New Trends in Separation Techniques of Lithium Isotopes: A Review of Chemical Separation Methods
Abstract
1. Introduction
2. Challenges in Development of Separation Methods for Lithium Isotopes Using Chemical Exchange Methods in Two Liquid Phases
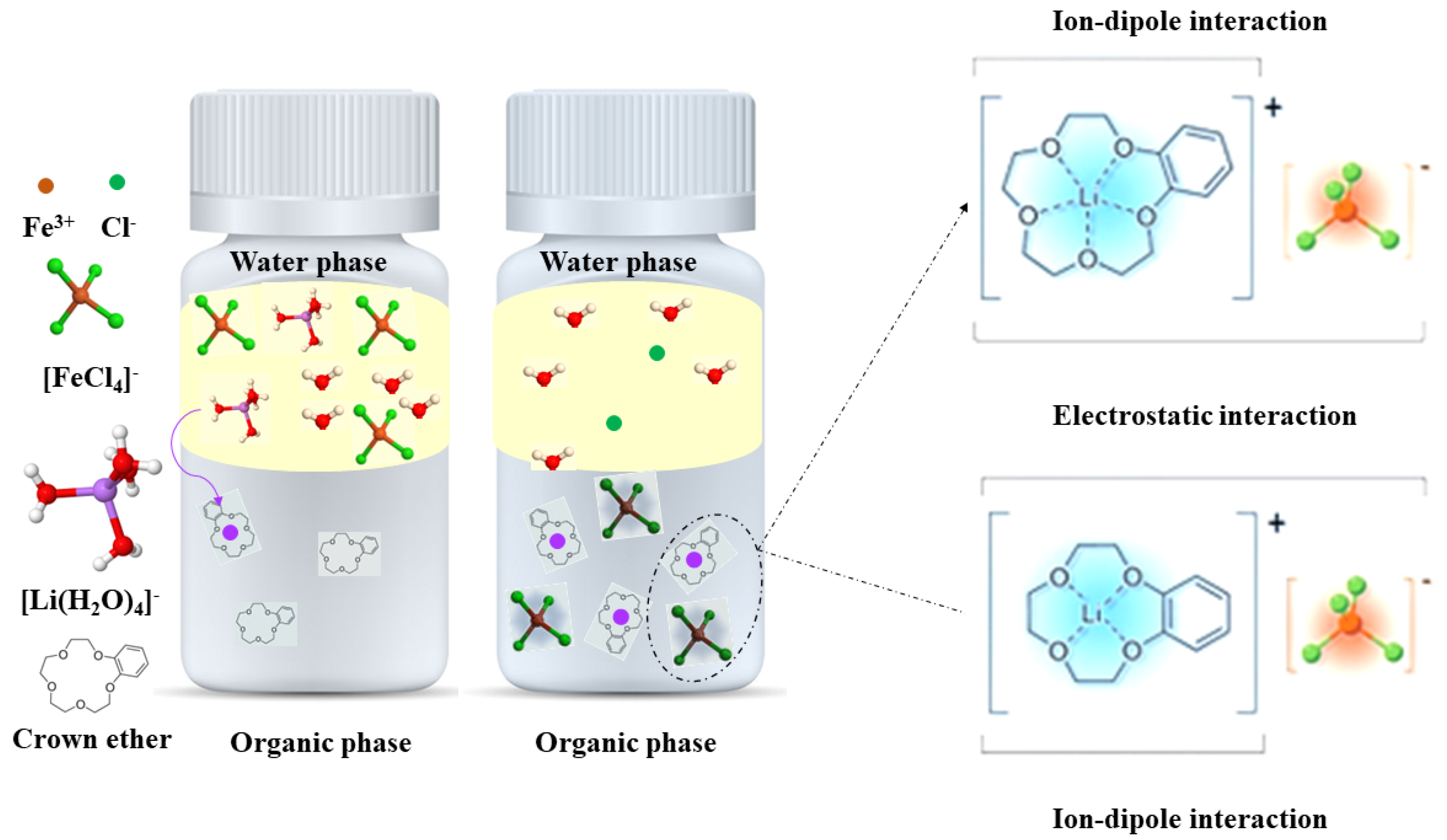
2.1. Separation Using Crown Ethers
2.2. Challenges in Development of Separation Methods for Lithium Isotopes Using Electrochemical Exchange Methods
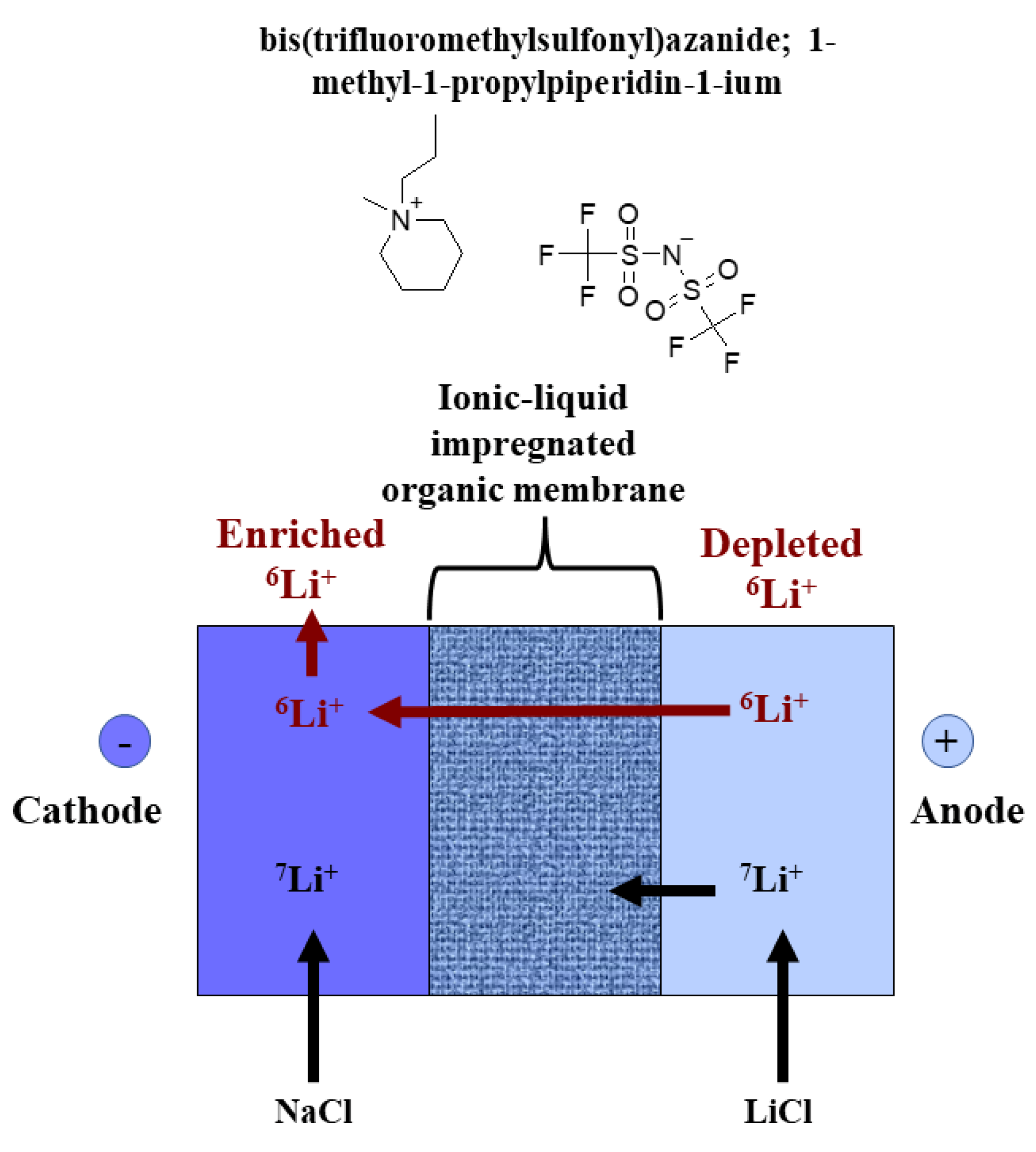
2.2.1. Challenges in Development of Separation Methods for Lithium Isotopes with Electromigration
2.2.2. Challenges in Development of Separation Methods for Lithium Isotopes Using Electrodialysis Methods
2.3. Challenges in Development of Separation Methods for Lithium Isotopes Using Displacement Chromatography Methods
2.3.1. Inorganic Microstructure Methods
2.3.2. Ion-Exchange Methods Using Resin
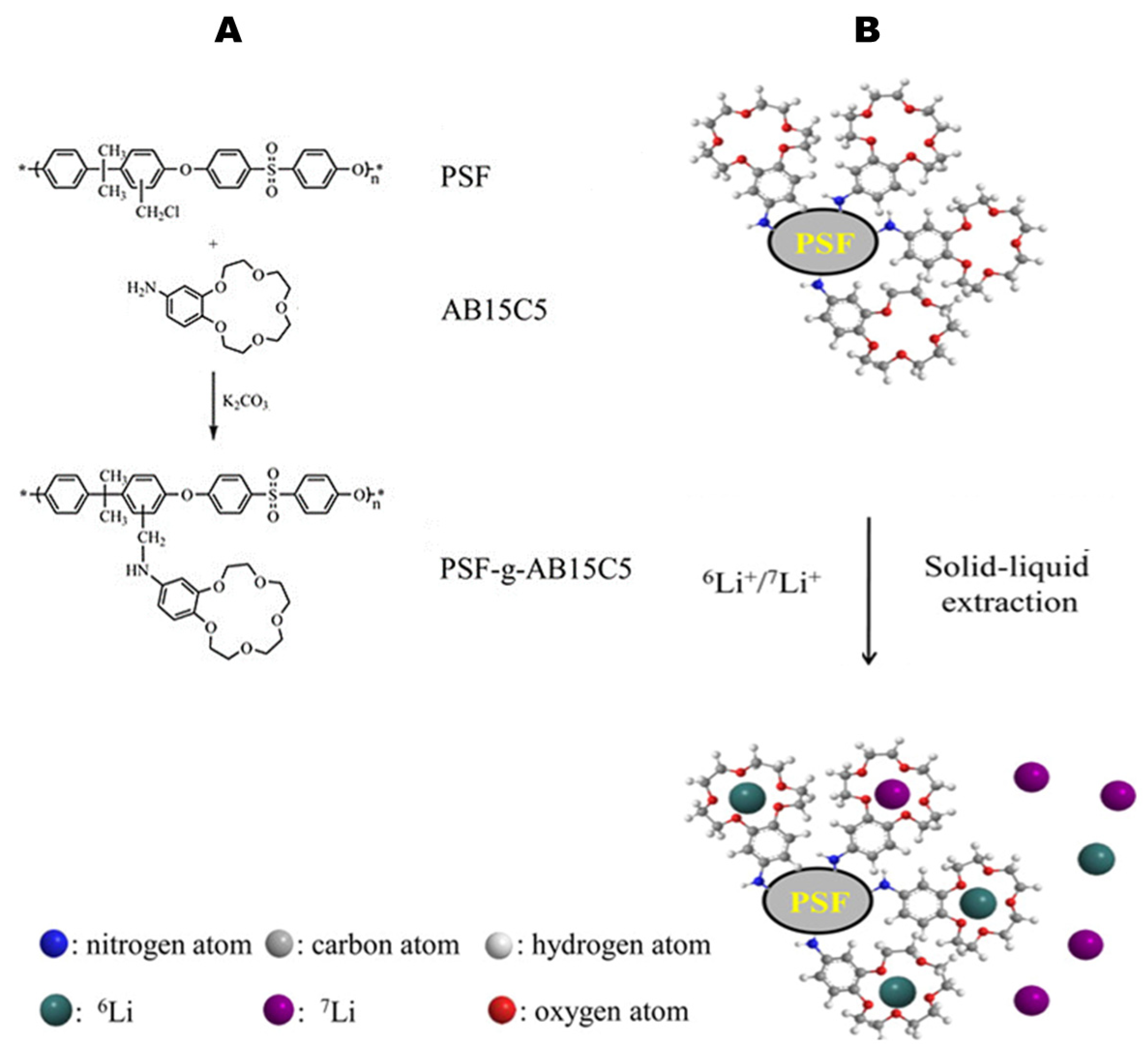
2.3.3. Methods Involving Resin-Supported Complexing Agents
2.3.4. Methods Involving Ion-Exchange Membranes
2.4. Challenges in Development of Analysis Methods for Lithium Isotopes Using Spectrometric and Spectroscopic Methods
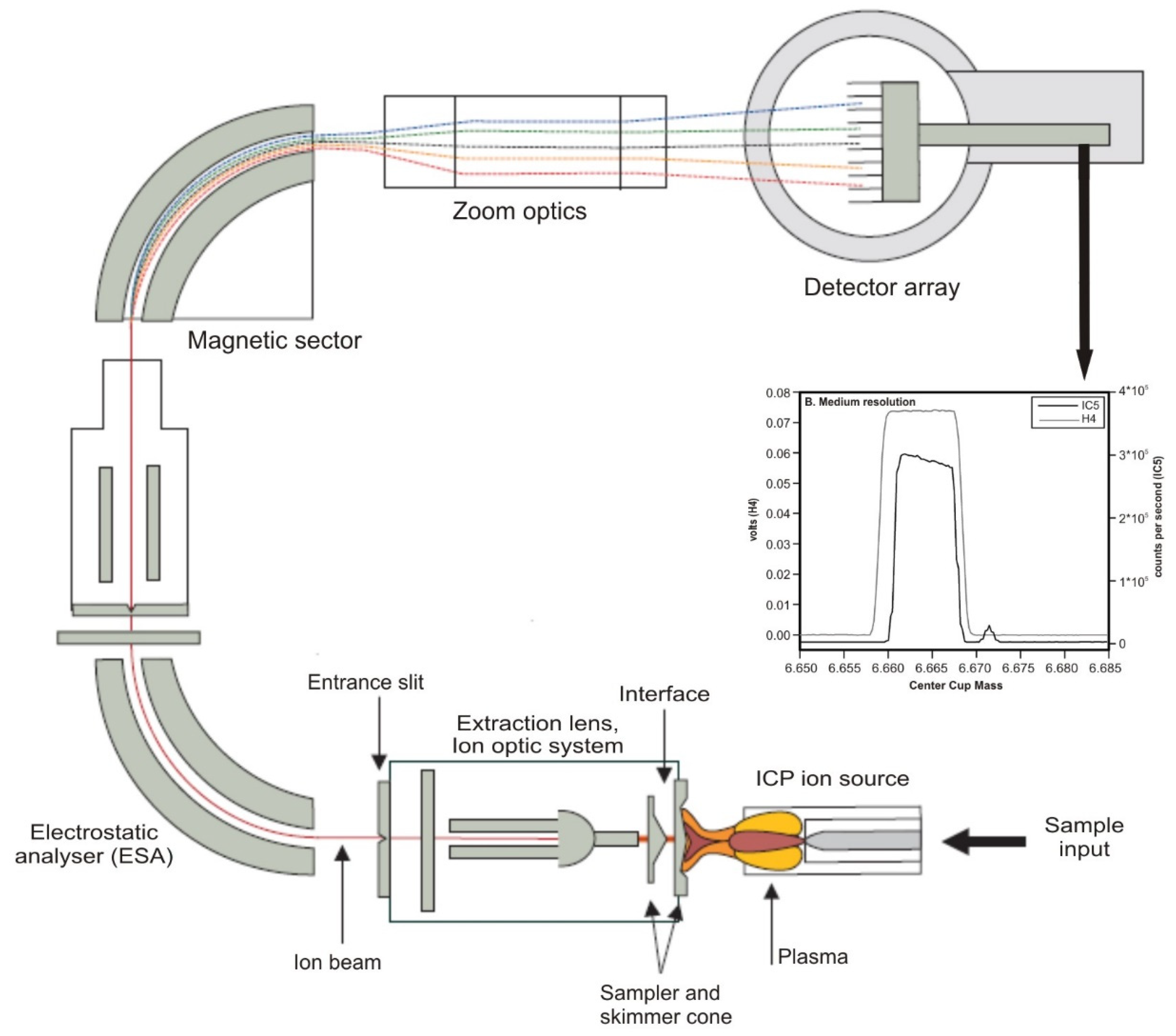
2.4.1. Analysis Methods for Lithium Isotopes Using ICP-MS Methods
2.4.2. Analysis Methods for Lithium Isotopes Using MC-ICP-MS Methods
2.4.3. Analysis Methods for Lithium Isotopes Using Thermal Ionisation Mass Spectrometry
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, F.; Dellinger, M.; Hilton, R.G.; Yu, J.; Allen, M.B.; Densmore, A.L.; Sun, H.; Jin, Z. Hydrological Control of River and Seawater Lithium Isotopes. Nat. Commun. 2022, 13, 3359. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.-F.; Xu, Y.; Tong, F.; Jin, Z. Li Isotopic Seasonality in a Small Catchment at the Northeastern Tibetan Plateau: Roles of Hydrology and Temperature Dependency. Sci. Total Environ. 2023, 870, 161896. [Google Scholar] [CrossRef] [PubMed]
- Desaulty, A.M.; Monfort Climent, D.; Lefebvre, G.; Cristiano-Tassi, A.; Peralta, D.; Perret, S.; Urban, A.; Guerrot, C. Tracing the Origin of Lithium in Li-Ion Batteries Using Lithium Isotopes. Nat. Commun. 2022, 13, 4172. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Liu, J.; Ju, Y.; Liu, J.; Yang, Z.; Shi, Y. Equilibrium Lithium Isotope Fractionation in Li-Bearing Minerals. Geochim. Cosmochim. Acta 2018, 235, 360–375. [Google Scholar] [CrossRef]
- Giegerich, T.; Battes, K.; Schwenzer, J.C.; Day, C. Development of a Viable Route for Lithium-6 Supply of DEMO and Future Fusion Power Plants. Fusion Eng. Des. 2019, 149, 111339. [Google Scholar] [CrossRef]
- Baker, P.S.; Duncan, F.R.; Greene, H.B. Preparation of High-Purity Lithium Metal by Vacuum Distillation. Science 1953, 118, 778–780. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, C.S.; Lee, N.S.; Ryu, H.I. Chromatographic Separation of Lithium and Magnesium Isotopes by Manganese(IV) Oxide. Radiochim. Acta 2002, 90, 179–183. [Google Scholar] [CrossRef]
- Kim, D.W.; Kang, B.M.; Jeon, B.K.; Jeon, Y.S. Separation of Lithium Isotopes by Elution Chromatography with an AB18C6 Bonded Merrifield Peptide Resin. J. Radioanal. Nucl. Chem. 2003, 256, 81–85. [Google Scholar] [CrossRef]
- Zeng, Y.; Pei, H.; Wang, Z.; Yan, F.; Li, J.; Cui, Z.; He, B. Chitosan-Graft-Benzo-15-Crown-5-Ether/PVA Blend Membrane with Sponge-like Pores for Lithium Isotope Adsorptive Separation. ACS Omega 2018, 3, 554–561. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Huang, C.; Zhang, Q.; Ju, H.; Xue, Z.; Shao, F.; Li, B.; Mao, L.; Jing, Y.; et al. Electromigration Separation of Lithium Isotopes: The Effect of Electrode Solutions. J. Electrochem. Soc. 2022, 169, 016516. [Google Scholar] [CrossRef]
- Sun, X.L.; Zhou, W.; Gu, L.; Qiu, D.; Ren, D.H.; Gu, Z.G.; Li, Z. Liquid–Liquid Extraction to Lithium Isotope Separation Based on Room-Temperature Ionic Liquids Containing 2,2′-Binaphthyldiyl-17-Crown-5. J. Nucl. Sci. Technol. 2014, 52, 332–341. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, C.; Xue, Z.; Mao, L.; Sun, J.; Shao, F.; Qi, M.; Jing, Y.; Jia, Y. Extraction Separation of Lithium Isotopes with Bromobenzene-15-Crown-5/Ionic Liquids System: Experimental and Theoretical Study. J. Mol. Liq. 2022, 364, 120020. [Google Scholar] [CrossRef]
- Yudaev, P.; Chistyakov, E. Chelating Extractants for Metals. Metals 2022, 12, 1275. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Ionic Liquids as Components of Systems for Metal Extraction. Chem. Eng. 2022, 6, 6. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Ju, H.; Xue, Z.; Zhou, X.; Mao, L.; Shao, F.; Zou, X.; Jing, Y.; Jia, Y.; et al. Electromigration Separation of Lithium Isotopes: The Multiple Roles of Crown Ethers. Chem. Phys. Lett. 2022, 787, 139265. [Google Scholar] [CrossRef]
- Murali, A.; Zhang, Z.; Free, M.L.; Sarswat, P.K. A Comprehensive Review of Selected Major Categories of Lithium Isotope Separation Techniques. Phys. Status Solidi A 2021, 218, 2100340. [Google Scholar] [CrossRef]
- Misra, S.; Froelich, P.N. Measurement of Lithium Isotope Ratios by Quadrupole-ICP-MS: Application to Seawater and Natural Carbonates. J. Anal. At. Spectrom. 2009, 24, 1524–1533. [Google Scholar] [CrossRef]
- Nishizawa, K.; Ishino, S.I.; Watanabe, H.; Shinagawa, M. Lithium Isotope Separation by Liquid-Liquid Extraction Using Benzo-15-Crown-5. J. Nucl. Sci. Technol. 1984, 21, 694–701. [Google Scholar] [CrossRef]
- Nishizawa, K.; Takano, T.; Ikeda, I.; Okahara, M. Extractive Separation Of Lithium Isotopes By Crown Ethers. Sep. Sci. Technol. 1988, 23, 333–345. [Google Scholar] [CrossRef]
- Sun, H.; Jia, Y.; Liu, B.; Jing, Y.; Zhang, Q.; Shao, F.; Yao, Y. Separation of Lithium Isotopes by Using Solvent Extraction System of Crown Ether-Ionic Liquid. Fusion Eng. Des. 2019, 149, 111338. [Google Scholar] [CrossRef]
- Torrejos, R.E.C.; Nisola, G.M.; Song, H.S.; Limjuco, L.A.; Lawagon, C.P.; Parohinog, K.J.; Koo, S.; Han, J.W.; Chung, W.J. Design of Lithium Selective Crown Ethers: Synthesis, Extraction and Theoretical Binding Studies. Chem. Eng. J. 2017, 326, 921–933. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Liu, W.; Tong, B.; Li, M.; Yao, Y.; Zhu, M.; Zhao, Z.; Liu, W.; et al. Density Functional Theory Study of Selectivity of Crown Ethers to Li+ in Spent Lithium-Ion Batteries Leaching Solutions. Chin. J. Chem. Phys. 2019, 32, 343–348. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Ye, G.; Pu, N.; Wu, F.; Wang, Z.; Huo, X.; Xu, J.; Chen, J. Macrocyclic Ligand Decorated Ordered Mesoporous Silica with Large-Pore and Short-Channel Characteristics for Effective Separation of Lithium Isotopes: Synthesis, Adsorptive Behavior Study and DFT Modeling. Dalton Trans. 2016, 45, 16492–16504. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, S.; Kang, J.; Yin, C.; Guo, Y.; He, H.; Cheng, F. A Novel Ion-Pair Strategy for Efficient Separation of Lithium Isotopes Using Crown Ethers. Sep. Purif. Technol. 2021, 274, 118989. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Zhang, P.; Huang, C.; Zhang, Q.; Shao, F.; Jing, Y.; Jia, Y. Lithium Isotope Separation by Electromigration. Chem. Phys. Lett. 2020, 746, 137290. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Meng, Q.; Xue, Z.; Zhou, X.; Ju, H.; Mao, L.; Shao, F.; Jing, Y.; Jia, Y.; et al. Electromigration Separation of Lithium Isotopes: The Effect of Electrolytes. J. Environ. Chem. Eng. 2023, 11, 109933. [Google Scholar] [CrossRef]
- Hoshino, T.; Terai, T. High-Efficiency Technology for Lithium Isotope Separation Using an Ionic-Liquid Impregnated Organic Membrane. Fusion Eng. Des. 2011, 86, 2168–2171. [Google Scholar] [CrossRef]
- Shin-mura, K.; Tokuyoshi, R.; Tazoe, H.; Sasaki, K. Temperature Effect on Lithium Isotope Separation by Electrodialysis Using La0.57Li0.29TiO3 Electrolyte. Fusion Eng. Des. 2021, 171, 112577. [Google Scholar] [CrossRef]
- Ounissi, T.; Ammar, R.B.; Larchet, C.; Chaabane, L.; Baklouti, L.; Dammak, L.; Hmida, E.S.B.H. Lithium-Sodium Separation by a Lithium Composite Membrane Used in Electrodialysis Process: Concept Validation. Membranes 2022, 12, 244. [Google Scholar] [CrossRef]
- Bazrgar Bajestani, M.; Moheb, A.; Dinari, M. Preparation of Lithium Ion-Selective Cation Exchange Membrane for Lithium Recovery from Sodium Contaminated Lithium Bromide Solution by Electrodialysis Process. Desalination 2020, 486, 114476. [Google Scholar] [CrossRef]
- Ishikawa, A.; Sasaki, M.; Narita, S.; Takeuchi, A.; Ohki, H.; Yoshino, K. Chromatographic Formation of a Triadic Band of Lithium in Hydrated LTA Zeolite: An Investigation on Lithium Isotope Separation Effects by Ion Exchange. Microporous Mesoporous Mater. 2017, 248, 115–121. [Google Scholar] [CrossRef]
- Košler, J.; Kučera, M.; Sylvester, P. Precise Measurement of Li Isotopes in Planktonic Foraminiferal Tests by Quadrupole ICPMS. Chem. Geol. 2001, 181, 169–179. [Google Scholar] [CrossRef]
- Karami, H.; Sadeghi, M.; Nemati, S.; Zeinali, B. Chemical Separation of Li from Na+ and K+ Ions Using Ion Exchange Resin (AG 50W-X8). Anal. Chem. Indian J. 2009, 8, 109–113. [Google Scholar]
- Ivan Taylor, T.; Urey, H.C. On the Electrolytic and Chemical Exchange Methods for the Separation of the Lithium Isotopes. J. Chem. Phys. 1937, 5, 597. [Google Scholar] [CrossRef]
- Oi, T.; Kondoh, A.; Ohno, E.; Hosoe, M. Lithium Isotope Effects in Cation Exchange Chromatography of Lithium Lactate in Water-Dimethyl Sulfoxide and Water-Acetone Mixed Solvent Media. Z. Naturforsch. 1993, 48, 811–818. [Google Scholar] [CrossRef]
- Suzuki, T.; Tachibana, Y.; Matsumoto, K.; Putra, A.R.; Aikawa, F.; Sanduinjud, U.; Tanaka, M. Lithium Isotope Separation Using Cation Exchange Resin with High Cross-Linkage. Energy Procedia 2017, 131, 146–150. [Google Scholar] [CrossRef]
- Putra, A.R.; Tachibana, Y.; Tanaka, M.; Suzuki, T. Lithium Isotope Separation Using Displacement Chromatography by Cation Exchange Resin with High Degree of Cross-Linkage. Fusion Eng. Des. 2018, 136, 377–380. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vidyadharan Nair, V.; Mohan, S.; Suzuki, T. Chromatographic Fractionation of Lithium Isotope in Aqueous Solution Using Bifunctional Ion-Exchange Resin. Sep. Sci. Technol. 2019, 55, 2183–2192. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Sherrill, C.D. Effects of Heteroatoms on Aromatic π-π Interactions: Benzene-Pyridine and Pyridine Dimer. J. Phys. Chem. A 2009, 113, 878–886. [Google Scholar] [CrossRef]
- Qiao, L.; Rodriguez Peña, S.; Martínez-Ibañez, M.; Santiago, A.; Aldalur, I.; Lobato, E.; Sanchez-Diez, E.; Zhang, Y.; Manzano, H.; Zhu, H.; et al. Anion π- π Stacking for Improved Lithium Transport in Polymer Electrolytes. J. Am. Chem. Soc. 2022, 144, 9806–9816. [Google Scholar] [CrossRef]
- Tachibana, Y.; Rahma Putra, A.; Hashimoto, S.; Suzuki, T.; Tanaka, M. Lithium Isotope Fractionation in Weak Basic Solution Using Cation Exchange Chromatography. J. Ion Exch. 2018, 29, 41–47. [Google Scholar] [CrossRef]
- Nishizawa, K.; Watanabe, H.; Ishino, S.; Shinagawa, M. Lithium Isotope Separation by Cryptand (2B, 2, 1) Polymer. J. Nucl. Sci. Technol. 1984, 21, 133–138. [Google Scholar] [CrossRef]
- Yan, F.; Pei, H.; Pei, Y.; Li, T.; Li, J.; He, B.; Cheng, Y.; Cui, Z.; Guo, D.; Cui, J. Preparation and Characterization of Polysulfone-Graft-4′-Aminobenzo-15-Crown-5-Ether for Lithium Isotope Separation. Ind. Eng. Chem. Res. 2015, 54, 3473–3479. [Google Scholar] [CrossRef]
- Pei, H.; Yan, F.; Wang, Z.; Liu, C.; Hou, S.; Ma, X.; Li, J.; Cui, Z.; He, B.; Wickramsinghe, S.R. Polysulfone-Graft-4′-Aminobenzo-15-Crown-5-Ether Based Tandem Membrane Chromatography for Efficient Adsorptive Separation of Lithium Isotopes. J. Chromatogr. A 2019, 1602, 206–216. [Google Scholar] [CrossRef]
- Grégoire, D.C.; Acheson, B.M.; Taylor, R.P. Measurement of Lithium Isotope Ratios by Inductively Coupled Plasma Mass Spectrometry: Application to Geological Materials. J. Anal. At. Spectrom. 1996, 11, 765–772. [Google Scholar] [CrossRef]
- Jeffcoate, A.B.; Elliott, T.; Thomas, A.; Bouman, C. Precise/ Small Sample Size Determinations of Lithium Isotopic Compositions of Geological Reference Materials and Modern Seawater by MC-ICP-MS. Geostand. Geoanal. Res. 2004, 28, 161–172. [Google Scholar] [CrossRef]
- Tomascak, P.B.; Carlson, R.W.; Shirey, S.B. Accurate and Precise Determination of Li Isotopic Compositions by Multi-Collector Sector ICP-MS. Chem. Geol. 1999, 158, 145–154. [Google Scholar] [CrossRef]
- Liu, X.M.; Li, W. Optimization of Lithium Isotope Analysis in Geological Materials by Quadrupole ICP-MS. J. Anal. At. Spectrom. 2019, 34, 1708–1717. [Google Scholar] [CrossRef]
- Juzer, S.; Tanwar, N.; Misra, S. Precise Determination of Lithium Isotope Ratios at the Sub-Nanogram Level by QQQ-ICP-MS: Application to Natural Waters and Carbonates. J. Anal. At. Spectrom. 2022, 37, 1541–1553. [Google Scholar] [CrossRef]
- Rosner, M.; Ball, L.; Peucker-Ehrenbrink, B.; Blusztajn, J.; Bach, W.; Erzinger, J. A Simplified, Accurate and Fast Method for Lithium Isotope Analysis of Rocks and Fluids, and Δ7Li Values of Seawater and Rock Reference Materials. Geostand. Geoanal. Res. 2007, 31, 77–88. [Google Scholar] [CrossRef]
- Millot, R.; Guerrot, C.; Vigier, N. Accurate and High-Precision Measurement of Lithium Isotopes in Two Reference Materials by MC-ICP-MS. Geostand. Geoanal. Res. 2004, 28, 153–159. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Y.; Hu, Z.; Yang, L.; Chen, K.; Chen, H.; Zong, K.; Gao, S. Accurate Determination of Lithium Isotope Ratios by MC-ICP-MS without Strict Matrix-Matching by Using a Novel Washing Method. J. Anal. At. Spectrom. 2016, 31, 390–397. [Google Scholar] [CrossRef]
- Choi, M.S.; Ryu, J.S.; Park, H.Y.; Lee, K.S.; Kil, Y.; Shin, H.S. Precise Determination of the Lithium Isotope Ratio in Geological Samples Using MC-ICP-MS with Cool Plasma. J. Anal. At. Spectrom. 2013, 28, 505–509. [Google Scholar] [CrossRef]
- Greber, N.D.; Van Zuilenc, K. Multi-Collector Inductively Coupled Plasma Mass Spectrometry: New Developments and Basic Concepts for High-Precision Measurements of Mass-Dependent Isotope Signatures. Chimia 2022, 76, 18. [Google Scholar] [CrossRef]
- Scott, S.R.; Shafer, M.M. A new method for measurement of lithium isotopes with nuclear applications. Prog. Nucl. Energy 2020, 120, 103234. [Google Scholar] [CrossRef]
- Huang, K.F.; You, C.F.; Liu, Y.H.; Wang, R.M.; Lin, P.Y.; Chung, C.H. Low-Memory, Small Sample Size, Accurate and High-Precision Determinations of Lithium Isotopic Ratios in Natural Materials by MC-ICP-MS. J. Anal. At. Spectrom. 2010, 25, 1019–1024. [Google Scholar] [CrossRef]
- Tian, S.; Hou, Z.; Su, A.; Hou, K.; Hu, W.; Li, Z.; Zhao, Y.; Gao, Y.; Li, Y.; Yang, D.; et al. Separation and Precise Measurement of Lithium Isotopes in Three Reference Materials Using Multi Collector-Inductively Coupled Plasma Mass Spectrometry. Acta Geol. Sin. (Engl. Ed.) 2012, 86, 1297–1305. [Google Scholar] [CrossRef]
- Balaram, V.; Rahaman, W.; Roy, P. Recent Advances in MC-ICP-MS Applications in Earth and Environmental Sciences: Challenges and Solutions. GeoGeo 2022, 1, 100019. [Google Scholar] [CrossRef]
- Jackson, S.E.; Günther, D. The Nature and Sources of Laser Induced Isotopic Fractionation in Laser Ablation-Multicollector-Inductively Coupled Plasma-Mass Spectrometry. J. Anal. At. Spectrom. 2003, 18, 205–212. [Google Scholar] [CrossRef]
- Horn, I.; von Blanckenburg, F. Investigation on Elemental and Isotopic Fractionation during 196 Nm Femtosecond Laser Ablation Multiple Collector Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 410–422. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Z. A Critical Review of Isotopic Fractionation and Interference Correction Methods for Isotope Ratio Measurements by Laser Ablation Multi-Collector Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2020, 171, 105929. [Google Scholar] [CrossRef]
- Steinmann, L.K.; Oeser, M.; Horn, I.; Seitz, H.M.; Weyer, S. In Situ High-Precision Lithium Isotope Analyses at Low Concentration Levels with Femtosecond-LA-MC-ICP-MS. J. Anal. At. Spectrom. 2019, 34, 1447–1458. [Google Scholar] [CrossRef]
- Flesch, G.D.; Anderson, A.R.; Svec, H.J. A Secondary Isotopic Standard for 6Li/7Li Determinations. Int. J. Mass Spectrom. Ion Phys. 1973, 12, 265–272. [Google Scholar] [CrossRef]
- Chan, L.H. Lithium Isotope Analysis by Thermal Ionization Mass Spectrometry of Lithium Tetraborate. Anal. Chem. 1987, 59, 2662–2665. [Google Scholar] [CrossRef]
- Green, L.W.; Leppiner, J.J.; Elliot, N.L. Isotopic Analysis of Lithium as Thermal Dilithium Fluoride Ions. Anal. Chem. 1988, 60, 34–37. [Google Scholar] [CrossRef]
- Moriguti, T.; Nakamura, E. Precise Lithium Isotopic Analysis by Thermal Ionization Mass Spectrometry Using Lithium Phosphate as an Ion Source Material. Proc. Jpn. Acad. Ser. B 1993, 69, 123–128. [Google Scholar] [CrossRef]
- Ahmed, S.; Jabeen, N.; Ur Rehman, E. Determination of Lithium Isotopic Composition by Thermal Ionization Mass Spectrometry. Anal. Chem. 2002, 74, 4133–4135. [Google Scholar] [CrossRef]
- Arienzo, I.; Liotta, M.; Brusca, L.; D’Antonio, M.; Lupone, F.; Cucciniello, C. Analytical Method for Lithium Isotopes Determination by Thermal Ionization Mass Spectrometry: A Useful Tool for Hydrogeochemical Applications. Water 2020, 12, 2182. [Google Scholar] [CrossRef]
- Bhushan, K.S.; Goswami, P.G.; Rao, R.M. Precise Determination of 6Li/7Li Isotopic Ratio with NaLiBO2+ Ion Using Total Evaporation and Ion Integration by Thermal Ionization Mass Spectrometry(TIMS). Int. J. Mass Spectrom. 2021, 469, 116683. [Google Scholar] [CrossRef]
- Amelin, Y.; Merle, R. Isotopic Analysis of Potassium by Total Evaporation and Incipient Emission Thermal Ionisation Mass Spectrometry. Chem. Geol. 2021, 559, 119976. [Google Scholar] [CrossRef]

| Method Type | Main Advantages/Disadvantages | |||
|---|---|---|---|---|
| Separation Factor | Eco Friendly | Complexity of Methodology | Costs | |
| Chemical exchange methods in two liquid phases | High | Very little | Low | Very high |
| Electrochemical exchange methods | Low | Moderate | High | Moderate |
| Displacement chromatography methods | Low | Moderate | Relatively low | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badea, S.-L.; Niculescu, V.-C.; Iordache, A.-M. New Trends in Separation Techniques of Lithium Isotopes: A Review of Chemical Separation Methods. Materials 2023, 16, 3817. https://doi.org/10.3390/ma16103817
Badea S-L, Niculescu V-C, Iordache A-M. New Trends in Separation Techniques of Lithium Isotopes: A Review of Chemical Separation Methods. Materials. 2023; 16(10):3817. https://doi.org/10.3390/ma16103817
Chicago/Turabian StyleBadea, Silviu-Laurentiu, Violeta-Carolina Niculescu, and Andreea-Maria Iordache. 2023. "New Trends in Separation Techniques of Lithium Isotopes: A Review of Chemical Separation Methods" Materials 16, no. 10: 3817. https://doi.org/10.3390/ma16103817
APA StyleBadea, S.-L., Niculescu, V.-C., & Iordache, A.-M. (2023). New Trends in Separation Techniques of Lithium Isotopes: A Review of Chemical Separation Methods. Materials, 16(10), 3817. https://doi.org/10.3390/ma16103817







