Investigation of the Effect of Double-Filler Atoms on the Thermoelectric Properties of Ce-YbCo4Sb12
Abstract
1. Introduction
2. Materials and Methods
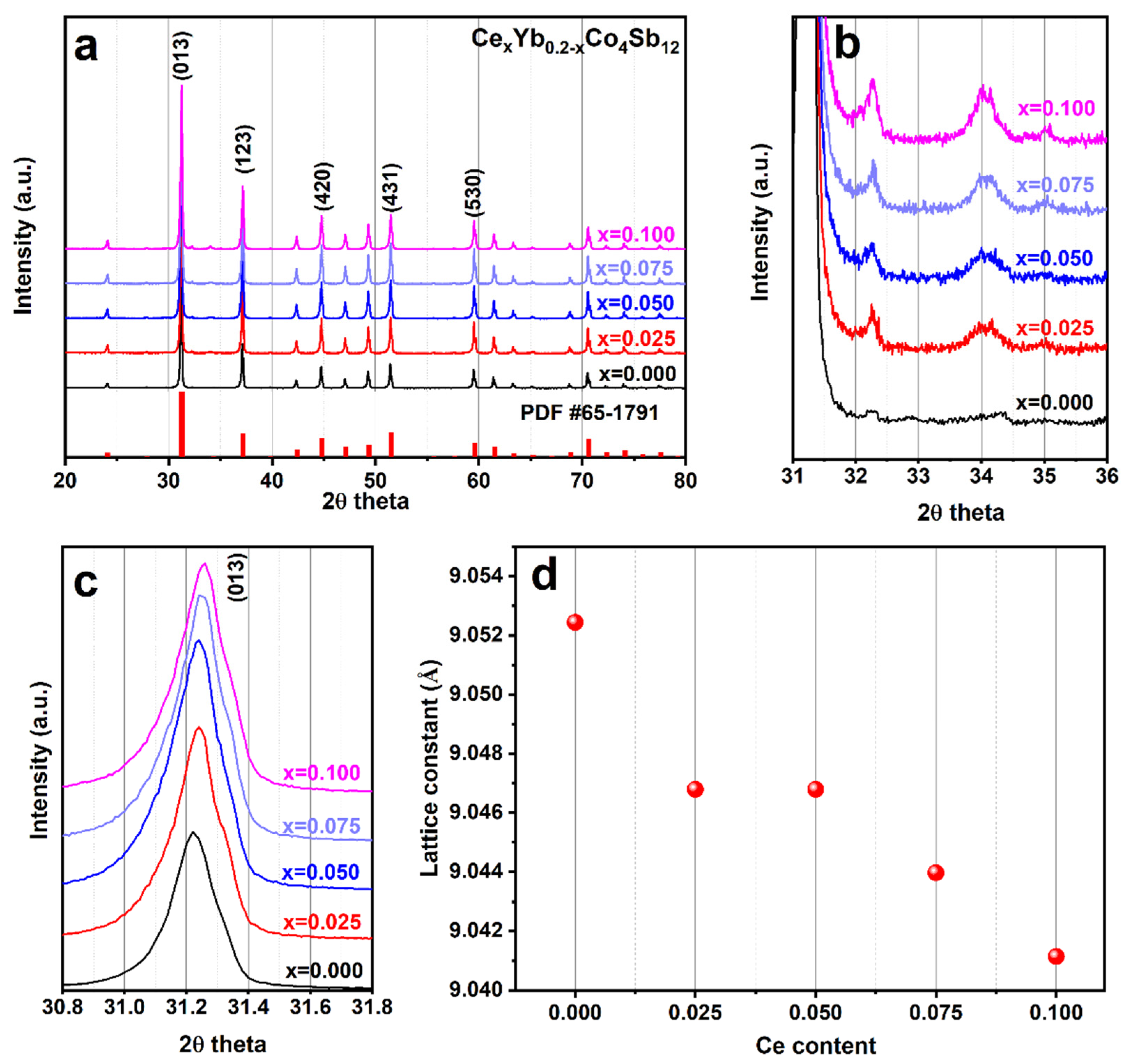
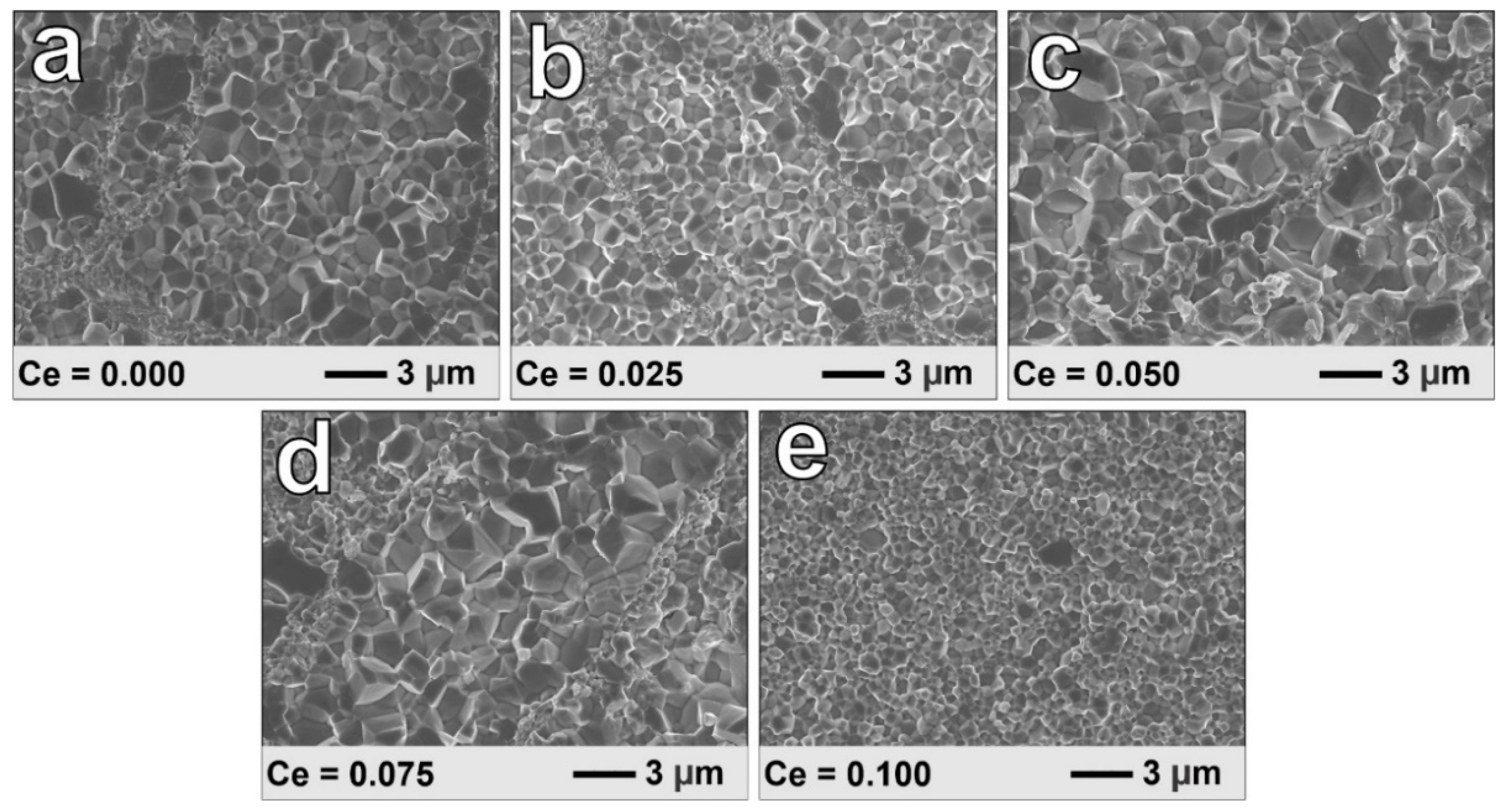
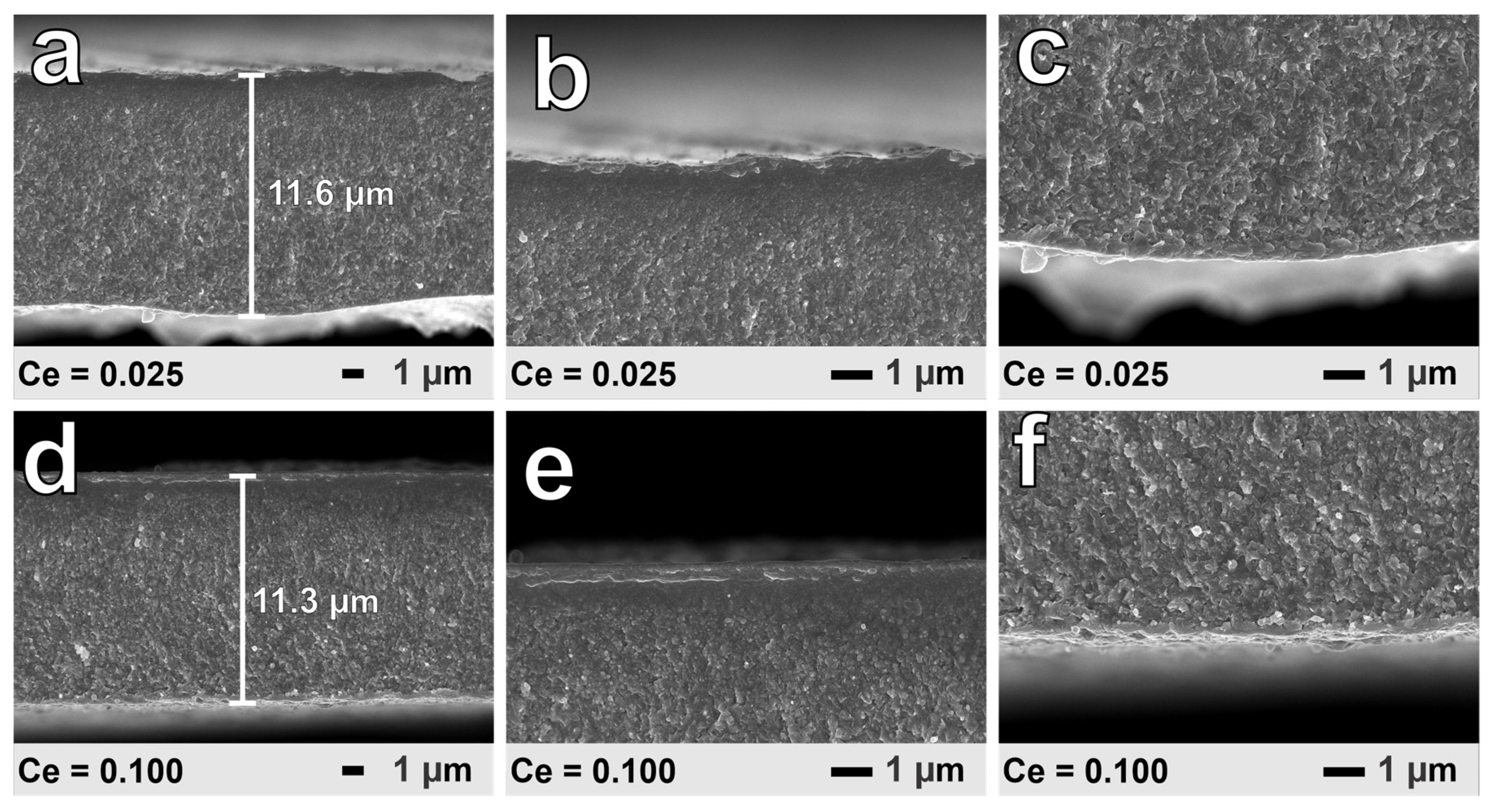
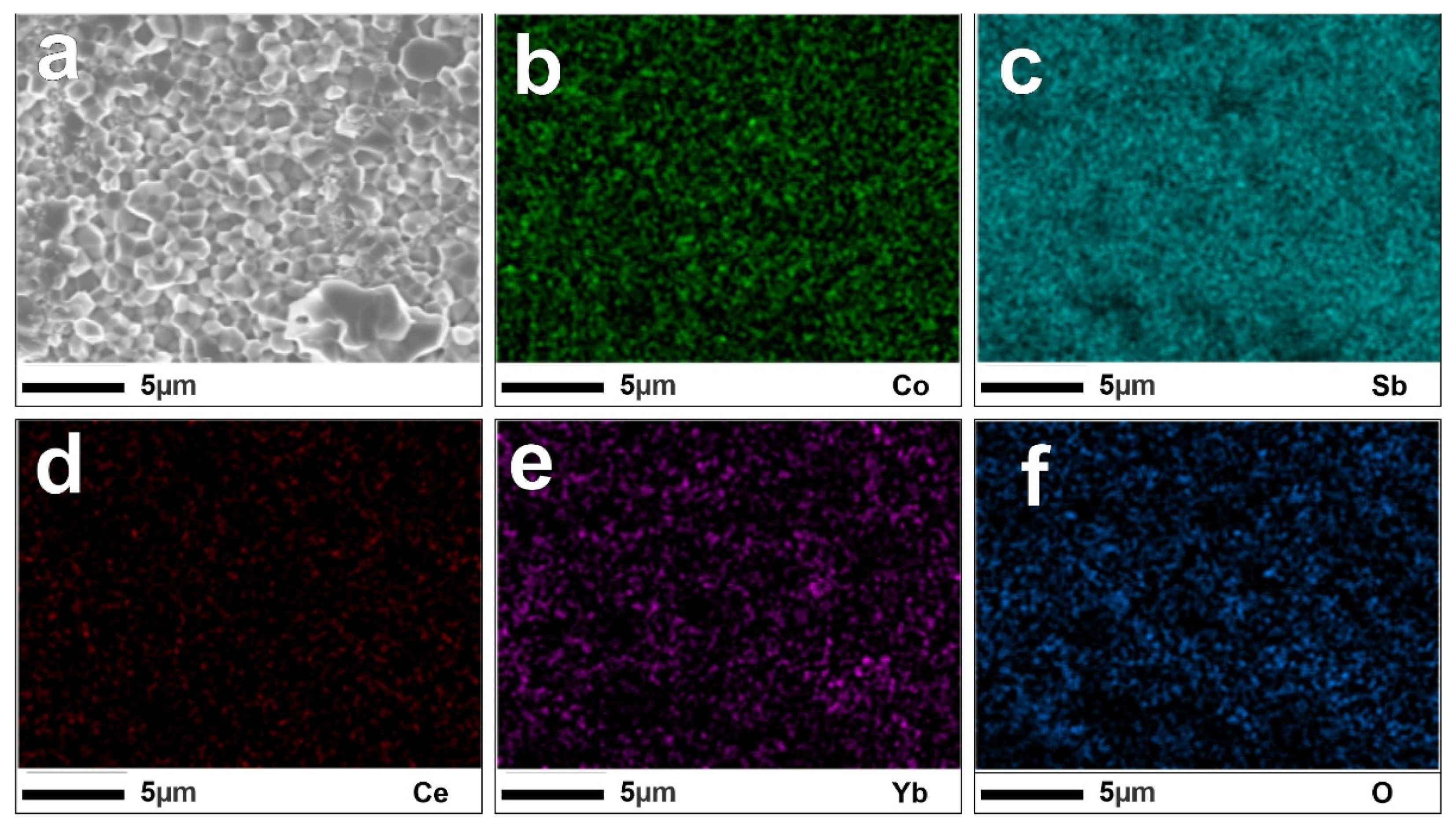
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riffat, S.B.; Ma, X. Thermoelectrics: A review of present and potential applications. Appl. Ther. Eng. 2003, 23, 913–935. [Google Scholar] [CrossRef]
- Tritt, T.M.; Subramanian, M.A. Thermoelectric materials, phenomena, and applications: A bird’s eye view. MRS Bull. 2006, 31, 188–198. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 101–110. [Google Scholar]
- Sales, B.; Mandrus, D.; Williams, R.K. Filled skutterudite antimonides: A new class of thermoelectric materials. Science 1996, 272, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Dughaish, Z. Lead telluride as a thermoelectric material for thermoelectric power generation. Phys. B Condens. Matter 2002, 322, 205–223. [Google Scholar] [CrossRef]
- Rull-Bravo, M.; Moure, A.; Fernandez, J.; Martín-González, M. Skutterudites as thermoelectric materials: Revisited. RSC Adv. 2015, 5, 41653–41667. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Rogl, P.; Royanian, E.; Bauer, E.; Horky, J.; Setman, D.; Schafler, E.; Zehetbauer, M. Dependence of thermoelectric behaviour on severe plastic deformation parameters: A case study on p-type skutterudite DD0.60Fe3CoSb12. Acta Mater. 2013, 61, 6778–6789. [Google Scholar] [CrossRef]
- Dahal, T.; Kim, H.S.; Gahlawat, S.; Dahal, K.; Jie, Q.; Liu, W.; Lan, Y.; White, K.; Ren, Z. Transport and mechanical properties of the double-filled p-type skutterudites La0.68Ce0.22Fe4−xCoxSb12. Acta Mater. 2016, 117, 13–22. [Google Scholar] [CrossRef]
- Music, D.; Geyer, R.W.; Keuter, P. Thermomechanical response of thermoelectrics. Appl. Phys. Lett. 2016, 109, 223903. [Google Scholar] [CrossRef]
- Nolas, G.; Slack, G.; Morelli, D.; Tritt, T.; Ehrlich, A. The effect of rare-earth filling on the lattice thermal conductivity of skutterudites. J. Appl. Phys. 1996, 79, 4002–4008. [Google Scholar] [CrossRef]
- Keppens, V.; Mandrus, D.; Sales, B.C.; Chakoumakos, B.; Dai, P.; Coldea, R.; Maple, M.; Gajewski, D.; Freeman, E.; Bennington, S. Localized vibrational modes in metallic solids. Nature 1998, 395, 876–878. [Google Scholar] [CrossRef]
- Hermann, R.P.; Grandjean, F.; Long, G.J. Einstein oscillators that impede thermal transport. Am. J. Phys. 2005, 73, 110–118. [Google Scholar] [CrossRef]
- Rowe, D.M. CRC Handbook of Thermoelectrics; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Sales, B.; Mandrus, D.; Chakoumakos, B.; Keppens, V.; Thompson, J. Filled skutterudite antimonides: Electron crystals and phonon glasses. Phys. Rev. B 1997, 56, 15081. [Google Scholar] [CrossRef]
- Tang, Y.; Gibbs, Z.M.; Agapito, L.A.; Li, G.; Kim, H.-S.; Nardelli, M.B.; Curtarolo, S.; Snyder, G.J. Convergence of multi-valley bands as the electronic origin of high thermoelectric performance in CoSb3 skutterudites. Nat. Mater. 2015, 14, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.; Yang, J.; Wang, H.; Shi, X. Double-filled skutterudites of the type YbxCayCo4Sb12: Synthesis and properties. J. Appl. Phys. 2010, 107, 043705. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, W.; Bai, S.; Mei, Z.; Chen, L. Dual-frequency resonant phonon scattering in BaxRyCo4Sb12 (R = La, Ce, and Sr). Appl. Phys. Lett. 2007, 90, 192111. [Google Scholar] [CrossRef]
- Wang, S.; Salvador, J.R.; Yang, J.; Wei, P.; Duan, B.; Yang, J. High-performance n-type YbxCo4Sb12: From partially filled skutterudites towards composite thermoelectrics. NPG Asia Mater. 2016, 8, e285. [Google Scholar] [CrossRef]
- Hu, P.; Wei, T.-R.; Qiu, P.; Cao, Y.; Yang, J.; Shi, X.; Chen, L. Largely enhanced Seebeck coefficient and thermoelectric performance by the distortion of electronic density of states in Ge2Sb2Te5. ACS Appl. Mater. Interfaces 2019, 11, 34046–34052. [Google Scholar] [CrossRef]
- Peng, J.; Alboni, P.; He, J.; Zhang, B.; Su, Z.; Holgate, T.; Gothard, N.; Tritt, T. Thermoelectric properties of (In, Yb) double-filled CoSb3 skutterudite. J. Appl. Phys. 2008, 104, 053710. [Google Scholar] [CrossRef]
- Shi, X.; Kong, H.; Li, C.-P.; Uher, C.; Yang, J.; Salvador, J.; Wang, H.; Chen, L.; Zhang, W. Low thermal conductivity and high thermoelectric figure of merit in n-type BaxYbyCo4Sb12 double-filled skutterudites. Appl. Phys. Lett. 2008, 92, 182101. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Li, H.; Tang, X.; Zhang, Q.; Uher, C. Rapid preparation method of bulk nanostructured Yb0.3Co4Sb12+y compounds and their improved thermoelectric performance. Appl. Phys. Lett. 2008, 93, 252109. [Google Scholar] [CrossRef]
- Jie, Q.; Wang, H.; Liu, W.; Wang, H.; Chen, G.; Ren, Z. Fast phase formation of double-filled p-type skutterudites by ball-milling and hot-pressing. Phys. Chem. Chem. Phys. 2013, 15, 6809–6816. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, X.; Su, X.; Zhang, Q. Preparation and thermoelectric properties of high-performance Sb additional Yb0.2Co4Sb12+y bulk materials with nanostructure. Appl. Phys. Lett. 2008, 92, 202114. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, W.; Zhou, H.; Wei, P.; Zhang, Q. Rapid preparation and thermoelectric properties of Ba and In double-filled p-type skutterudite bulk materials. Scr. Mater. 2013, 68, 643–646. [Google Scholar] [CrossRef]
- Tan, G.; Liu, W.; Wang, S.; Yan, Y.; Li, H.; Tang, X.; Uher, C. Rapid preparation of CeFe4Sb12 skutterudite by melt spinning: Rich nanostructures and high thermoelectric performance. J. Mater. Chem. A 2013, 1, 12657–12668. [Google Scholar] [CrossRef]
- Salvador, J.R.; Waldo, R.A.; Wong, C.A.; Tessema, M.; Brown, D.N.; Miller, D.J.; Wang, H.; Wereszczak, A.A.; Cai, W. Thermoelectric and mechanical properties of melt spun and spark plasma sintered n-type Yb-and Ba-filled skutterudites. Mater. Sci. Eng. B 2013, 178, 1087–1096. [Google Scholar] [CrossRef]
- Thompson, D.R.; Liu, C.; Yang, J.; Salvador, J.R.; Haddad, D.B.; Ellison, N.D.; Waldo, R.A.; Yang, J. Rare-earth free p-type filled skutterudites: Mechanisms for low thermal conductivity and effects of Fe/Co ratio on the band structure and charge transport. Acta Mater. 2015, 92, 152–162. [Google Scholar] [CrossRef]
- He, J.; Sootsman, J.R.; Girard, S.N.; Zheng, J.-C.; Wen, J.; Zhu, Y.; Kanatzidis, M.G.; Dravid, V.P. On the origin of increased phonon scattering in nanostructured PbTe based thermoelectric materials. J. Am. Chem. Soc. 2010, 132, 8669–8675. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Rogl, P.; Bauer, E.; Hochenhofer, M.; Anbalagan, R.; Mallik, R.; Schafler, E. Nanostructuring of p- and n-type skutterudites reaching figures of merit of approximately 1.3 and 1.6, respectively. Acta Mater. 2014, 76, 434–448. [Google Scholar] [CrossRef]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-Saving Pathways for Thermoelectric Nanomaterial Synthesis: Hydrothermal/Solvothermal, Microwave-Assisted, Solution-Based, and Powder Processing. Adv. Sci. 2022, 9, 2106052. [Google Scholar] [CrossRef]
- Emsley, J. Nature’s Building Blocks: An AZ Guide to the Elements; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Tang, Y.; Hanus, R.; Chen, S.-W.; Snyder, G.J. Solubility design leading to high figure of merit in low-cost Ce-CoSb3 skutterudites. Nat. Commun. 2015, 6, 7584. [Google Scholar] [CrossRef] [PubMed]
- Khovaylo, V.; Korolkov, T.; Voronin, A.; Gorshenkov, M.; Burkov, A. Rapid preparation of InxCo4Sb12 with a record-breaking ZT = 1.5: The role of the In overfilling fraction limit and Sb overstoichiometry. J. Mater. Chem. A 2017, 5, 3541–3546. [Google Scholar] [CrossRef]
- Li, H.; Tang, X.; Su, X.; Zhang, Q.; Uher, C. Nanostructured bulk YbxCo4Sb12 with high thermoelectric performance prepared by the rapid solidification method. J. Phys. D Appl. Phys. 2009, 42, 145409. [Google Scholar] [CrossRef]
- Aversano, F.; Branz, S.; Bassani, E.; Fanciulli, C.; Ferrario, A.; Boldrini, S.; Baricco, M.; Castellero, A. Effect of rapid solidification on the synthesis and thermoelectric properties of Yb-filled Co4Sb12 skutterudite. J. Alloys Compd. 2019, 796, 33–41. [Google Scholar] [CrossRef]
- Kruszewski, M.; Zybała, R. Review of rapid fabrication methods of skutterudite materials. Mater. Today Proc. 2021, 44, 3475–3482. [Google Scholar] [CrossRef]
- Yang, K.; Cheng, H.; Hng, H.; Ma, J.; Mi, J.; Zhao, X.; Zhu, T.; Zhang, Y. Synthesis and thermoelectric properties of double-filled skutterudites CeyYb0.5−yFe1.5Co2.5Sb12. J. Alloys Compd. 2009, 467, 528–532. [Google Scholar] [CrossRef]
- Bérardan, D.; Godart, C.; Alleno, E.; Bauer, E. Chemical properties and thermopower of the new series of skutterudite Ce1−pYbpFe4Sb12. J. Alloys Compd. 2003, 351, 18–23. [Google Scholar] [CrossRef]
- Berardan, D.; Alleno, E.; Godart, C.; Puyet, M.; Lenoir, B.; Lackner, R.; Bauer, E.; Girard, L.; Ravot, D. Improved thermoelectric properties in double-filled Cey/2Yby/2Fe4−x(Co/Ni)xSb12 skutterudites. J. Appl. Phys. 2005, 98, 033710. [Google Scholar] [CrossRef]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 2008, 321, 554–557. [Google Scholar] [CrossRef]
- Fisk, Z.; Sarrao, J.; Smith, J.; Thompson, J. The physics and chemistry of heavy fermions. Proc. Natl. Acad. Sci. USA 1995, 92, 6663–6667. [Google Scholar] [CrossRef]
- Cho, J.Y.; Thomas, E.L.; Nambu, Y.; Capan, C.; Karki, A.B.; Young, D.P.; Kuga, K.; Nakatsuji, S.; Chan, J.Y. Crystal Growth, Structure, and Physical Properties of Ln(Cu,Ga)13−x(Ln = La−Nd, Eu; x ≈ 0.2). Chem. Mater. 2009, 21, 3072–3078. [Google Scholar] [CrossRef]
- Steglich, F. Superconductivity and magnetism in heavy-fermion compounds. J. Phys. Soc. Jpn. 2005, 74, 167–177. [Google Scholar] [CrossRef]
- Miyake, K.; Narikiyo, O.; Onishi, Y. Superconductivity of Ce-based heavy fermions under pressure: Valence fluctuation mediated pairing associated with valence instability of Ce. Phys. B Condens. Matter 1999, 259, 676–677. [Google Scholar] [CrossRef]
- Kim, H.-S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
- Goto, Y.; Miyao, S.; Kamihara, Y.; Matoba, M. Electrical/thermal transport and electronic structure of the binary cobalt pnictides CoPn2 (Pn = As and Sb). AIP Adv. 2015, 5, 067147. [Google Scholar] [CrossRef]
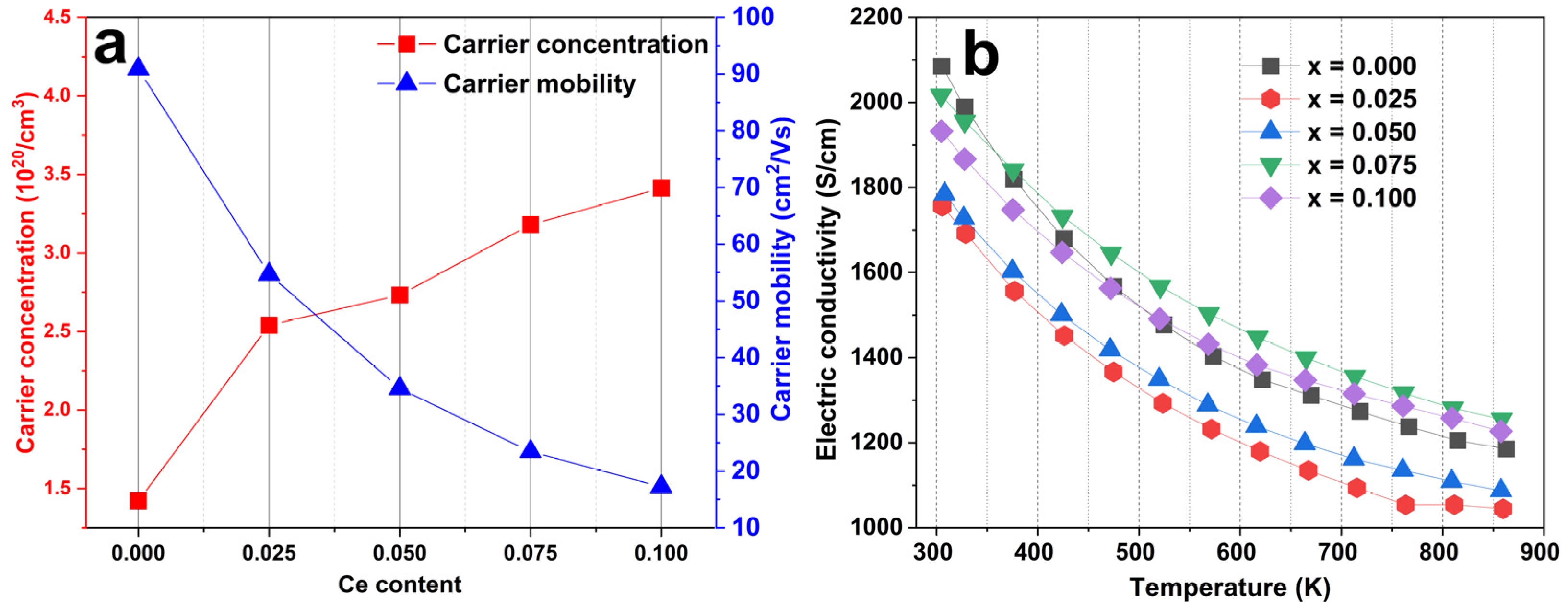



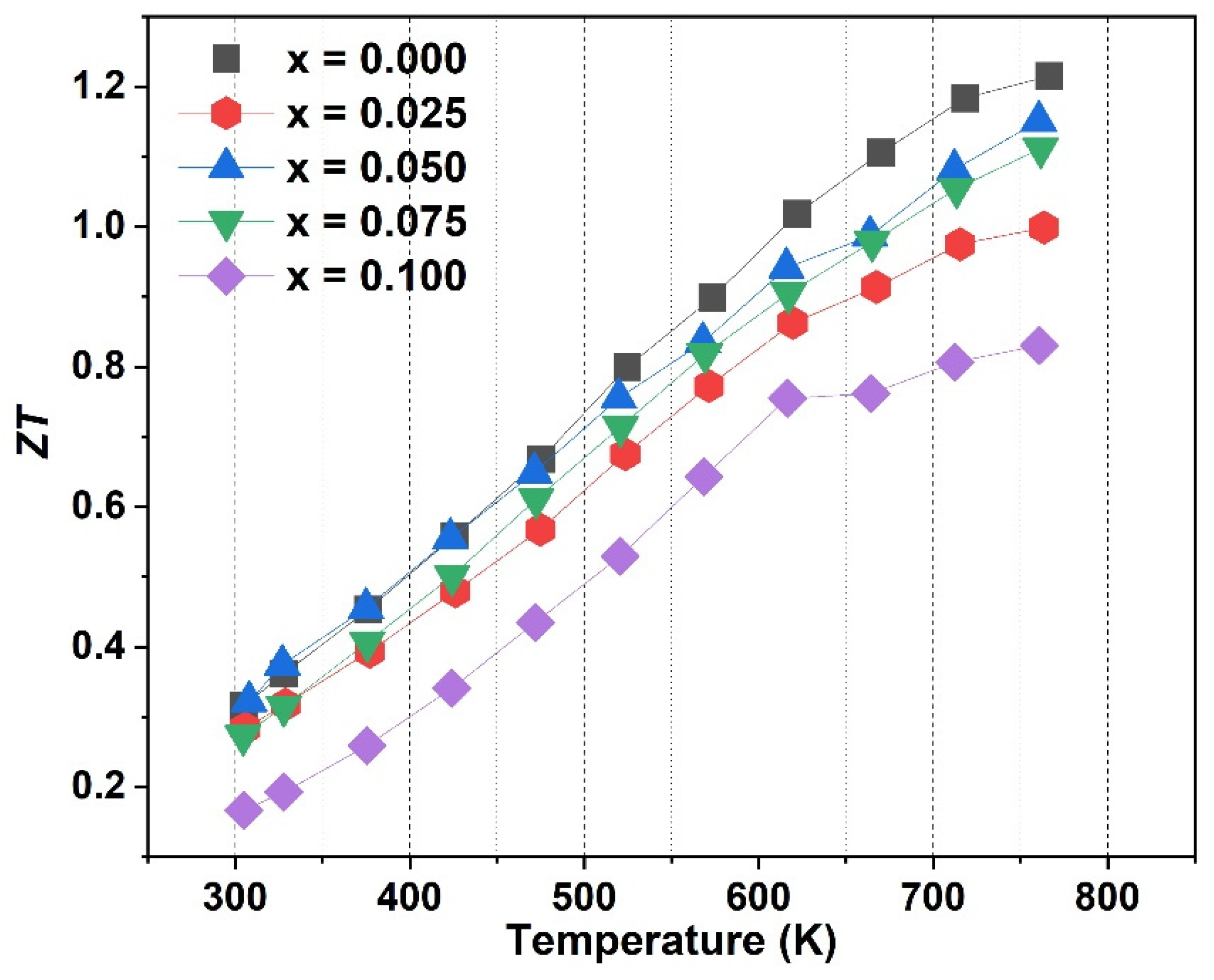
| Composition | a (Å) | n (×1020 cm−3) | σ (Sm−1) | S (µVK−1) | k (Wm−1K−1) | m*/m0 |
|---|---|---|---|---|---|---|
| Yb0.2Co4Sb12 | 9.052 | 1.4 | 2085.5 | −133.1 | 2.4 | 1.77 |
| Ce0.025Yb0.175Co4Sb12 | 9.047 | 2.5 | 1756.1 | −143.2 | 3.0 | 2.77 |
| Ce0.05Yb0.15Co4Sb12 | 9.047 | 2.7 | 1783.9 | −141.8 | 2.5 | 2.92 |
| Ce0.075Yb0.125Co4Sb12 | 9.044 | 3.2 | 2016.9 | −128.9 | 2.6 | 2.94 |
| Ce0.1Yb0.1Co4Sb12 | 9.041 | 3.4 | 1932.2 | −123.2 | 4.3 | 2.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binh, N.V.; Van Du, N.; Lee, N.; Kang, M.; Ryu, S.H.; Lee, M.; Seo, D.; Nam, W.H.; Roh, J.W.; Lee, S.; et al. Investigation of the Effect of Double-Filler Atoms on the Thermoelectric Properties of Ce-YbCo4Sb12. Materials 2023, 16, 3819. https://doi.org/10.3390/ma16103819
Binh NV, Van Du N, Lee N, Kang M, Ryu SH, Lee M, Seo D, Nam WH, Roh JW, Lee S, et al. Investigation of the Effect of Double-Filler Atoms on the Thermoelectric Properties of Ce-YbCo4Sb12. Materials. 2023; 16(10):3819. https://doi.org/10.3390/ma16103819
Chicago/Turabian StyleBinh, Nguyen Vu, Nguyen Van Du, Nayoung Lee, Minji Kang, So Hyeon Ryu, Munhwi Lee, Deokcheol Seo, Woo Hyun Nam, Jong Wook Roh, Soonil Lee, and et al. 2023. "Investigation of the Effect of Double-Filler Atoms on the Thermoelectric Properties of Ce-YbCo4Sb12" Materials 16, no. 10: 3819. https://doi.org/10.3390/ma16103819
APA StyleBinh, N. V., Van Du, N., Lee, N., Kang, M., Ryu, S. H., Lee, M., Seo, D., Nam, W. H., Roh, J. W., Lee, S., Kim, S. Y., Koo, S.-M., Shin, W. H., & Cho, J. Y. (2023). Investigation of the Effect of Double-Filler Atoms on the Thermoelectric Properties of Ce-YbCo4Sb12. Materials, 16(10), 3819. https://doi.org/10.3390/ma16103819









