The Preparations of Fluorographene Nanosheets and Research in Tribological Properties in High Vacuum
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Fluorinated Graphene
2.2. Dispersion of Fluorinated Graphene in IL and Preparation of a Composite Lubricating Film
2.3. Testing Methods
3. Results
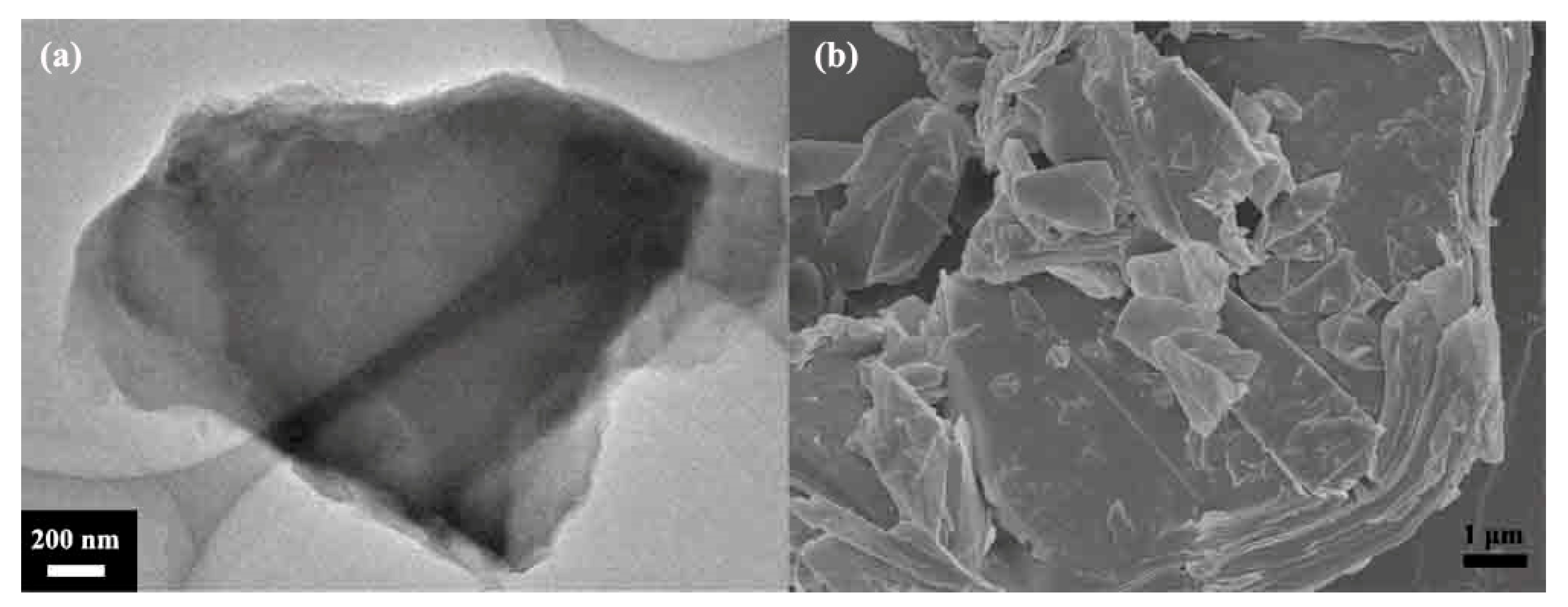
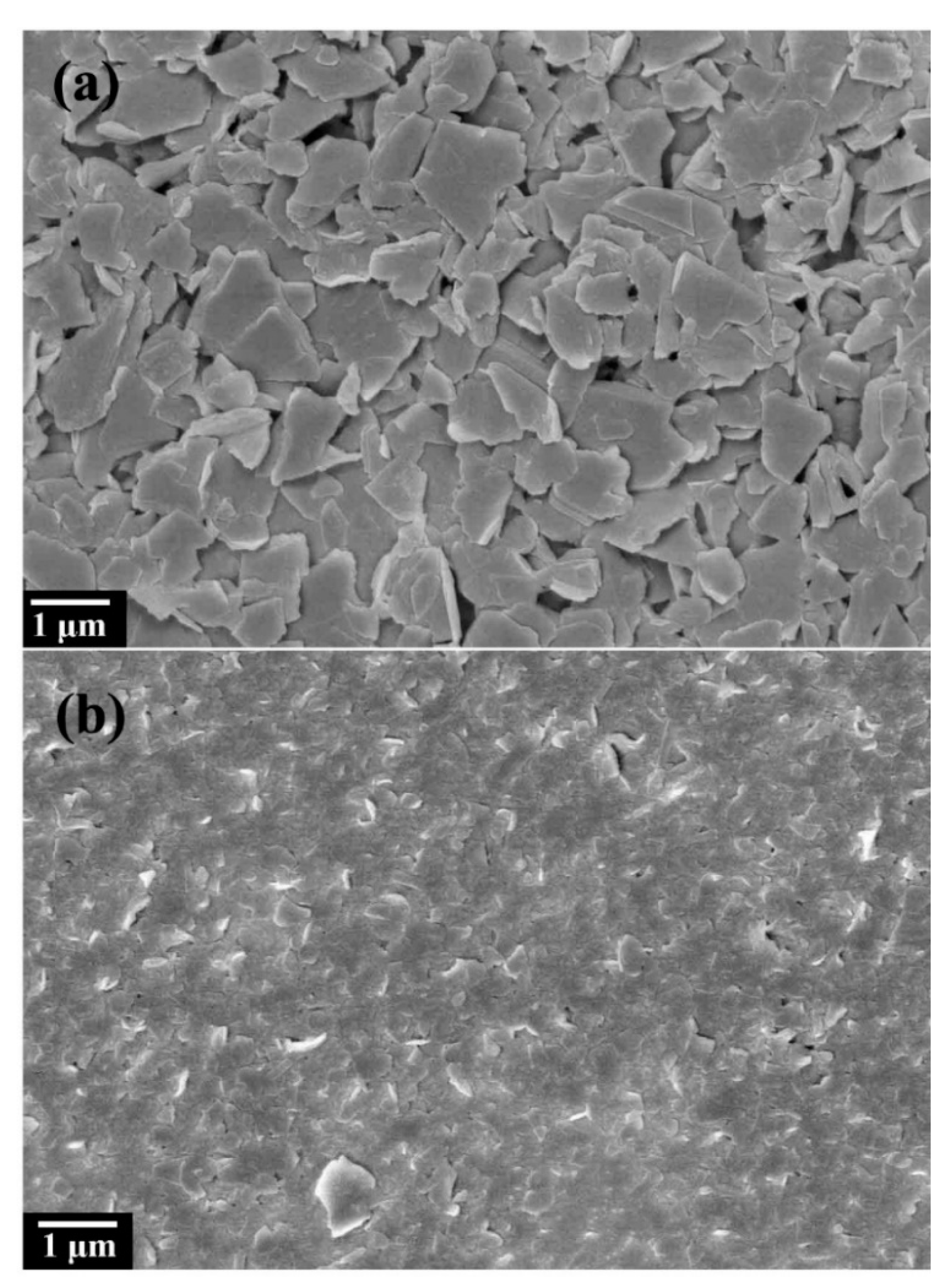
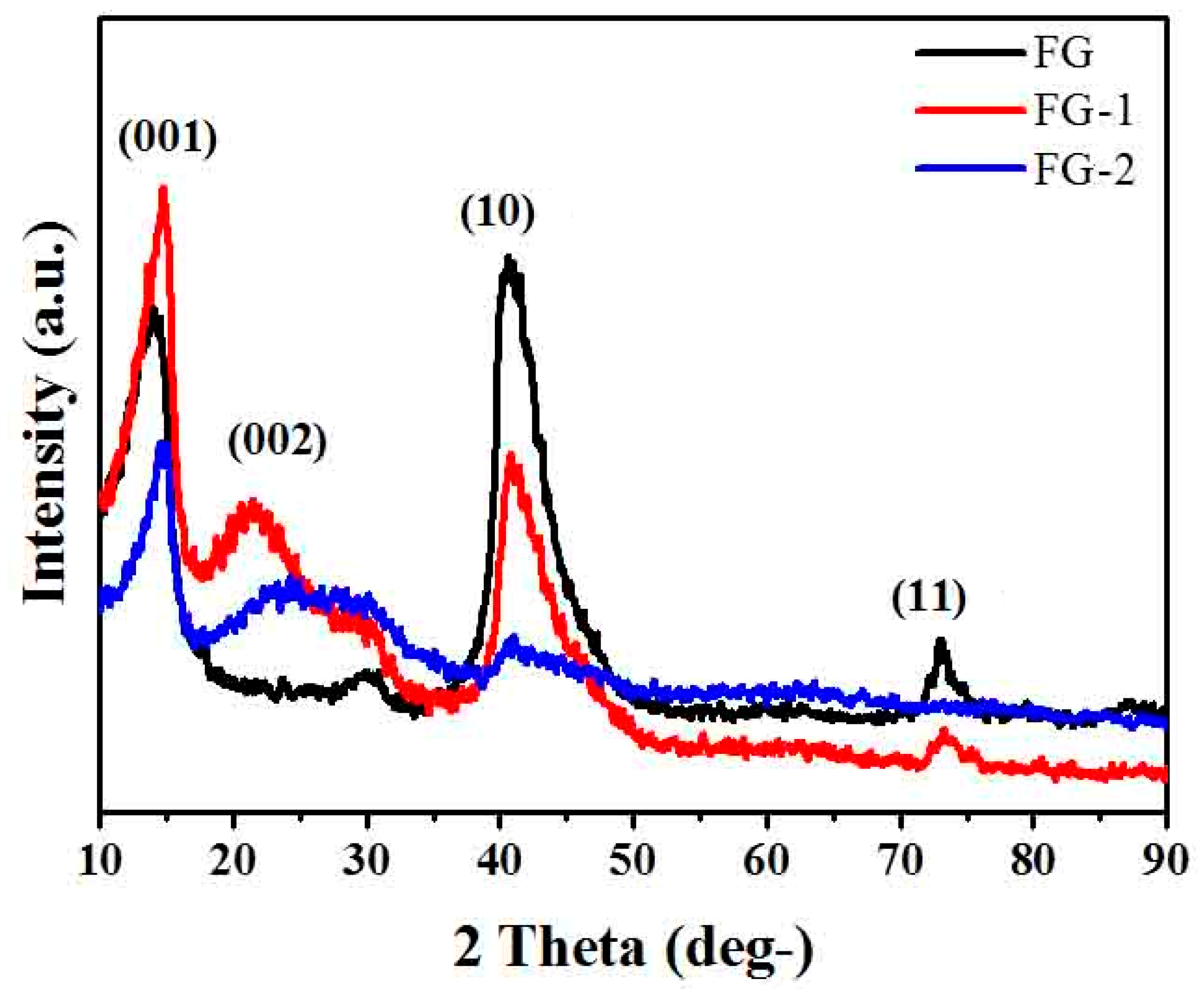
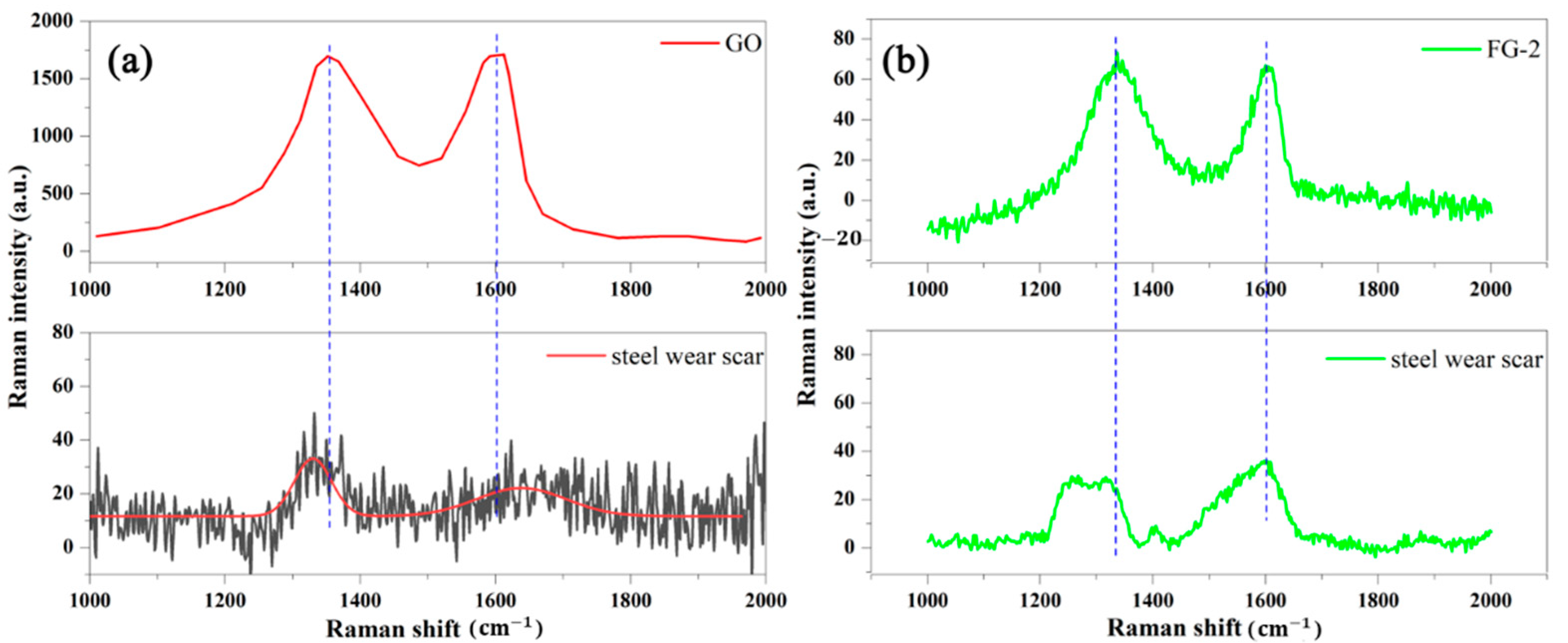
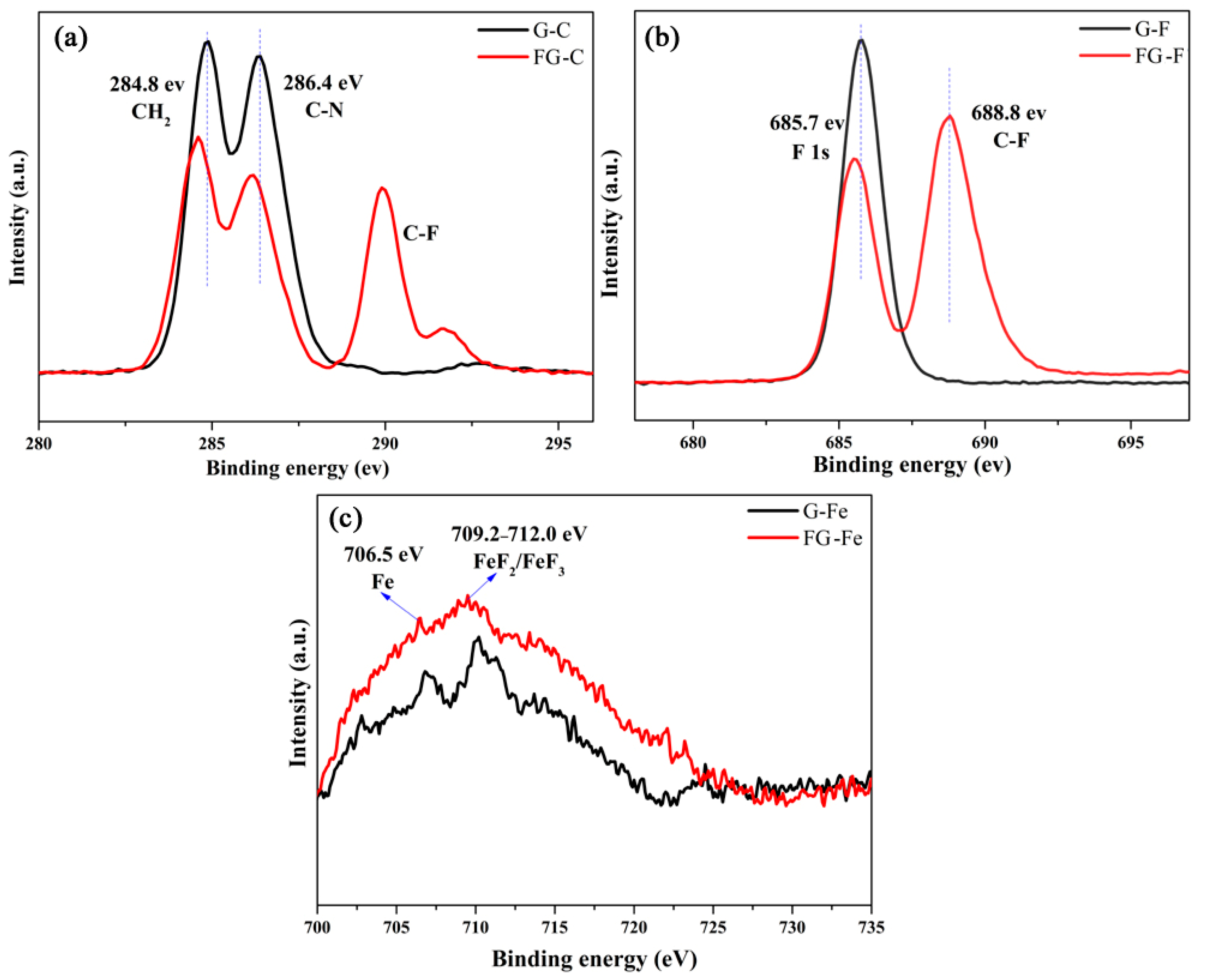
3.1. Structural Analysis of Fluorinated Graphene
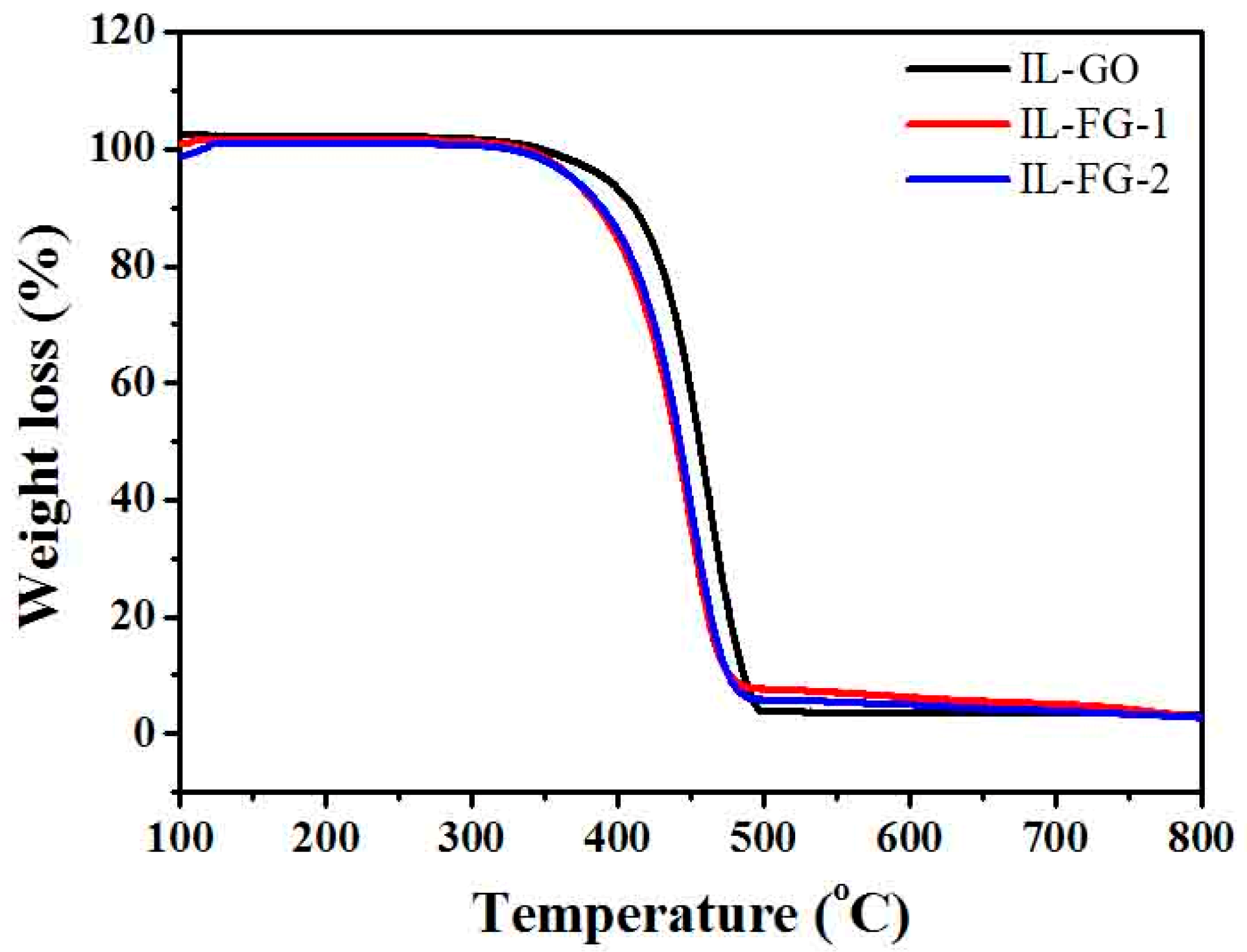
3.2. Thermal Stability of IL-GO, IL-FG-1, and IL-FG-2
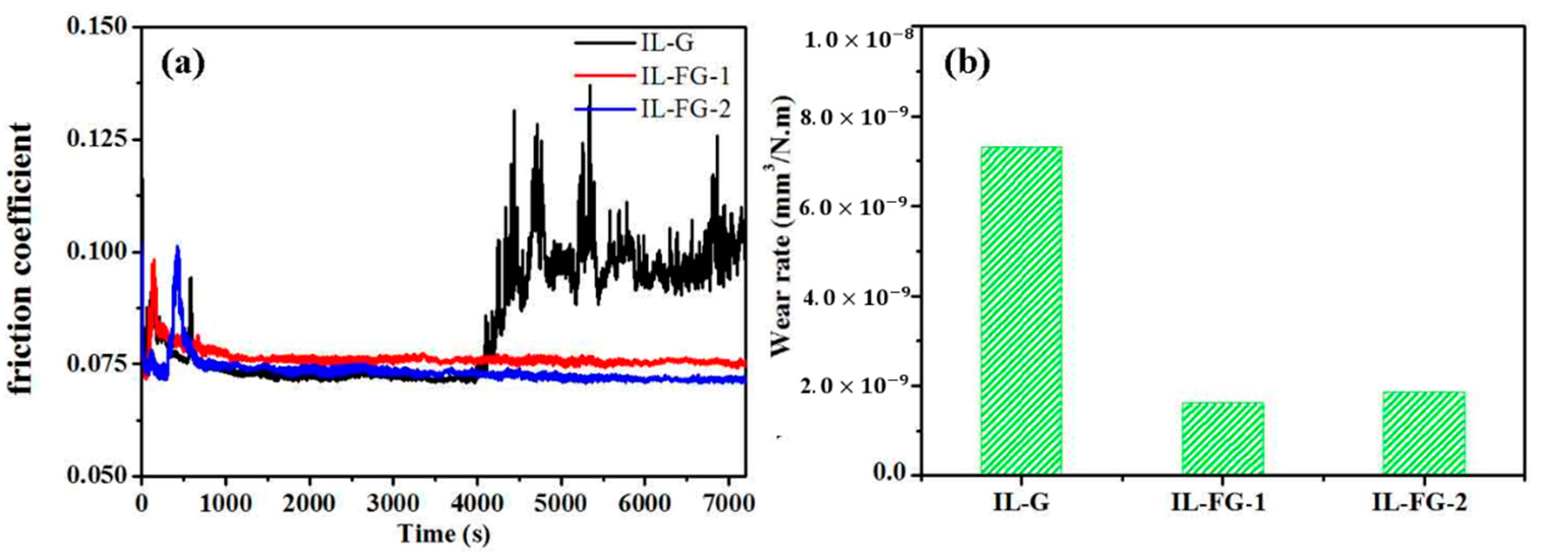
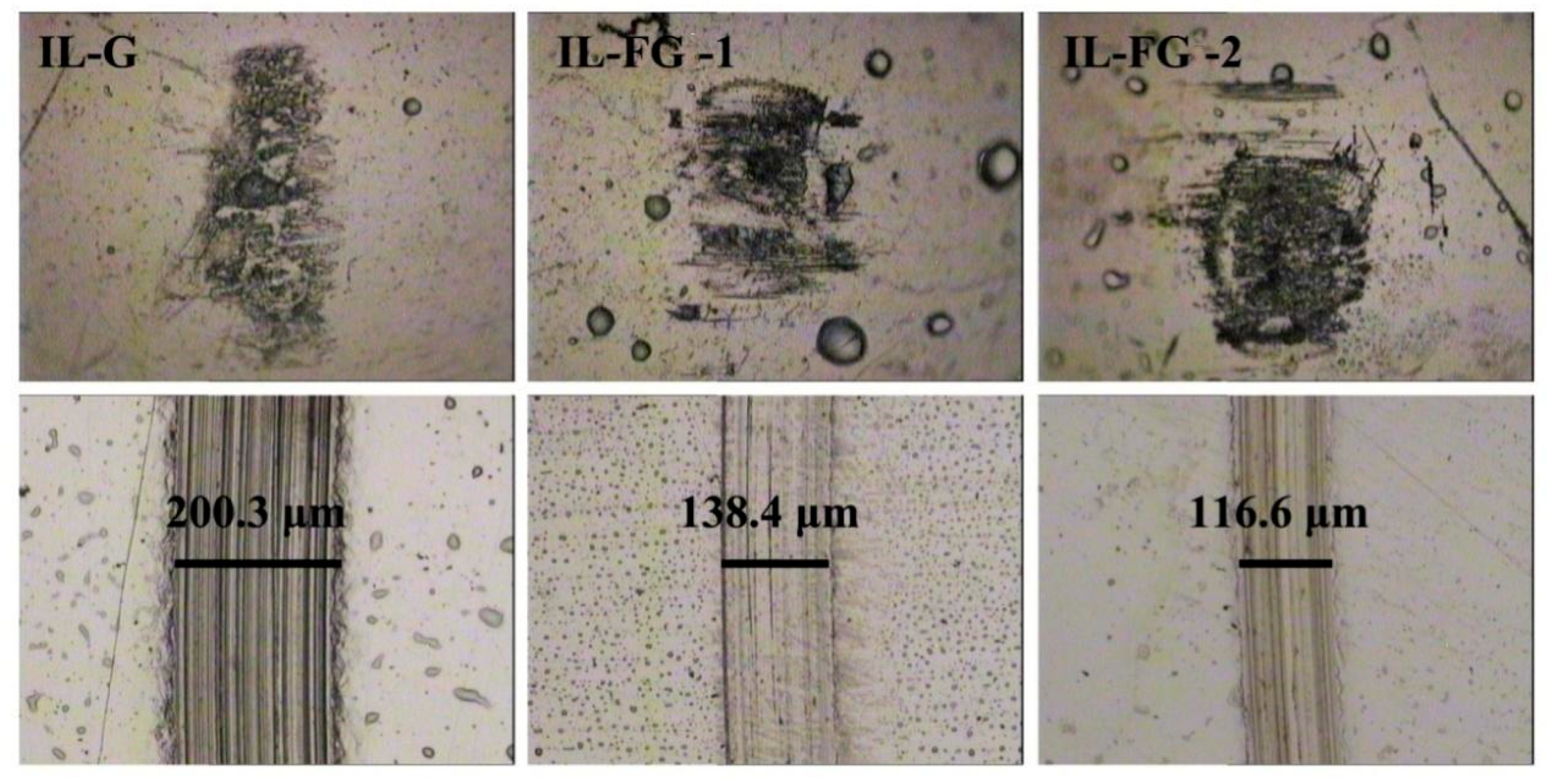
3.3. Tribological Properties
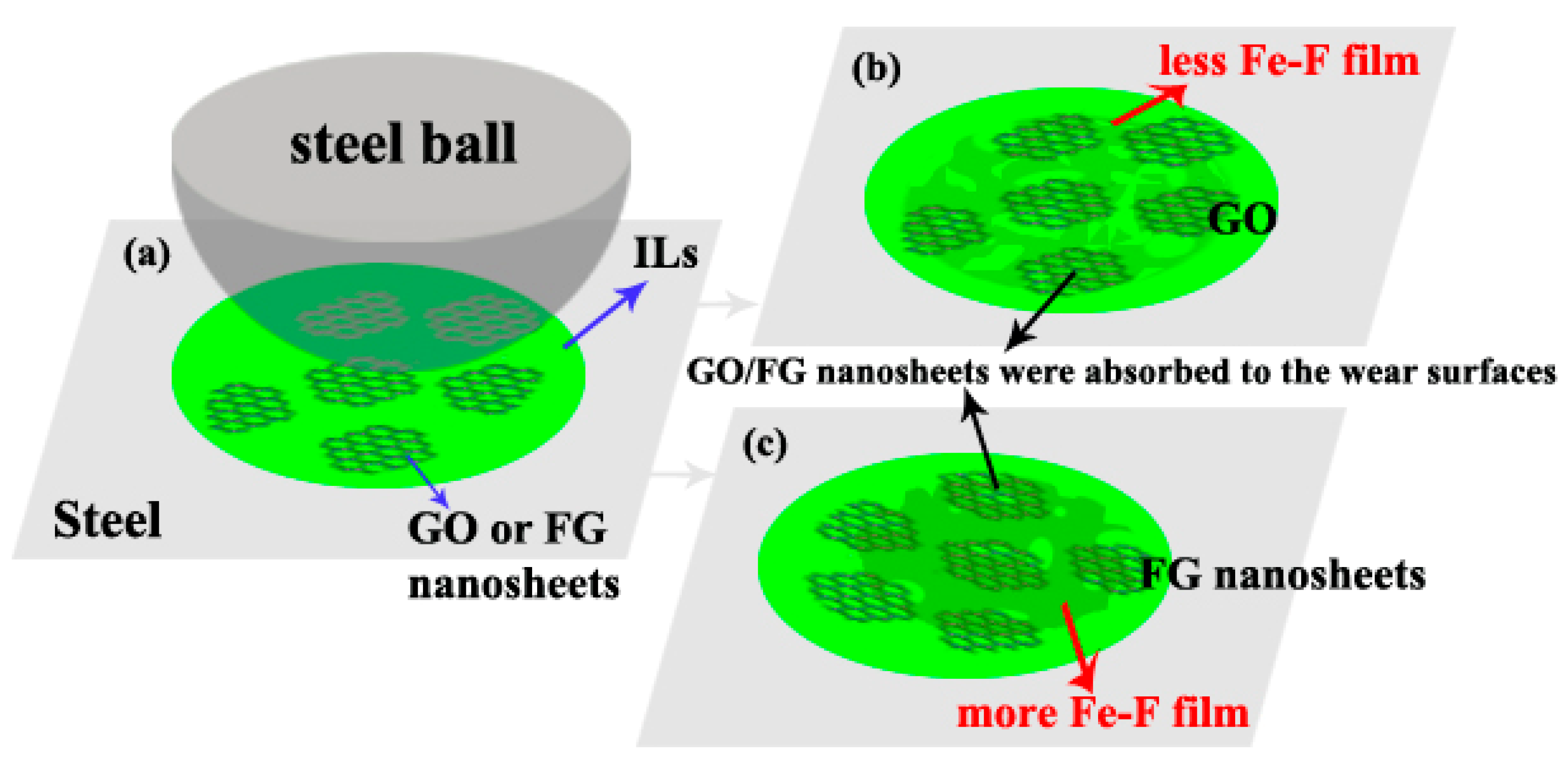
3.4. Related Wear Mechanisms
4. Conclusions
- Using fluorinated graphite as the raw material, fluorinated graphene was successfully prepared by liquid-phase ultrasonic exfoliation. This preparation method is simple, and fluorinated graphene of different sheet sizes can be obtained under different centrifugal separation speeds.
- The tribological properties of FG nanosheets were studied. Compared with IL-G, IL-FG nanosheets have better tribological properties for steel/steel friction pairs; during the friction process, FG nanosheets are adsorbed on the interface of steel/steel friction pairs to form a protective layer to avoid friction. During the friction process, the FG-2 nanosheets with smaller lamellae can be deposited on the microscopic defect area of the worn surface under the action of compressive stress, thus playing a good role in repairing the worn surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Huang, Y.Y.; He, Y.Y.; Shi, Y.J. Nanolubricant additives: A review. Friction 2021, 9, 891–917. [Google Scholar] [CrossRef]
- Zeng, Q. Superlow friction and diffusion behaviors of a steel-related system in the presence of nano lubricant additive in PFPE oil. J. Adhes. Sci. Technol. 2019, 33, 1001–1018. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.L.; Sun, W.D.; Guo, F. Effects of Nano-TiO2 additives on the film-forming properties of low viscosity oil. Lubr. Eng. 2022, 47, 69–75. [Google Scholar]
- Jia, X.; Huang, J.; Li, Y.; Yang, J.; Song, H. Monodisperse Cu nanoparticles @MoS2 nanosheets as a lubricant additive for improved tribological properties. Appl. Surf. Sci. 2019, 494, 430–439. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Guo, F.; Sheng, H.; Xia, K.; Liu, W.; Wen, J.; Shi, Y.; Erdemir, A.; Luo, J. Catalytically active oil-based lubricant additives enabled by calcining Ni-Al layered double hydroxides. J. Phys. Chem. Lett. 2020, 11, 113–120. [Google Scholar] [CrossRef]
- Rengifo, S.; Zhang, C.; Harimkar, S.; Boesl, B.; Agarwal, A. Effect of WS2 addition on tribological behavior of aluminum at room and elevated temperatures. Tribol. Lett. 2017, 65, 76. [Google Scholar] [CrossRef]
- Song, W.; Yan, J.C.; Ji, H.B. Fabrication of GNS/MoS2 composite with different morphology and its tribological performance as a lubricant additive. Appl. Surf. Sci. 2019, 469, 226–235. [Google Scholar] [CrossRef]
- Kumar, N.; Saini, V.; Bijwe, J. Performance properties of lithium greases with PTFE particles as additive: Controlling parameter-size or shape? Tribol. Int. 2020, 148, 106302. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, J.; Wang, L.; Xue, Q. Synergistic effect of hybrid carbon nanotube-graphene oxide as nanoadditive enhancing the frictional Properties of Ionic Liquids in High Vacuum. ACS Appl. Mater. Interfaces 2015, 7, 8592. [Google Scholar] [CrossRef]
- Pu, J.; Zhang, L.; Wang, L. Frictional dependence of graphene and carbon nanotube in diamond-like carbon/ionic liquids hybrid films in vacuum. Carbon 2014, 80, 734–745. [Google Scholar]
- Zhao, J.; Li, Y.; He, Y.; Luo, J. In situ green synthesis of the new sandwichlike nanostructure of Mn3O4/Graphene as lubricant additives. ACS Appl. Mater. Interfaces 2019, 11, 36931–36938. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Chen, G.; Zhao, J.; He, Y.; Luo, J. An investigation on the tribological behaviors of steel/copper and steel/steel friction pairs via lubrication with a graphene additive. Friction 2021, 9, 228–238. [Google Scholar] [CrossRef]
- Xie, H.; Jiang, B.; Dai, J.; Peng, C.; Li, C.; Li, Q.; Pan, F. Tribological behaviors of graphene and graphene oxide as water-based lubricant additives for magnesium alloy/steel contacts. Materials 2018, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xia, Y.F.; Song, H.J.; Chen, B.; Zhang, Z. Synthesis of the liquid-like graphene with excellent tribological properties. Tribol. Int. 2017, 105, 118–124. [Google Scholar] [CrossRef]
- Wang, L.; Gong, P.W.; Li, W.; Luo, T.; Cao, B. Mono-dispersed Ag/Graphene nanocomposite as lubricant additive to reduce friction and wear. Tribol. Int. 2020, 146, 106228. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.; Song, H.; Chen, B.; Zhang, Z. In situ graphene formation induced by tribochemical reaction for sustainable lubrication. ACS Sustain. Chem. Eng. 2023, 11, 2238–2248. [Google Scholar]
- Luo, J.; Xu, S.H.; Xiang, S. Study on dispersion and tribological properties of fluorinated graphene. Contemp. Chem. Ind. 2020, 49, 2472–2476. [Google Scholar]
- Zeng, X.; Wang, Y.; Wu, X.; Ren, T.; He, Z. Alkyloxy-s-Triazine, derivatives with and without fluorine-containing substituents as lubricants for steel−steel contact. J. Synth. Lubr. 2006, 23, 1–10. [Google Scholar] [CrossRef]
- Nair, R.R.; Ren, W.; Jalil, R.; Riaz, I.; Kravets, V.G.; Britnell, L.; Blake, P.; Schedin, F.; Mayorov, A.S.; Yuan, S.; et al. Fluorographene: A two-dimensional counterpart of teflon. Small 2015, 6, 2877–2884. [Google Scholar] [CrossRef]
- Narayanan, T.N.; Biroju, R.K.; Renugopalakrishnan, V. Fluorographene: Synthesis and sensing applications. J. Mater. Res. 2017, 32, 2848–2859. [Google Scholar] [CrossRef]
- Jeon, K.-J.; Lee, Z.; Pollak, E.; Moreschini, L.; Bostwick, A.; Park, C.-M.; Mendelsberg, R.; Radmilovic, V.; Kostecki, R.; Richardson, T.J.; et al. Fluorographene: A wide bandgap semiconductor with ultraviolet luminescence. ACS Nano 2011, 5, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Burgess, J.S.; Junkermeier, C.E.; Badescu, S.C.; Reinecke, T.L.; Perkins, F.K.; Zalalutdniov, M.K.; Baldwin, J.W.; Culbertson, J.C.; Sheehan, P.E.; et al. Properties of fluorinated Graphene films. Nano Lett. 2010, 10, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.S.; Zeng, X. Tribological behavior of nanocarbon materials with different dimensions in aqueous systems. Friction 2020, 1, 8. [Google Scholar] [CrossRef]
- Min, C.Y.; He, Z.B.; Song, H.J. Fluorinated graphene oxide nanosheet: A highly efficient water-based lubricated additive. Tribol. Int. 2019, 140, 105867–105876. [Google Scholar] [CrossRef]
- Zheng, S.Z.; Zhou, Q.; Yang, S.R.; Yang, Z.G.; Wang, J.Q. Preparation and tribological properties of fluorinated graphene nanosheets as additive in lubricating oil. Tribology 2017, 37, 402–408. [Google Scholar]
- Ci, X.; Zhao, W.; Luo, J.; Wu, Y.; Ge, T.; Xue, Q.; Gao, X.; Fang, Z. How the fluorographene replaced graphene as nanoadditive for improving tribological performances of GTL-8 based lubricant oil. Friction 2021, 9, 488–501. [Google Scholar] [CrossRef]
- Ye, X.; Ma, L.; Yang, Z.; Wang, J.; Wang, H.; Yang, S. Covalent functionalization of fluorinated Graphene and subsequent application as water-based lubricant additive. ACS Appl. Mater. Interfaces 2016, 7483. [Google Scholar] [CrossRef]
- Fan, K.; Liu, X.; Liu, Y.; Li, Y.; Chen, Y.; Meng, Y.; Liu, X.; Feng, W.; Luo, L. Covalent functionalization of fluorinated graphene through activation of dormant radicals for water-based lubricants-Science Direct. Carbon 2020, 167, 826–834. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of graphene’s properties by reversible hydrogenation: Evidence for graphane. Am. Assoc. Adv. Sci. 2009, 5914. [Google Scholar] [CrossRef]
- Chronopoulos, D.D.; Bakandritsos, A.; Pykal, M.; Zbořil, R.; Otyepka, M. Chemistry, properties, and applications of fluorographene. Appl. Mater. Today 2017, 9, 60–70. [Google Scholar] [CrossRef]
- Zbořil, R.; Karlický, F.; Bourlinos, A.B.; Steriotis, T.A.; Stubos, A.K.; Georgakilas, V.; Šafářová, K.; Jančík, D.; Trapalis, C.; Otyepka, M. Graphene Fluoride: A stable stoichiometric graphene derivative and its chemical conversion to graphene. Small 2010, 6, 2885–2891. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Yang, S.; Wang, Y.; Wang, Y.; Ling, L.; Feng, X. Fabrication of fully fluorinated Graphene nanosheets towards high-performance lithium storage. Adv. Mater. Interfaces 2014, 1, 274–276. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, G.; Chen, L.; Pan, S.; Jing, X.; Wang, J.; Han, S. Enhanced hydrogen storage performances of LiBH4 modified with three dimensional porous fluorinated graphene. J. Hydrogen Energy 2015, 17, 18185–18192. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, Y.H.; Che, J.F. Preparation and application of fluorographene. New Chem. Mater. 2017, 45, 4–6. [Google Scholar]
- Lei, F.; Yang, M.; Jiang, F. Preparation and liquid phase exfoliation of graphite fluoride towards graphene fluoroxide. RSC Adv. 2013, 3, 21869–21876. [Google Scholar]
- Lei, F.; Yang, M.; Jiang, F.; Zhang, H.; Zhang, Z.; Sun, D. Microwave-assisted liquid phase exfoliation of graphite fluoride into fluorographene. Chem. Eng. J. 2019, 360, 673–679. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Sun, W.; Wang, L.D.; Li, S.; Zhu, T.; Liu, G. Liquid–phase exfoliated fluorographene as a two dimensional coating filler for enhanced corrosion protection performance. Corros. Sci. 2016, 103, 312–318. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Zou, K.; Okino, F.; Gutierrez, H.R.; Gupta, A.; Shen, N.; Eklund, P.C.; Sofo, J.O.; Zhu, J. Reversible fluorination of graphene: Evidence of a two- dimensional wide bandgap semiconductor. Phys. Rev. B 2010, 81, 205435. [Google Scholar] [CrossRef]
- Chang, H.; Cheng, J.; Liu, X.; Gao, J.; Li, M.; Li, J.; Tao, X.; Ding, F.; Zheng, Z. Facile synthesis of wide-bandgap fluorinated graphene semiconductors. Chem. Eur. J. 2011, 17, 8896–8903. [Google Scholar] [CrossRef]
- Bordes, E.; Szala-Bilnik, J.; Padua, A. Exfoliation of Graphene and fluorographene in molecular and ionic liquids. Faraday Discuss. 2018, 206, 61–75. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, G.; Li, Y.; Liu, Z.; Huang, X. One-step preparation of fluorographene: A highly efficient, low-cost, and large-scale approach of exfoliating fluorographite. ACS Appl. Mater. Interfaces 2013, 5, 13478–13483. [Google Scholar] [CrossRef]
- Gong, P.; Wang, Z.; Wang, J.; Wang, H.; Li, Z.; Fan, Z.; Xu, Y.; Han, X.; Yang, S. One-pot sonochemical preparation of fluorographene and selective tuning of its fluorine coverage. J. Mater. Chem. 2012, 22, 16950–16956. [Google Scholar] [CrossRef]
- Khan, U.; Porwal, H.; O’neill, A.; Nawaz, K.; May, P.; Coleman, J.N. Solvent-exfoliated graphene at extremely high concentration. Langmuir 2011, 27, 9077–9082. [Google Scholar] [CrossRef]
- Biscoe, J.; Warren, B.E. An X-ray study of carbon black. J. Appl. Phys. 1942, 13, 364–371. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Liu, S.H.; Wang, Y. Exploring the influence of the type of anion in imidazolium ionic liquids on its thermal stability. J. Therm. Anal. Calorim. 2023, 1–11. [Google Scholar] [CrossRef]
- Belmonte, M.; Ramírez, C.; González-Julián, J.; Schneider, J.; Miranzo, P.; Osendi, M.I. The beneficial effect of graphene nanofillers on the tribological performance of ceramics. Carbon 2013, 61, 431–435. [Google Scholar] [CrossRef]
- Murray, P.T.; Dyhouse, V.J.; Grzulis, L. Physics and chemistry of materials with layered structures. Mater. Res. Soc. Symp. Proc. 1991, 201, 513–516.ss. [Google Scholar] [CrossRef]


| Materials | Manufacturer | Purity |
|---|---|---|
| ionic liquids (IL [BMIM]BF4) | State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics (Lanzhou, China) | 97% |
| graphene oxide (GO) | Nanjing XFNANO Materials Tech Co., Ltd. (Nanjing, China) | ~99% |
| fluorinated graphite (FG) | Shanghai Fubang Chemical Co., Ltd., http://carfluor.company.lookchem.cn | 99.99% |
| N-methyl pyrrolidone (NMP) | Tianjin Chemical Reagent Co., Ltd. | analytical grade 99.7% |
| acetone | Tianjin Chemical Reagent Co., Ltd. | analytical grade 99.7% |
| ethanol | Tianjin Chemical Reagent Co., Ltd. | analytical grade 99.7% |
| C (at.%) | N (at.%) | F (at.%) | Si (at.%) | Cr (at.%) | Fe (at.%) | Ni (at.%) | |
|---|---|---|---|---|---|---|---|
| IL-G | 59.66 | 11.14 | 12.14 | 0.11 | 3.34 | 12.40 | 1.21 |
| IL-FG | 60.04 | 13.63 | 21.41 | 0.04 | 3.47 | 12.49 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, Z.; Gao, X.; Matlan, S.J.; Taha, N.A. The Preparations of Fluorographene Nanosheets and Research in Tribological Properties in High Vacuum. Materials 2023, 16, 3929. https://doi.org/10.3390/ma16113929
Zhang L, Zhang Z, Gao X, Matlan SJ, Taha NA. The Preparations of Fluorographene Nanosheets and Research in Tribological Properties in High Vacuum. Materials. 2023; 16(11):3929. https://doi.org/10.3390/ma16113929
Chicago/Turabian StyleZhang, Lili, Zhengrui Zhang, Xi’an Gao, Siti Jahara Matlan, and Nazaruddin Abd Taha. 2023. "The Preparations of Fluorographene Nanosheets and Research in Tribological Properties in High Vacuum" Materials 16, no. 11: 3929. https://doi.org/10.3390/ma16113929
APA StyleZhang, L., Zhang, Z., Gao, X., Matlan, S. J., & Taha, N. A. (2023). The Preparations of Fluorographene Nanosheets and Research in Tribological Properties in High Vacuum. Materials, 16(11), 3929. https://doi.org/10.3390/ma16113929






