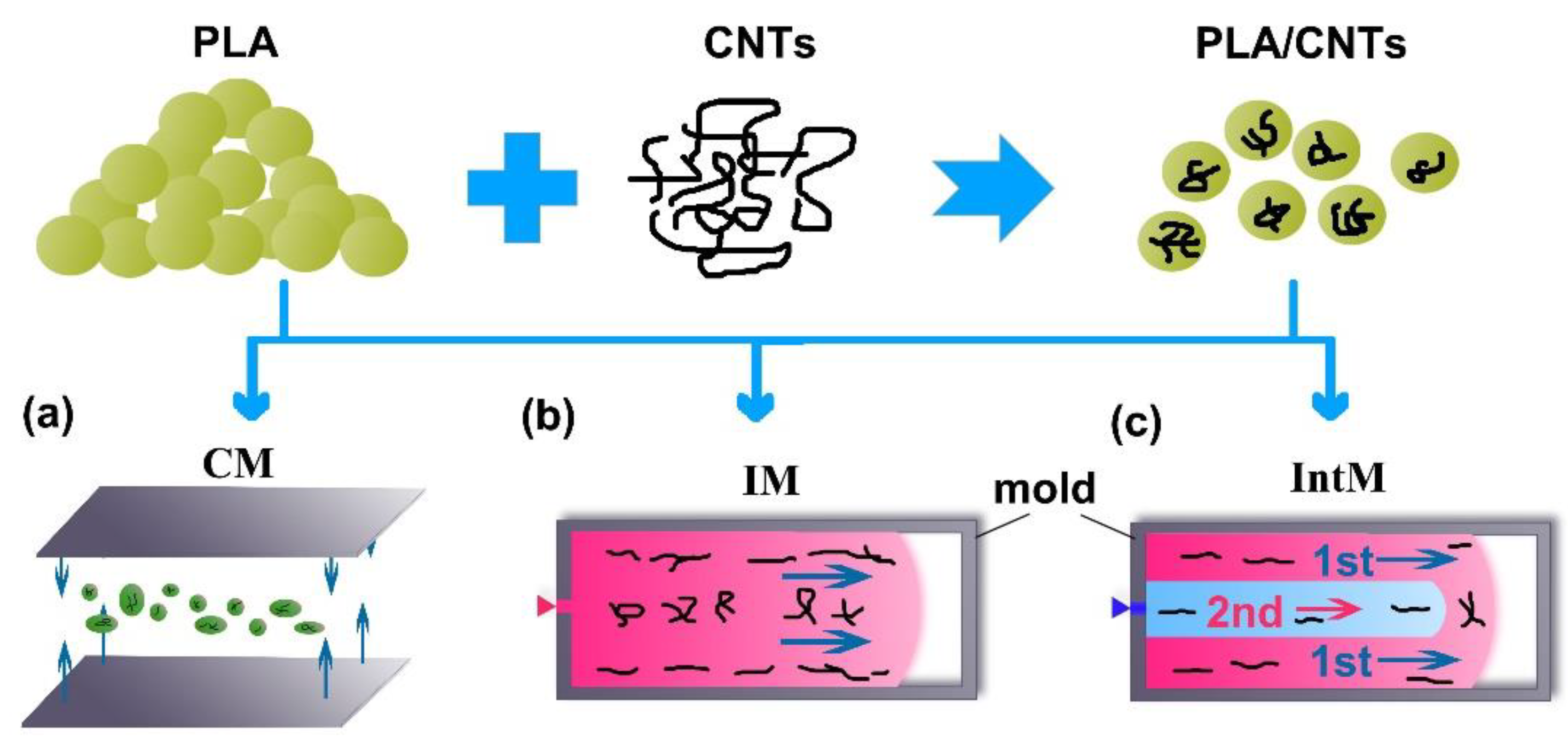




| Examples | PLA | CNT | CM/IM/IntM | UN-An/An80/An120 |
|---|---|---|---|---|
| 0CM | 100 wt.% | 0 | CM | UN-An |
| 6IntM80 | 94 wt.% | 6 wt.% | IntM | An80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, D.; Zhao, Z.; Bai, H. Improved Yield and Electrical Properties of Poly(Lactic Acid)/Carbon Nanotube Composites by Shear and Anneal. Materials 2023, 16, 4012. https://doi.org/10.3390/ma16114012
Mi D, Zhao Z, Bai H. Improved Yield and Electrical Properties of Poly(Lactic Acid)/Carbon Nanotube Composites by Shear and Anneal. Materials. 2023; 16(11):4012. https://doi.org/10.3390/ma16114012
Chicago/Turabian StyleMi, Dashan, Zhongguo Zhao, and Haiqing Bai. 2023. "Improved Yield and Electrical Properties of Poly(Lactic Acid)/Carbon Nanotube Composites by Shear and Anneal" Materials 16, no. 11: 4012. https://doi.org/10.3390/ma16114012
APA StyleMi, D., Zhao, Z., & Bai, H. (2023). Improved Yield and Electrical Properties of Poly(Lactic Acid)/Carbon Nanotube Composites by Shear and Anneal. Materials, 16(11), 4012. https://doi.org/10.3390/ma16114012






