Effect of Non-Isothermal Aging on the Mechanical Properties and Corrosion Resistance of 2A12 Aluminum Alloy
Abstract
1. Introduction
2. Materials and Methods
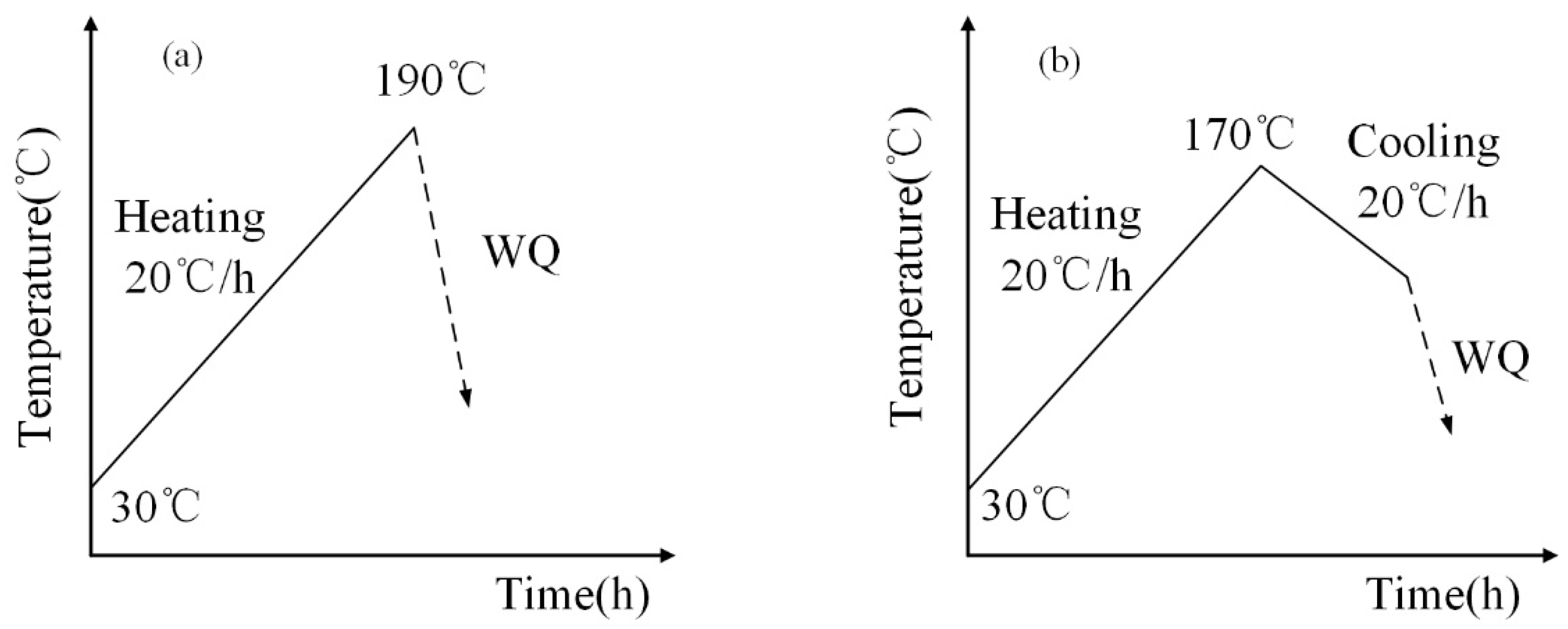
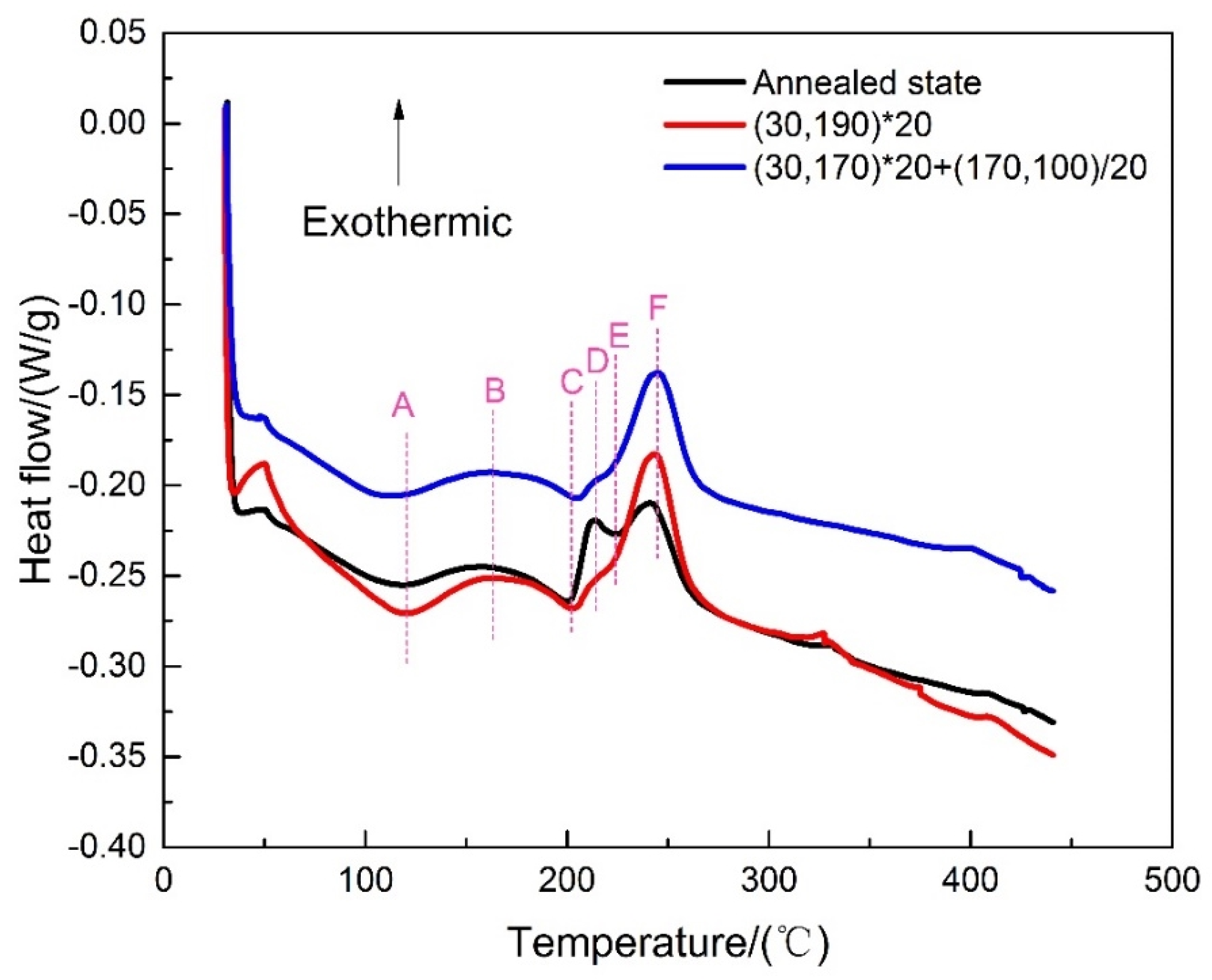
2.1. Thermal Property Analysis
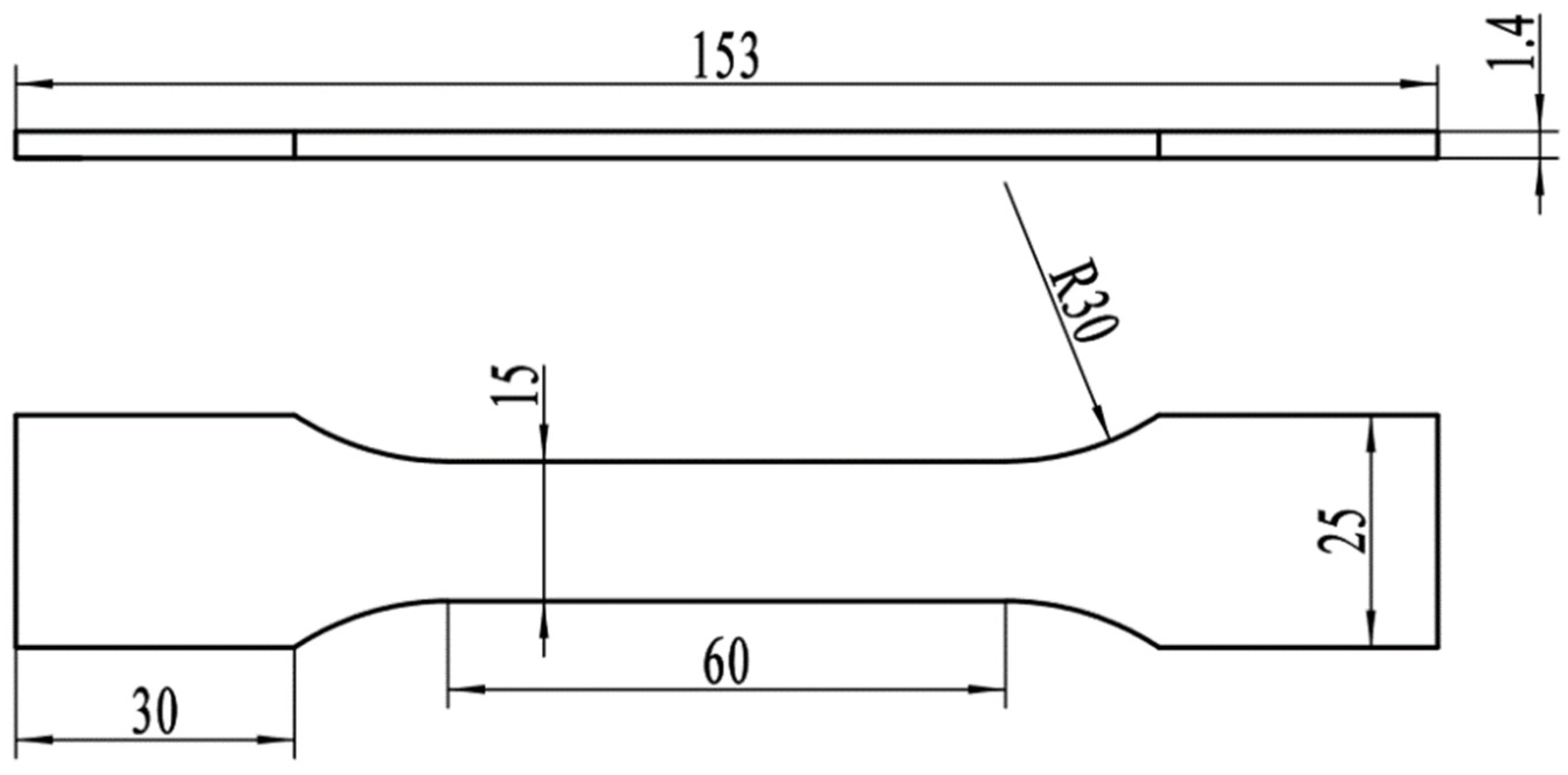
2.2. Tensile Test and Microhardness Analysis
2.3. Corrosion Test
3. Results and Discussion
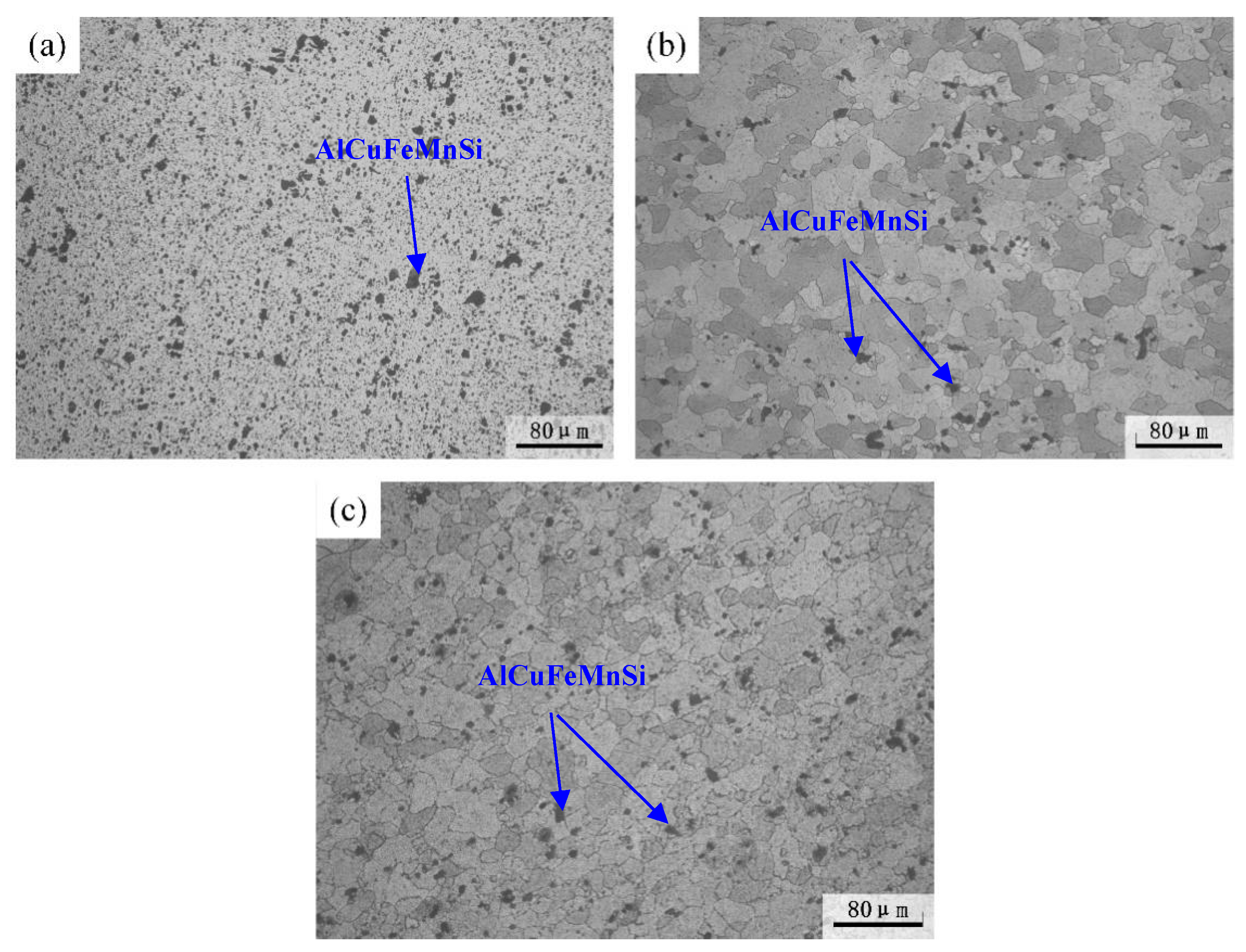
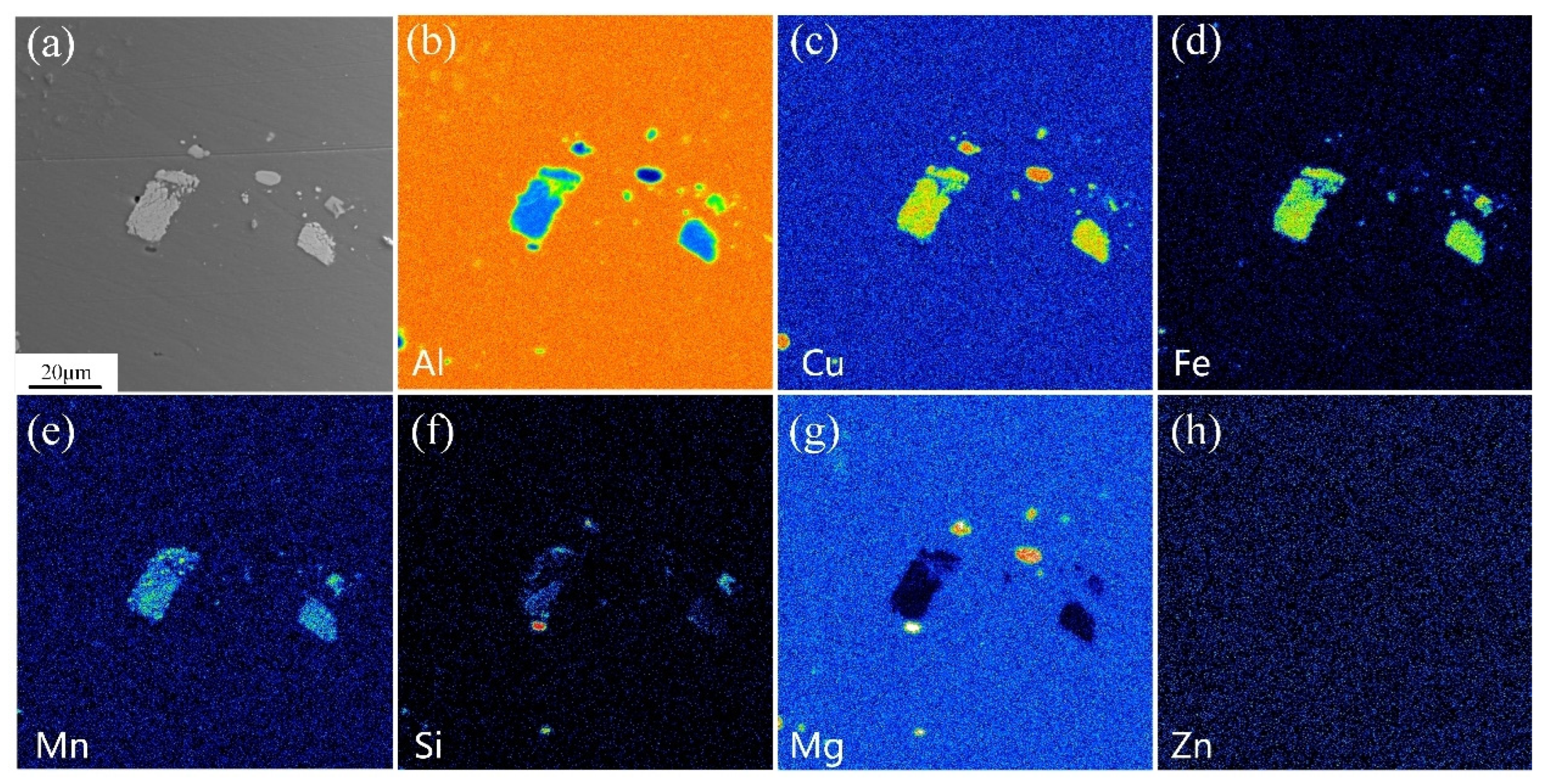
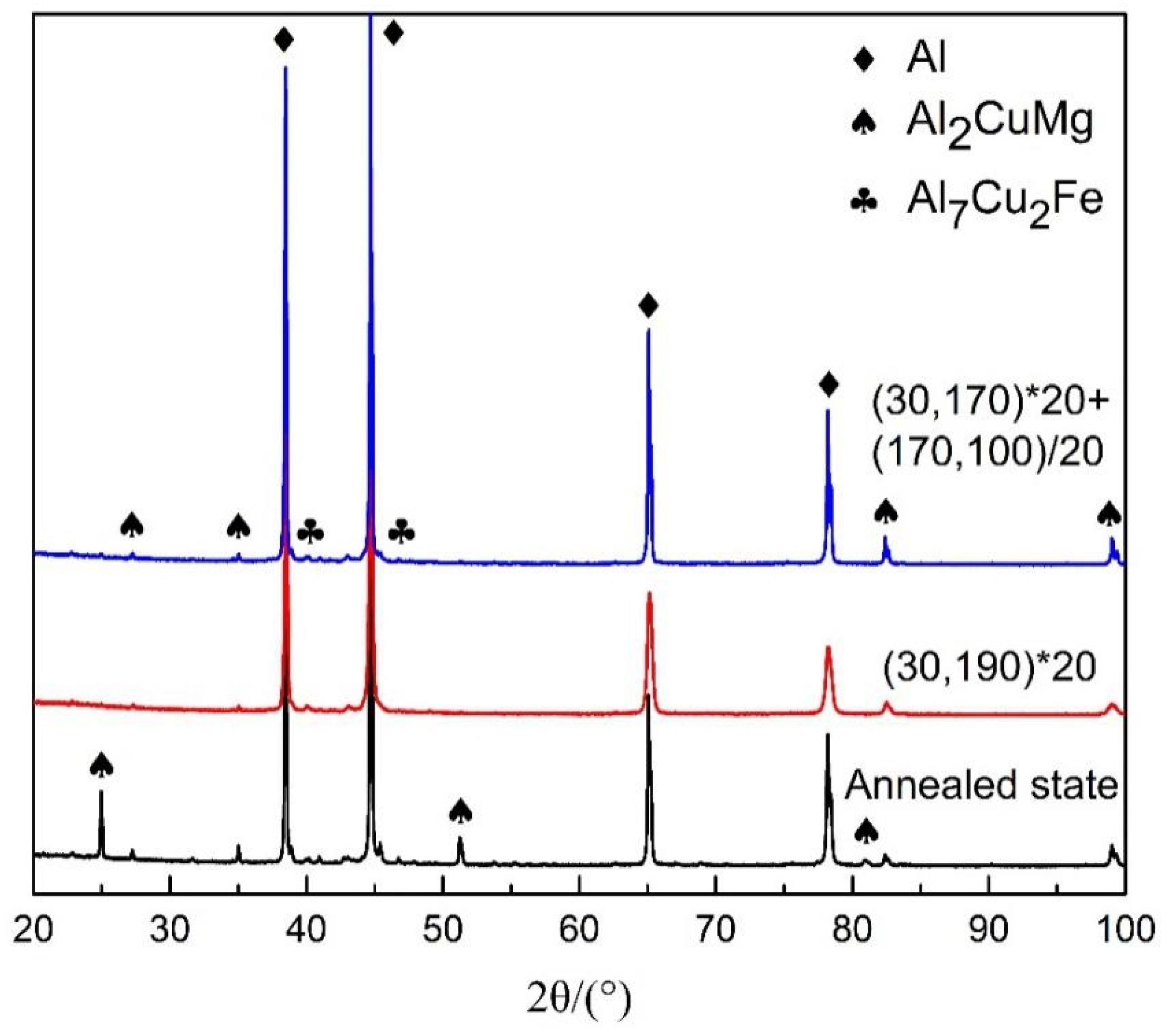
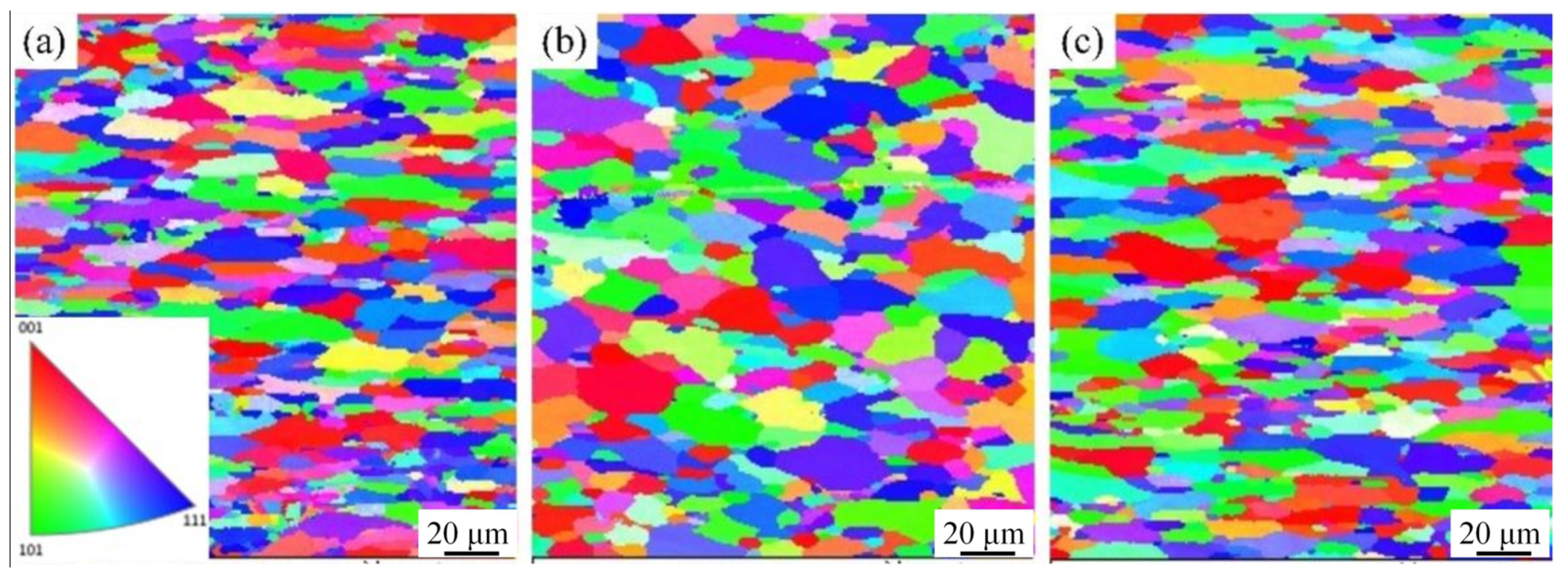
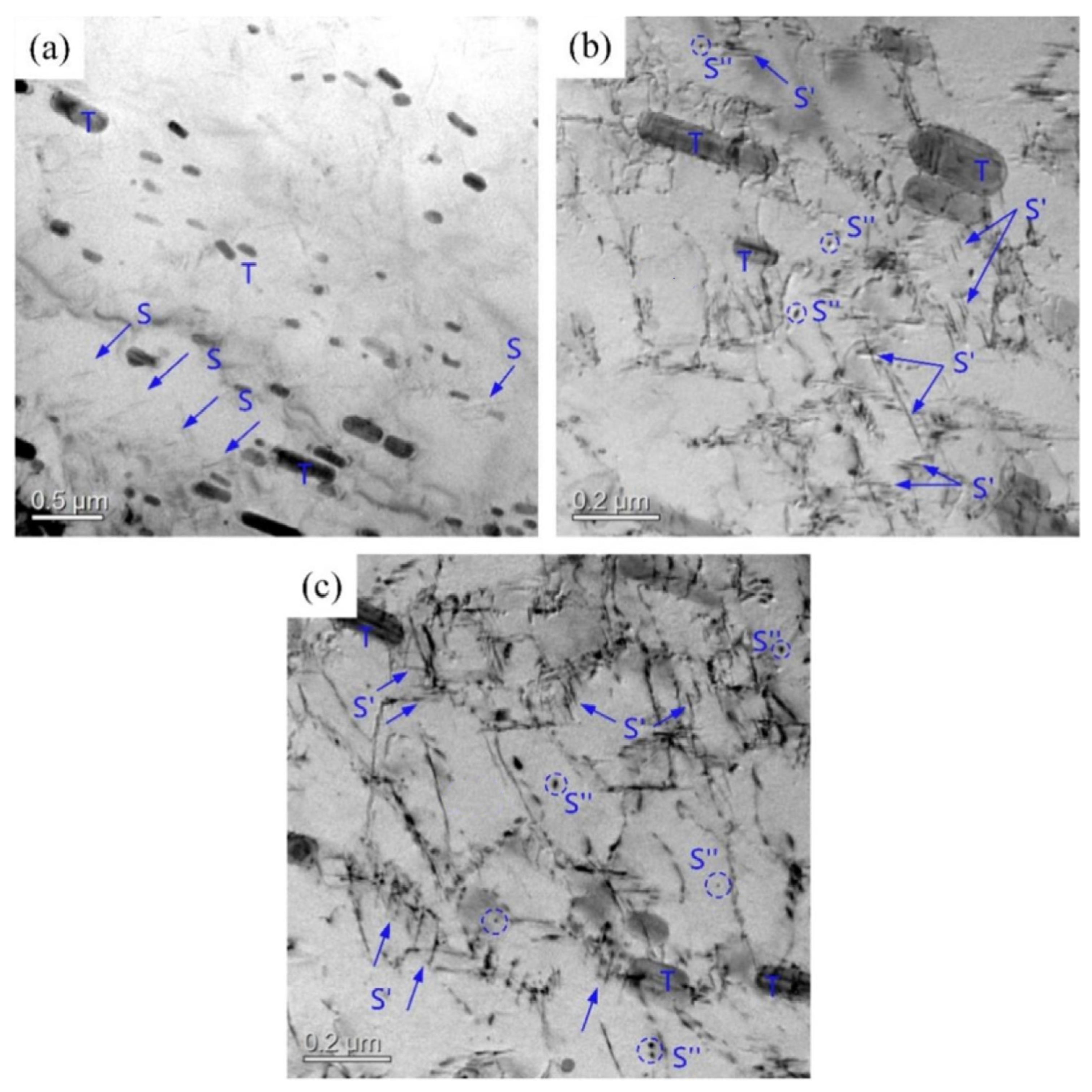
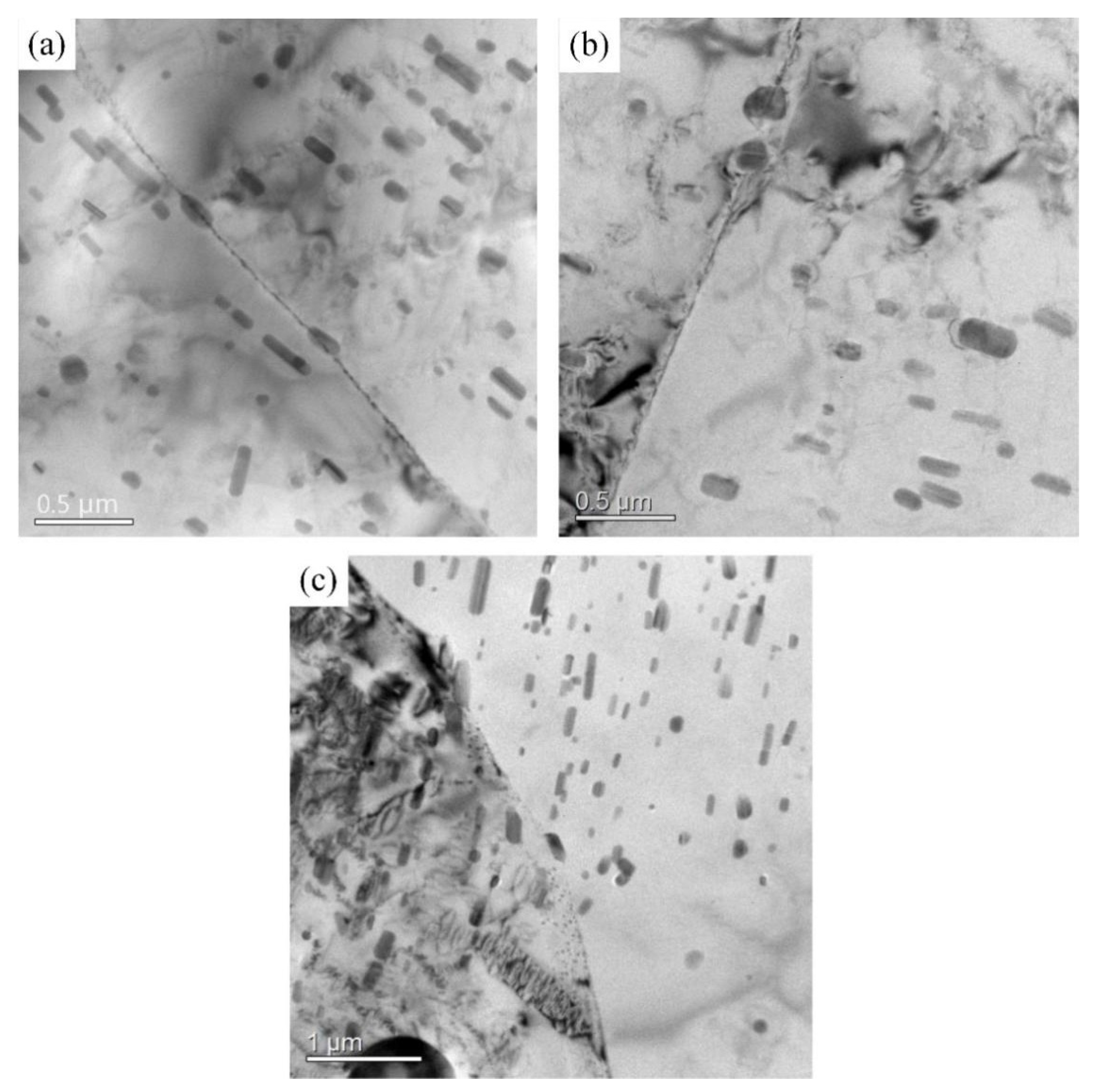
3.1. Microstructure
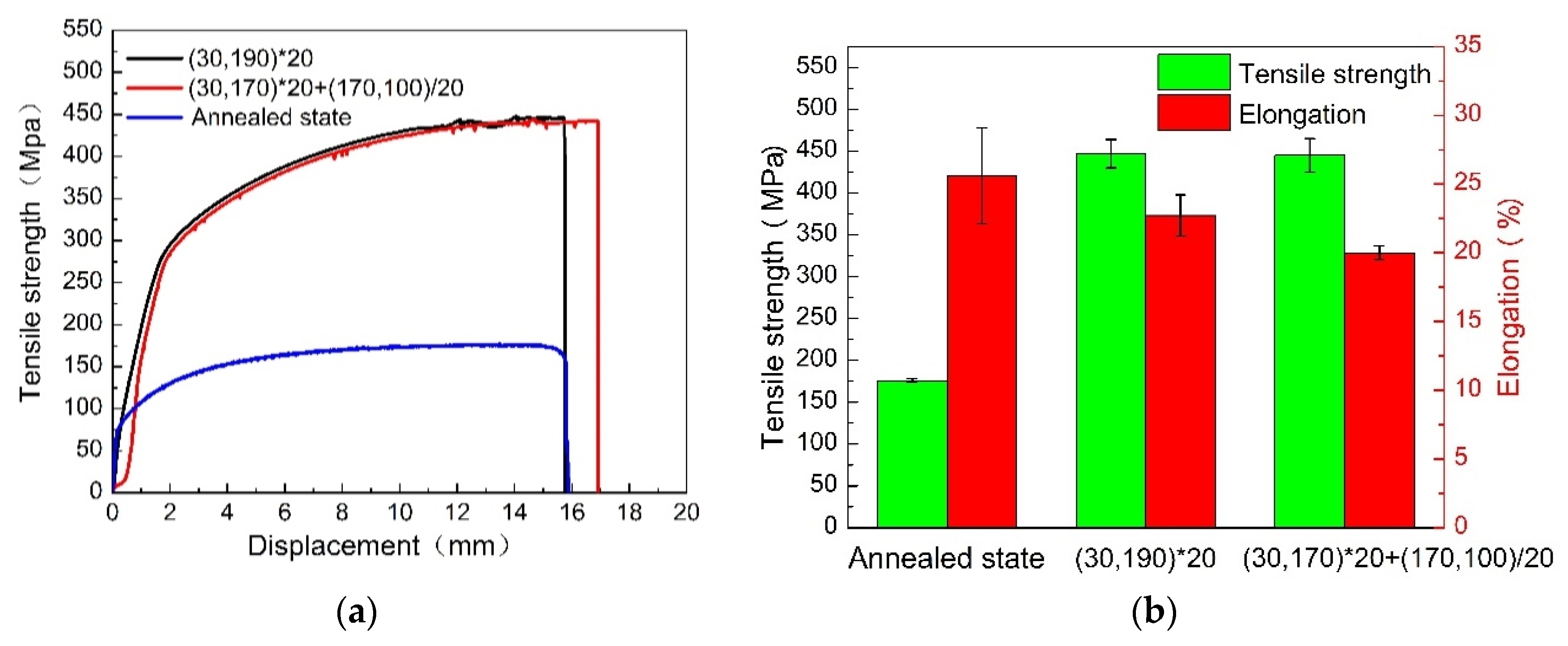
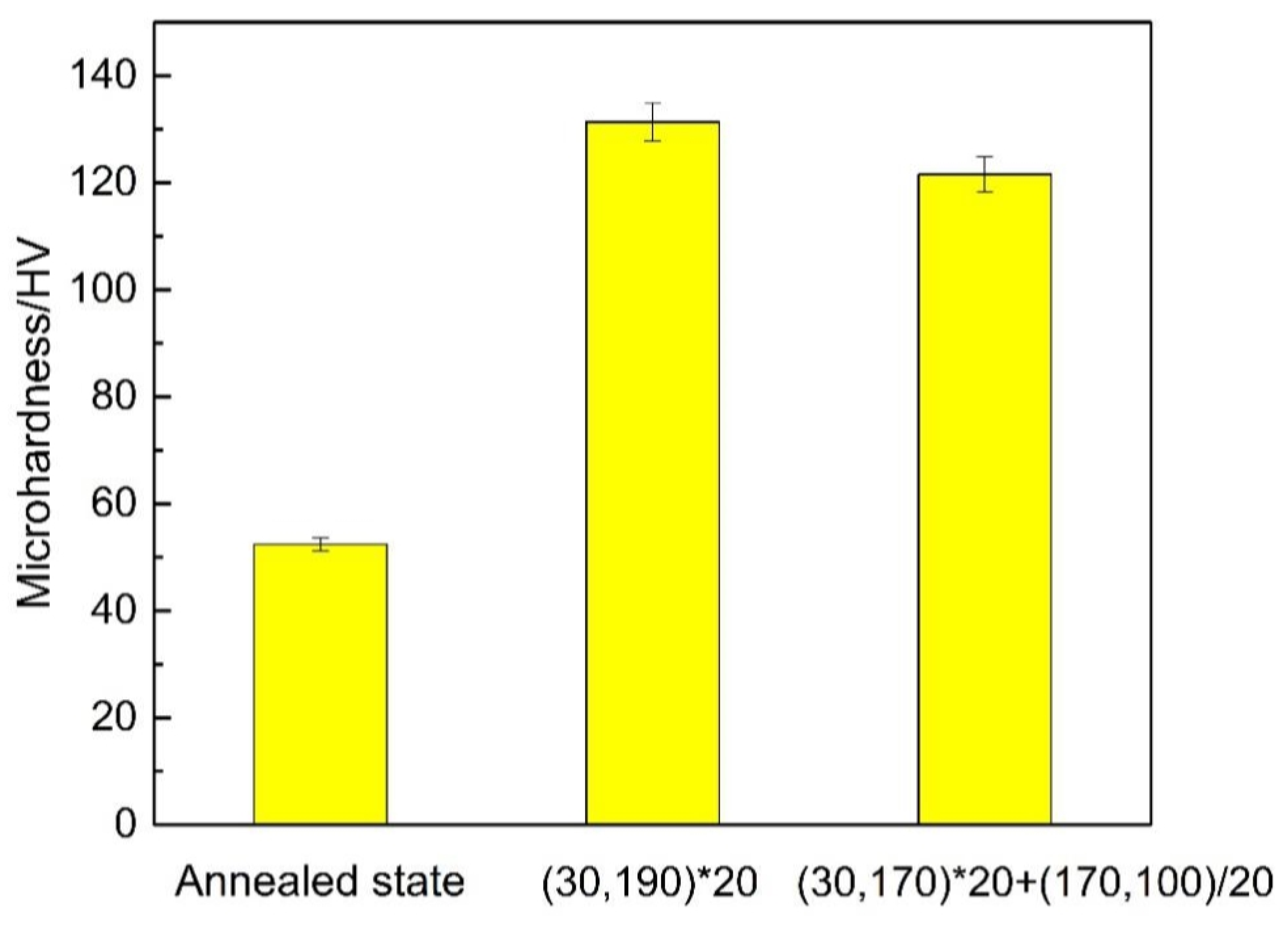
3.2. Mechanical Properties
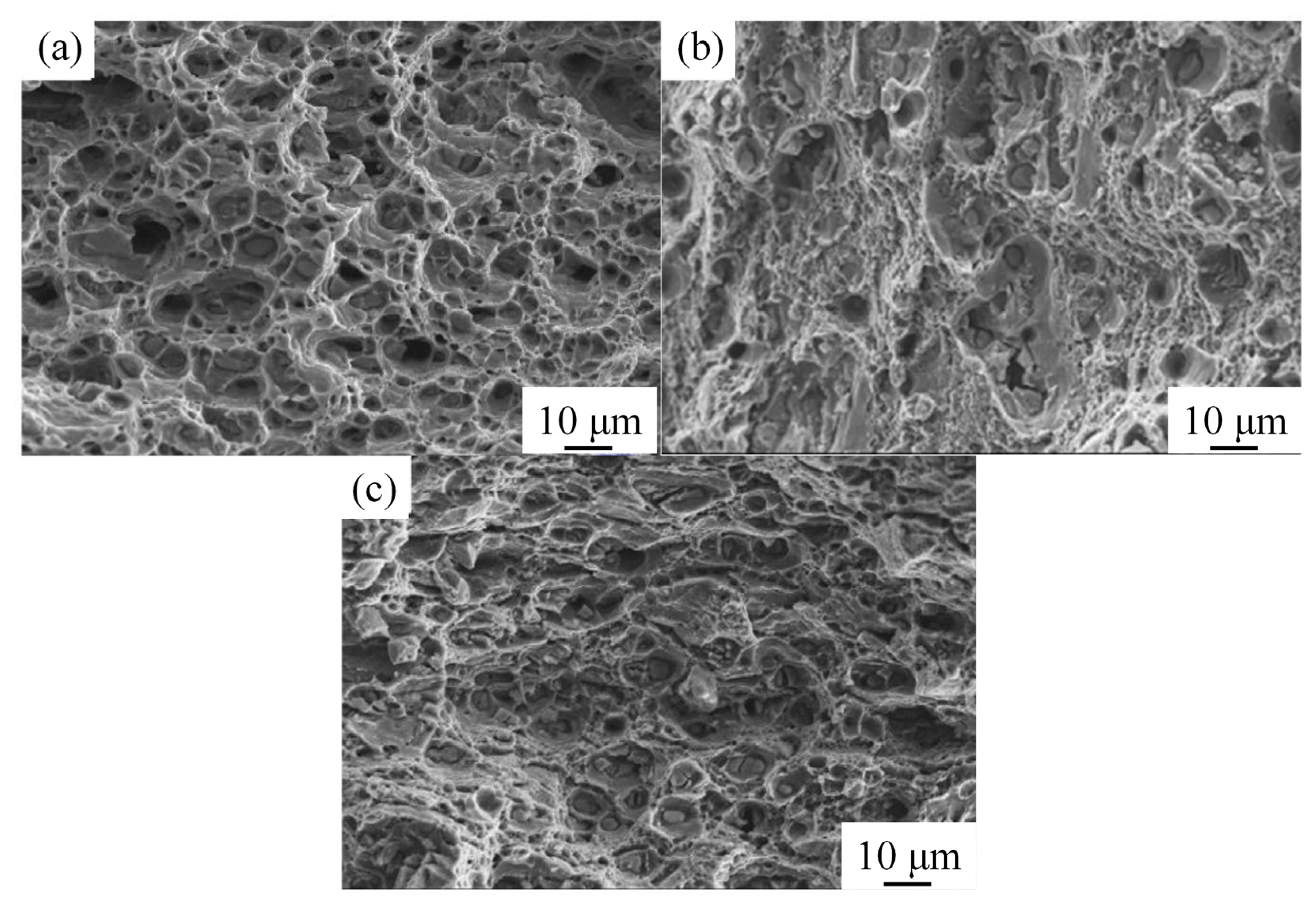
3.2.1. Tensile Properties
3.2.2. Microhardness
3.3. Corrosion Performance
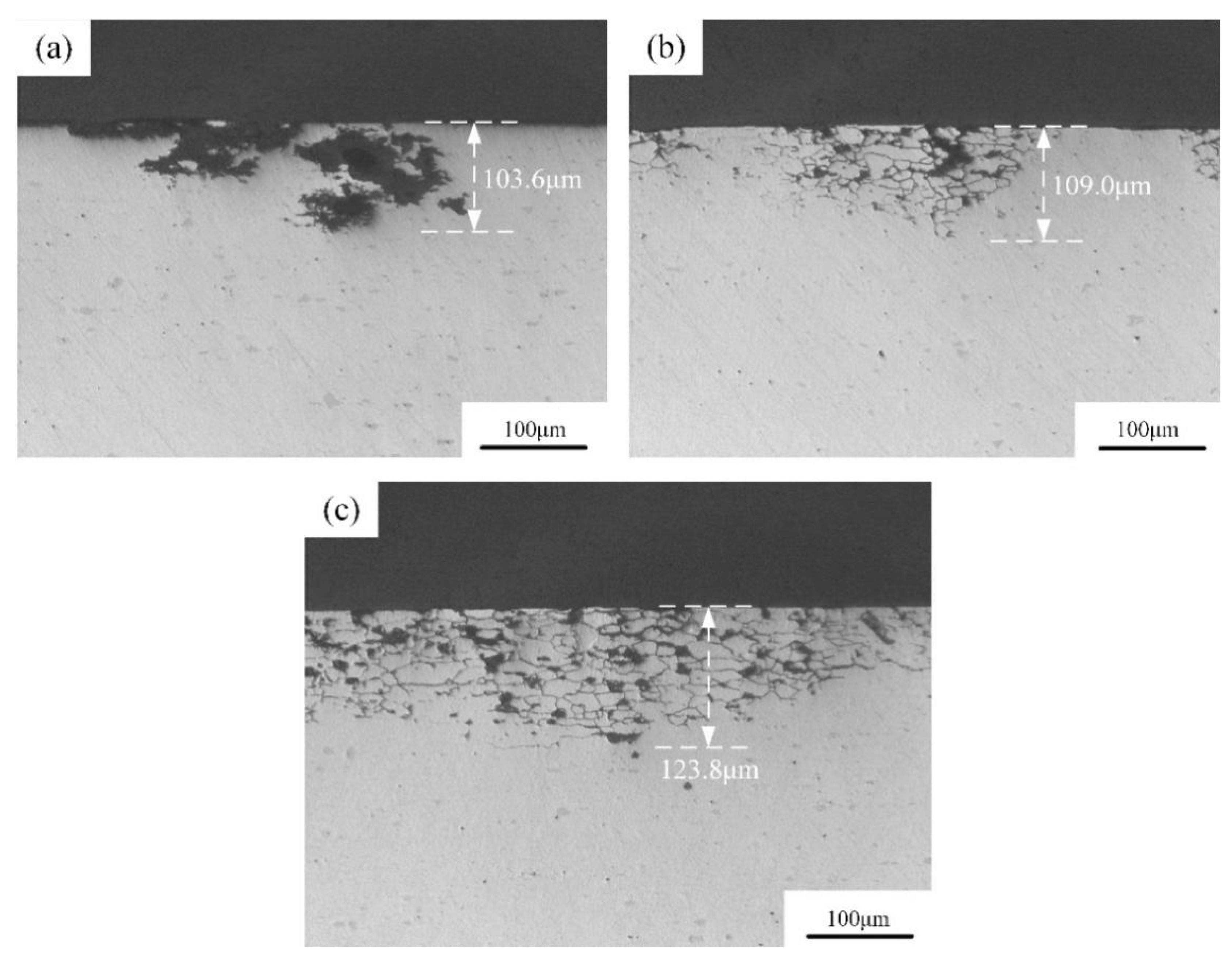
3.3.1. Intergranular Corrosion
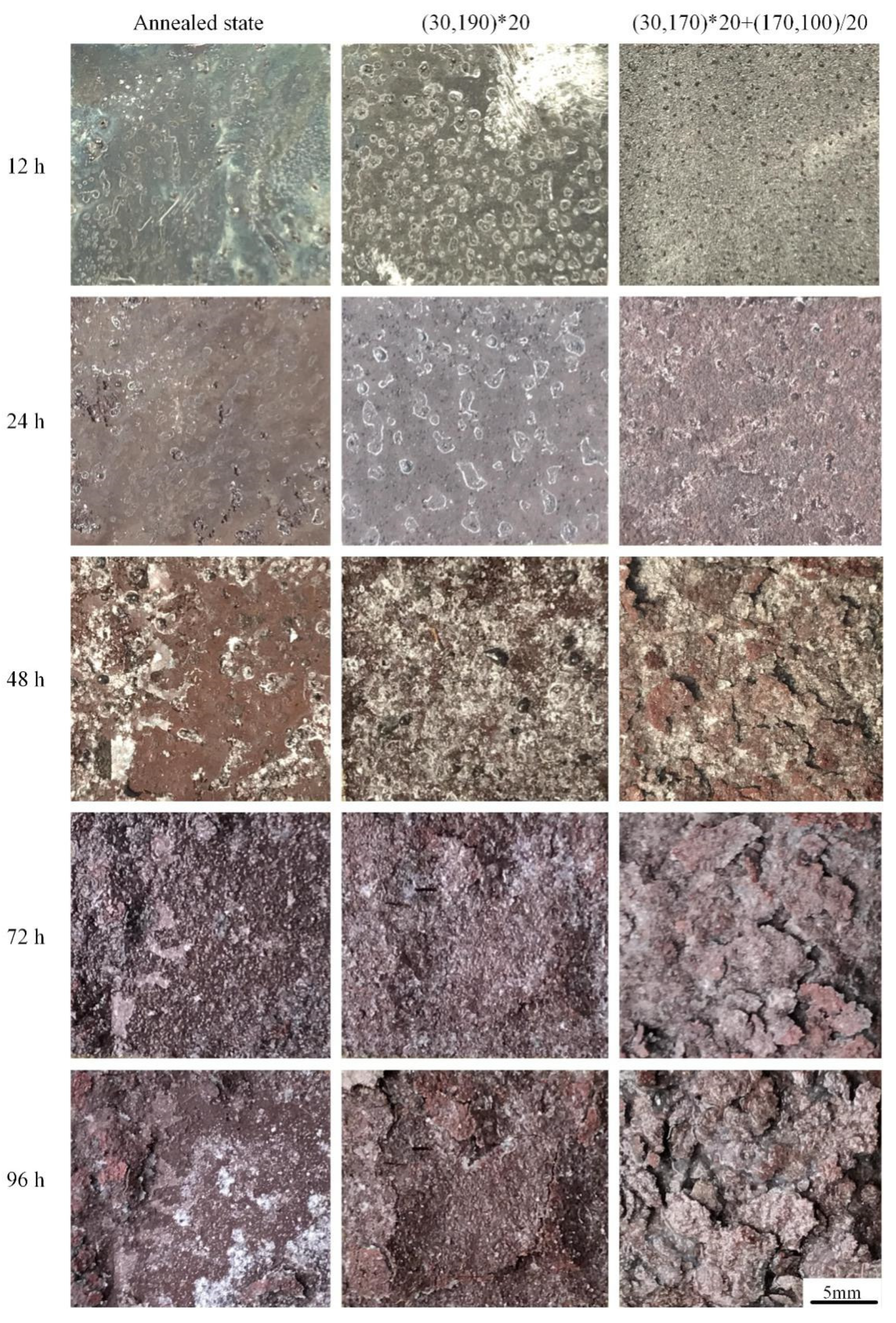
3.3.2. Exfoliation Corrosion
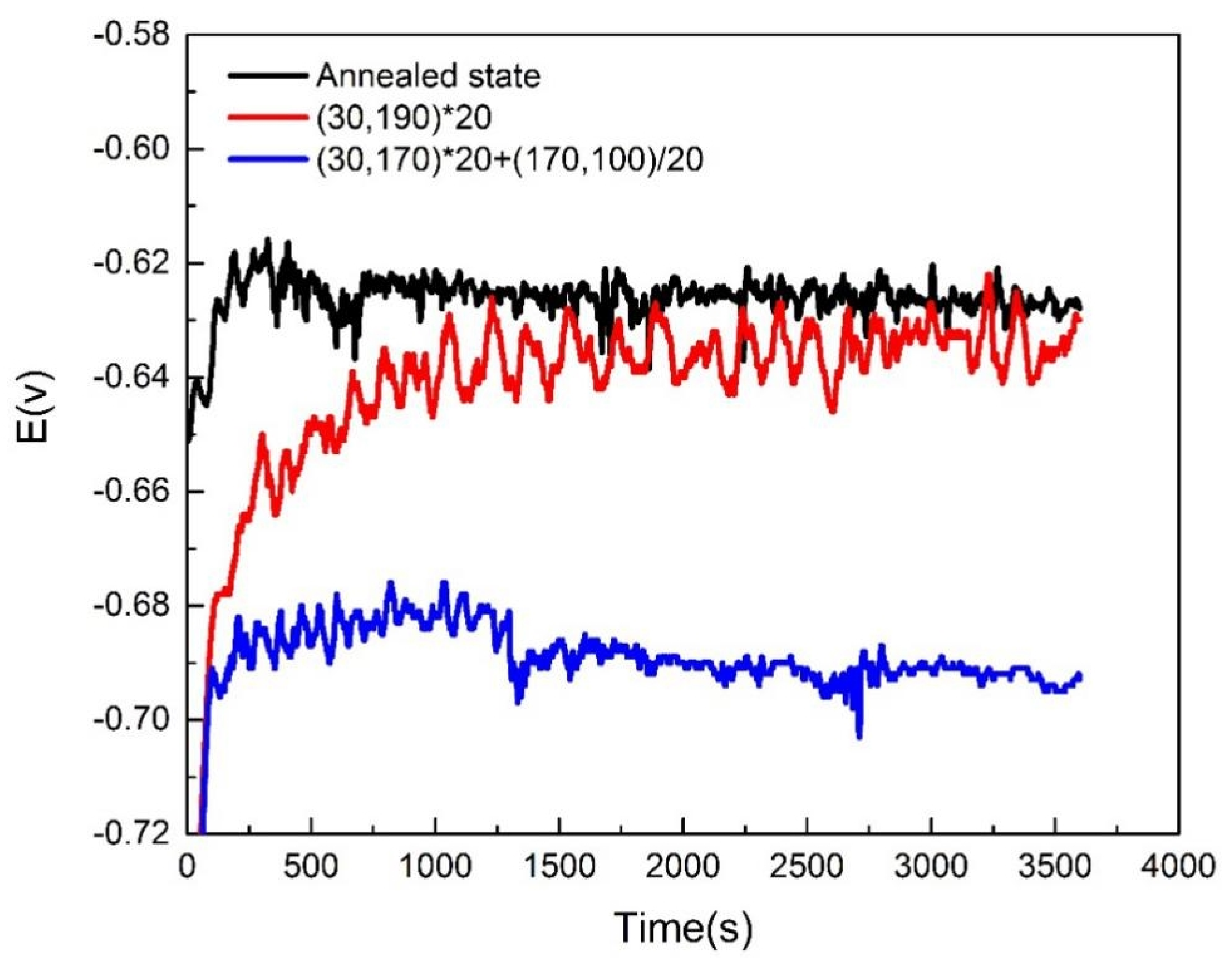
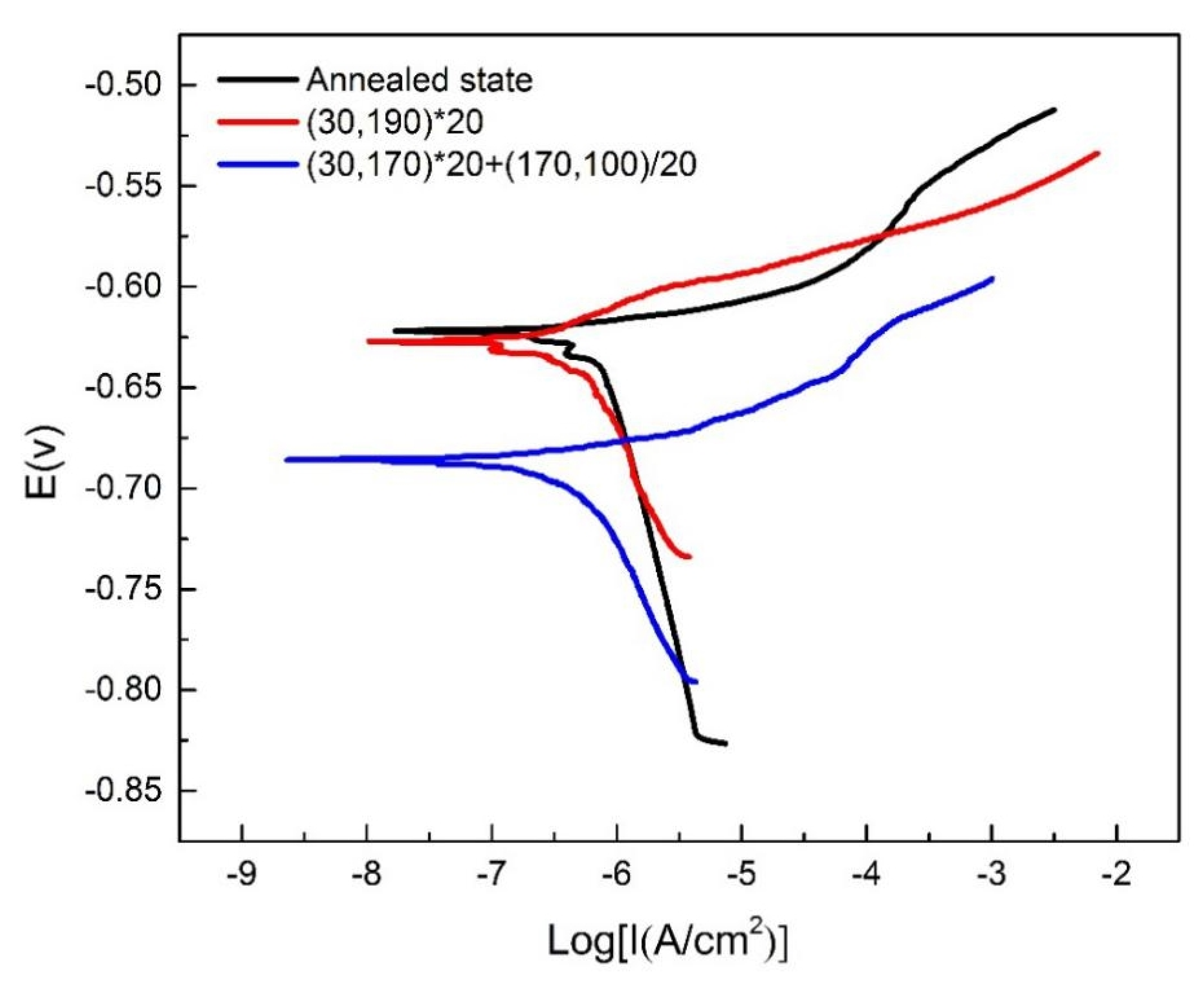
3.3.3. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- James, C.W.; Edgar, A.S., Jr. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar]
- Chen, G.; Fu, X.; Zhao, F.; Zhou, W. Microstructure and mechanical properties of 2A12 aluminum alloy after age forming. Trans. Nonferrous Met. Soc. 2012, 22, 1975–1980. [Google Scholar] [CrossRef]
- Ma, G.; Li, H.; Liu, Z.; Sun, S.; Wang, Z. High-Temperature oxidation properties of micro-arc oxidation film on 2a12 aluminum alloy. Rare Met. Mater. Eng. 2022, 51, 3166–3171. [Google Scholar]
- Rong, W.; Shan, Z.; Wang, B.; Wang, Y.; Wang, J. Microstructure evolution of 2A12 aluminum alloy under isothermal heat treatment direct writing process. Materials 2022, 15, 6279. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, L.; Zhang, X.; Han, Y. A hierarchically structured coating on 2A12-T4 aluminum alloy for anti-wear and corrosion. Corros. Sci. 2022, 207, 110598. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, X.; Wang, D.; Guo, L.; Zhang, Z. Obtaining Uniform High-Strength and Ductility of 2A12 Aluminum Alloy Cabin Components via Predeformation and Annular Channel Angular Extrusion. Coatings 2022, 12, 477. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Yu, M.; Du, R.; Rong, G.; Li, S. Effect of divalent metal ions on durability and anticorrosion performance of layered double hydroxides on anodized 2A12 aluminum alloy. Surf. Coat. Technol. 2019, 373, 56–64. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhao, Y.; Zeng, C.; Zhang, J. Effect of solution temperature on microstructure and properties of 2A97 alloy. Trans. Mater. Heat Treat. 2010, 31, 114–119. [Google Scholar]
- Lu, Z.; Pan, Q.; Chen, Q.; Cao, S.; Liu, X.; He, Y. Effects of Solution Treatment on Mechanical Properties and Microstructure of Al-Cu-Mg Alloy with Ag Addition. J. Aeronaut. Mater. 2011, 31, 24–29. [Google Scholar]
- Blankenship, C., Jr.; Starke, E.A., Jr. Structure-property relationships in Al-Li-Cu-Mg-Ag-Zr alloy X2095. Acta Metall. Mater. 1994, 42, 845–855. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, W.; Yang, W.; Shi, C.; Wang, H. Ageing response of a Al–Cu–Li 2198 alloy. Mater. Des. 2014, 63, 368–374. [Google Scholar] [CrossRef]
- Chen, P.; Bhat, B. Time-Temperature-Precipitation Behavior in Al-Li Alloy 2195; NASA Tech. Rep.; NASA: Greenbelt, MD, USA, 2002; p. 211548. [Google Scholar]
- Li, H.; Zhang, X.; Zhang, J.; Zheng, Z. Effect of multi-stage ageing treatments on microstructures and mechanical properties of new-type Al-Cu-Li alloy. Chin. J. Nonferrous Met. 2008, 108, 426–432. [Google Scholar]
- Wang, Z.; Liu, X.; Zhang, R.; Zhang, X.; Cui, H.; Gao, F. Effects of interrupted aging on corrosion resistance of Al-Cu-Mg-Ag new heat-resistant Al alloy. J. Cent. South Univ. (Sci. Technol.) 2017, 48, 1726–1733. [Google Scholar]
- Zhang, J.; Zhang, Y.; Zhu, B.; Wang, F.; Li, Z.; Li, X.; Xiong, B. Effect of ageing condition on microstructure and properties of Al-Cu-Mg-Ag-Zr alloy. Chin. J. Nonferrous Met. 2011, 21, 1235–1243. [Google Scholar]
- Staley, J.; Durham, N. Method and Process of Non-Isothermal Aging for Aluminum Alloys. U.S. Patent 20070267113A1, 12 March 2007. [Google Scholar]
- Jiang, D.; Liu, Y.; Liang, S.; Xie, W. The effects of non-isothermal aging on the strength and corrosion behavior of Al-Zn-Mg-Cu alloy. J. Alloys Compd. 2016, 681, 57–65. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Cai, X.; Jiang, J.; Shao, W.; Li, Y.; Zhen, L. Microstructure evolution and the resulted influence on localized corrosion in Al-Zn-Mg-Cu alloy during non-isothermal ageing. Materials 2018, 11, 720. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Peng, X.; Liu, S.; Deng, Y.; Liang, X. Effect of non-isothermal aging on microstructure and properties of Al–5.87Zn–2.07Mg–2.42Cu alloys. Trans. Nonferrous Met. Soc. China 2021, 31, 2899–2908. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, C.; Liu, M.; Zhang, Z.; Chen, L.; Zhao, G. Influence of isothermal and non-isothermal aging treatments on microstructure and properties of Al-Zn-Mg alloy helical profile. Mater. Charact. 2020, 169, 110613. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.; Wang, X.; Liu, Z. Quenching sensitivity of Al-Zn-Mg alloy after non-isothermal heat treatment. Materials 2019, 12, 1595. [Google Scholar] [CrossRef]
- Peng, X.; Guo, Q.; Liang, X.; Deng, Y.; Gu, Y.; Xu, G.; Yin, Z. Mechanical properties, corrosion behavior and microstructures of a non isothermal ageing treated Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2017, 688, 146–154. [Google Scholar] [CrossRef]
- GB/T 7998-2005; Test Method for Intergranular Corrosion of Aluminium Alloy. National Standard of the People’s Republic of China: Beijing, China, 2005.
- GB/T22639-2022; Test Method of Exfoliation Corrosion for Wrought Aluminium and Aluminium Alloys. National Standard of the People’s Republic of China: Beijing, China, 2022.
- Li, S.; Dong, H.; Li, P.; Chen, S. Effect of repetitious non-isothermal heat treatment on corrosion behavior of Al-Zn-Mg alloy. Corros. Sci. 2018, 131, 278–289. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.; Shi, L.; Li, P.; Ye, F. Corrosion behavior and mechanical properties of Al-Zn-Mg aluminum alloy weld. Corros. Sci. 2017, 123, 243–255. [Google Scholar] [CrossRef]
- Wang, S.; Starink, M. Precipitates and intermetallic phases in precipitation hardening Al–Cu–Mg–(Li) based alloys. Int. Mater. Rev. 2013, 50, 193–215. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Jin, Y. High resolution study of twins in Al20Cu2Mn3 phase. Acta Metall. Sin. (Chin. Ed.) 1992, 28, 1–5. [Google Scholar]
- Lu, H.; Shi, L.; Dong, H.; Li, S.; Guo, D.; Tao, C. Influence of flame rectification on mechanical properties of Al-Zn-Mg alloy. J. Alloys Compd. 2016, 689, 278–286. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Shi, L.; Li, P.; Li, S. Influence of cyclic non-isothermal heat treatment on microstructure, mechanical property and corrosion behavior of Al–Zn–Mg alloy. Mater. Res. Express. 2019, 6, 096501. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Y.; Wu, H. Effects of pre-ageing on thermomechanical treatment process of 2A12 aluminum alloy. Appl. Mech. Mater. 2012, 217, 283–287. [Google Scholar]
- Li, S.; Dong, H.; Shi, L.; Wang, X.; Liu, Z.; Shang, G.; Tian, Y. The Effects of Heat Straightening Temperature on the Microstructure and Properties of 7N01 Aluminum Alloy. Materials 2019, 12, 2949. [Google Scholar] [CrossRef]
- Beffort, O.; Solenthaler, C.; Speidel, M. Improvement of strength and fracture toughness of a spray-deposited Al-Cu-Mg-Ag-Mn-Ti-Zr alloy by optimized heat treatments and thermomechanical treatments. Mater. Sci. Eng. A 1995, 191, 113–120. [Google Scholar] [CrossRef]
| Element | Cu | Mg | Mn | Fe | Si | Zn | Ti | Ni | Other | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| Contents | 4.64 | 1.59 | 0.59 | 0.42 | 0.32 | 0.18 | 0.1 | 0.05 | 0.15 | Bal. |
| Conditions | Immersion Time | ||||
|---|---|---|---|---|---|
| 12 h | 24 h | 48 h | 72 h | 96 h | |
| Annealed state | PA | PB | PC | EA | EA |
| (30,190) × 20 | PA | PB | PC | EA | EB |
| (30,170) × 20 + (170,100)/20 | PB | PC | EB | EC | EC |
| Sample | Ecorr (mV (Ag/AgCl)) | Icorr (mA/cm2) |
|---|---|---|
| Annealed state | −621.95 | 2.2386 × 10−7 |
| (30,190) × 20 | −627.37 | 2.3613 × 10−7 |
| (30,170) × 20 + (170,100)/20 | −685.85 | 4.3455 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Liu, H.; Zeng, T.; Li, S.; Liu, Z.; Wu, T.; Gu, D. Effect of Non-Isothermal Aging on the Mechanical Properties and Corrosion Resistance of 2A12 Aluminum Alloy. Materials 2023, 16, 3921. https://doi.org/10.3390/ma16113921
Yang J, Liu H, Zeng T, Li S, Liu Z, Wu T, Gu D. Effect of Non-Isothermal Aging on the Mechanical Properties and Corrosion Resistance of 2A12 Aluminum Alloy. Materials. 2023; 16(11):3921. https://doi.org/10.3390/ma16113921
Chicago/Turabian StyleYang, Jie, Hongfeng Liu, Tao Zeng, Shuai Li, Zhongying Liu, Tingting Wu, and Dongdong Gu. 2023. "Effect of Non-Isothermal Aging on the Mechanical Properties and Corrosion Resistance of 2A12 Aluminum Alloy" Materials 16, no. 11: 3921. https://doi.org/10.3390/ma16113921
APA StyleYang, J., Liu, H., Zeng, T., Li, S., Liu, Z., Wu, T., & Gu, D. (2023). Effect of Non-Isothermal Aging on the Mechanical Properties and Corrosion Resistance of 2A12 Aluminum Alloy. Materials, 16(11), 3921. https://doi.org/10.3390/ma16113921







