Nanocomposite Electrocatalysts for Hydrogen Evolution Reactions (HERs) for Sustainable and Efficient Hydrogen Energy—Future Prospects
Abstract
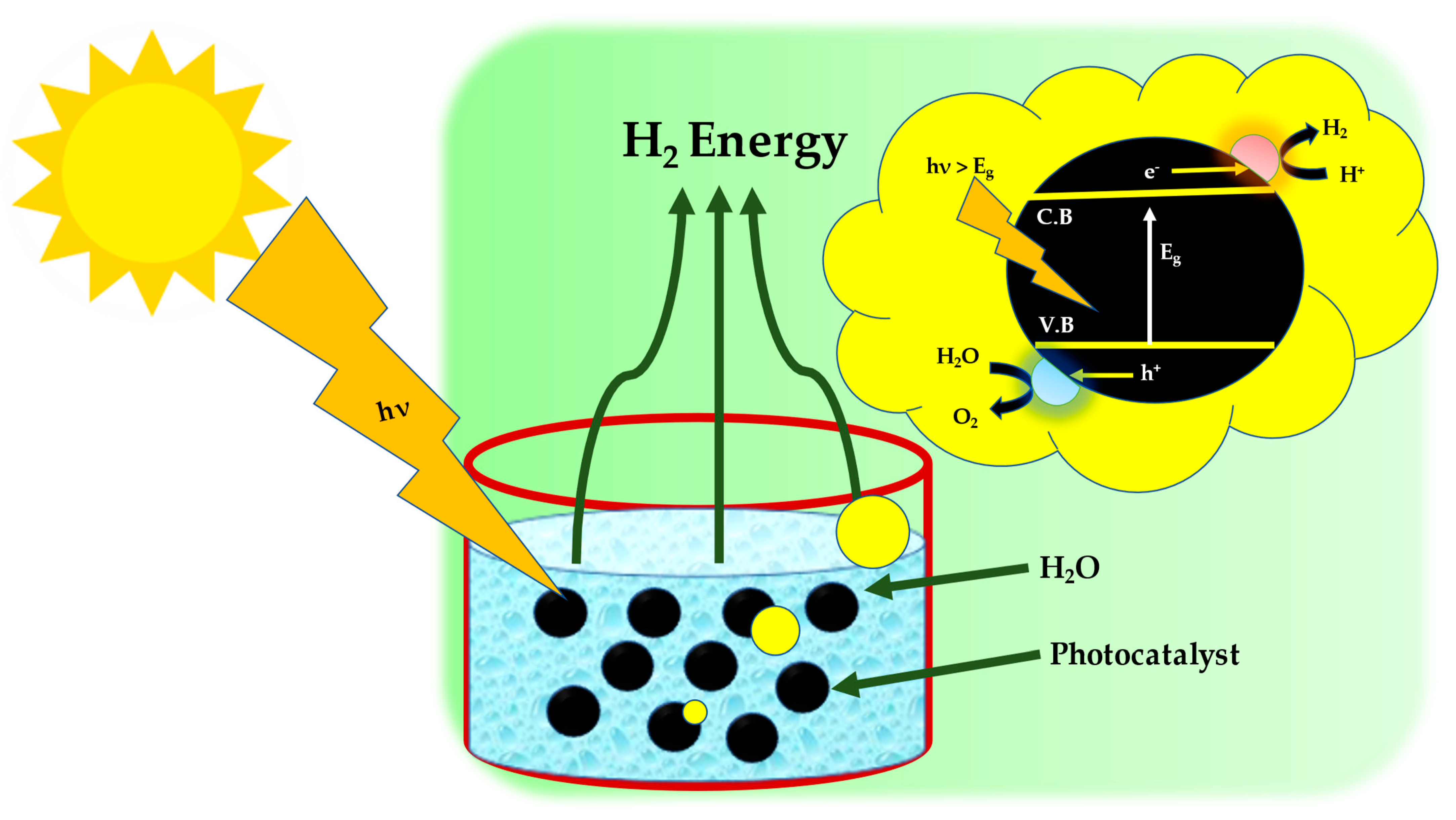
1. Introduction
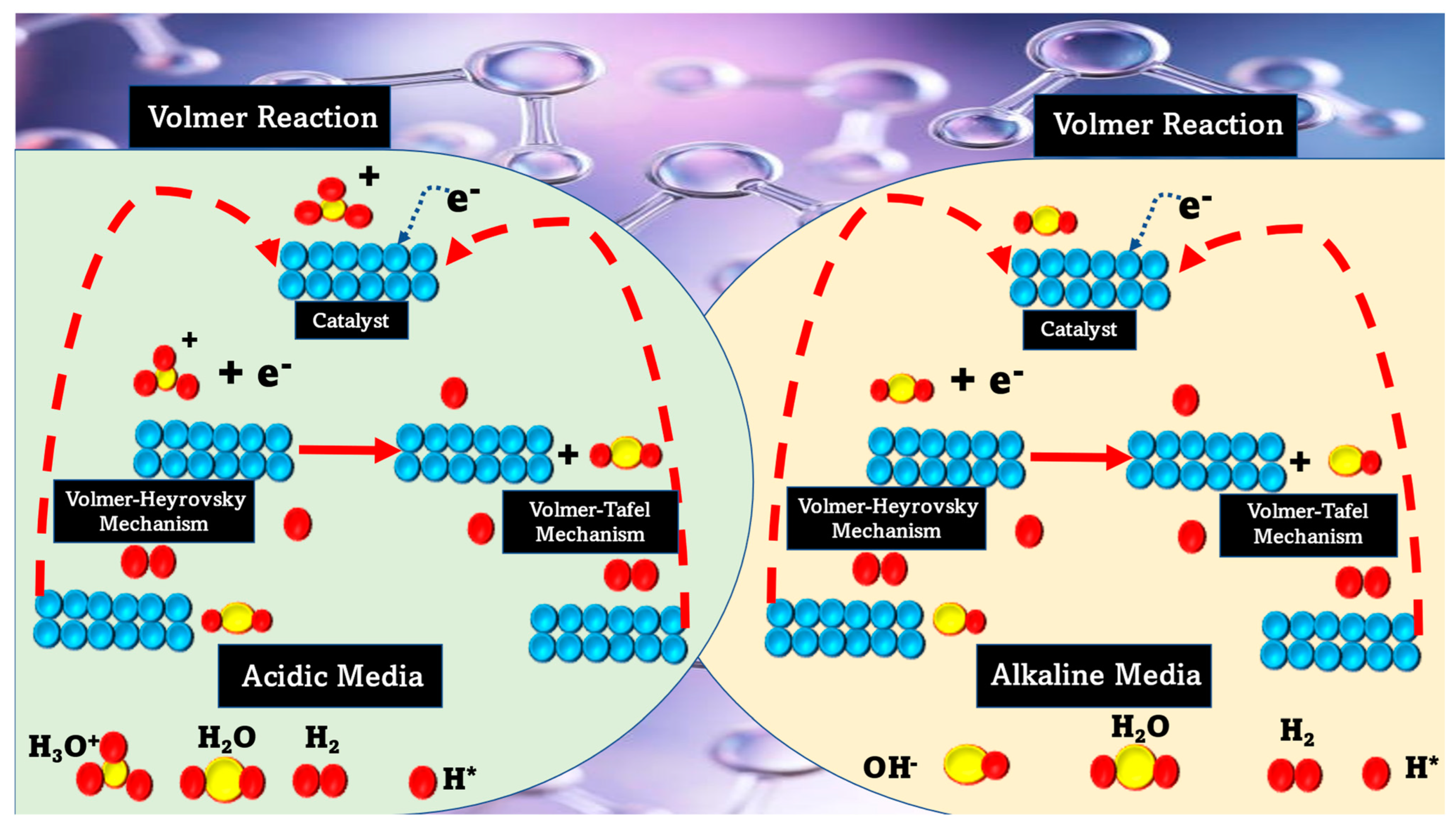
2. Basic Reactions Involved in HERs
3. Electrocatalysts in HERs
3.1. Noble Metal Electrocatalysts
3.2. Non-Noble Metal Electrocatalysts
4. Composite HER Electrocatalysts
4.1. Nanocomposites for HER Applications
4.1.1. Pt—Composites
4.1.2. Palladium-Based Composites
4.1.3. Nickel-Based Composites
| Catalysts | HER Performance | Stability | Reference |
|---|---|---|---|
| PtNi-Ni NA/CC | 10 I mA cm−2; 38η mV; Tafel slope of 42 mV dec−1 | 90 h | [16] |
| PtNi-O/C | 10 I mA cm−2; 39.8η mV; Tafel slope of 78.8 mV dec−1 | 10 h | [22] |
| PtNi(N) NW | 10 I mA cm−2; 13η mV; Tafel slope of 29 mV dec−1 | 10 h | [15] |
| Mo2C-R | 32 I mA cm−2; 200η mV; Tafel slope of 58 mV dec−1 | 2000 cycle | [25] |
| Mo2C-GNR | 10 I mA cm−2; 266η mV; Tafel slope of 74 mV dec−1 | 3000 cycles | [26] |
| Ni2P/Ti | 20 I mA cm−2; 130η mV; Tafel slope of 46 mV dec−1 | 500 cycles | [29] |
| NiCo2Px | 10 I mA cm−2; 63η mV; Tafel slope of 63.6 mV dec−1 | 5000 cycles | [30] |
| Defect rich MoS2 | 13 I mA cm−2; 200η mV; Tafel slope of 50 mV dec−1 | 10,000 s | [32] |
| CoS2 NW | 10 I mA cm−2; 145η mV; Tafel slope of 51.6 mV dec−1 | 3 h | [33] |
| CoS2 NW | 10 I mA cm−2; 158η mV; Tafel slope of 58 mV dec−1 | 41 h | [33] |
| Oxygenated MoS2 | 120η mV; Tafel slope of 55 mV dec−1 | 3000 cycles | [34] |
| Pt SAs over nanosheets of a two-dimensional inorganic material MXene-Mo2TiC2Tx | Overpotential of 77 mV is 8.3 Amg−1, 39.5 times more than commercial HER catalyst (40 wt% Pt/C, 0.21 Amg−1); Tafel slope of 30 mVdec−1 | 10,000 cycles | [58] |
| PdCu@Pd nanocube core@shell | 10 I mA cm−2; 65η mV; Tafel slope of 35 mV dec−1 | ND | [70] |
| Polyvinylpyrrolidone as a stabiliser to make Pd icosahedral NPs | 32 mV at 10 mA cm−2 | 130,000 cycles | [71] |
| Ni2P NW | 320 mV and a Tafel slope of 73 mV dec−1 | ND | [79] |
| Cu-Ni nanocages | Current density of 10 mA cm−2 (under an overpotential of 140 mV). | ND | [74] |
| Pt SA on graphene nanosheets | 10 times more active than commercial Pt/C | 150 cycles | [62] |
| Pt SAs and clusters on nitrogen-doped graphene nanosheets | 37 times more active than Pt/C | 50 and 100 cycles | [61] |
| Pt nanocubes on FTO/glass substrate | 1.77 A mg−1 at 50 mV and 0.54 A mg−1 at 100 mV | ND | [55] |
| Ni-HG-rGO/NF catalysts | −10 and −100 mA cm−2; overpotentials of −50 and −132 mV; a low Tafel slope of −48 mV dec−1 | ND | [43] |
| Cobalt SACs with a Co-N4 | 21 mV onset overpotential (h0) and a Tafel slope of 50 mV dec−1 | 10 h | [42] |
| NiFeMo alloy | Ultralow overpotentials of 33 and 249 mV; 500 mA cm−2 | 50 h | [46] |
| NiCo-nitrides/NiCo2O4/GF | Low Tafel slope of 58 mV dec−1; lowest overpotentials (η) of 71 and 180 mV to obtain current densities of 10 and 50 mA cm−2 | over 40 h | [51] |
| Ni@Pd/PEI–rGO stack structures | 10 I mA cm−2; 90η mV; Tafel slope of 54 mV dec−1 | ND | [81] |
| MWCNTs@Cu@MoS2 | 10 I mA cm−2; 184η mV; Tafel slope of 62 mV dec−1 | 1000 cycles | [82] |
| Nanoporous Ag2S/CuS | 10 I mA cm−2; 200η mV; Tafel slope of 75 mV dec−1 | 1000 cycles | [83] |
| Wl8O49@WS2 NRs | 10 I mA cm−2; 310η mV; Tafel slope of 86 mV dec−1 | 750 cycles | [84] |
| RuCo/Ti foil | 10 I mA cm−2; 387η mV; Tafel slope of 107 mV dec−1 | >12 h | [85] |
| Rh2S3–Thick HNP/C | 10 I mA cm−2; 122η mV; Tafel slope of 44 mV dec−1 | 10,000 cycles | [86] |
| Fe1-xCoxS2/CNT | 10 I mA cm−2; 158η mV; Tafel slope of 46 mV dec−1 | >40 h | [87] |
| MoS2@OMC | 10 I mA cm−2; 182η mV; Tafel slope of 60 mV dec−1 | ND | [88] |
| Pd2Te NWs/rGO | 10 I mA cm−2; 48η mV; Tafel slope of 63 mV dec−1 | 48 h | [89] |
| NiAu@Au NPs | Tafel slope of 36 mV dec−1 | 20,000 cycles | [90] |
5. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Dincă, M.; Surendranath, Y.; Nocera, D.G. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl. Acad. Sci. USA 2010, 107, 10337–10341. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhou, L.; Sun, L.; Xu, L.; Cheng, D.-G.; Chen, F.; Zhan, X.; Yang, Y. Boosting visible-light-driven hydrogen evolution from formic acid over AgPd/2D g-C3N4 nanosheets Mott-Schottky photocatalyst. Chem. Eng. J. 2020, 396, 125229. [Google Scholar] [CrossRef]
- Li, A.; Sun, Y.; Yao, T.; Han, H. Earth-Abundant Transition-Metal-Based Electrocatalysts for Water Electrolysis to Produce Renewable Hydrogen. Chem. Eur. J. 2018, 24, 18334–18355. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Sardar, K.; Petrucco, E.; Hiley, C.I.; Sharman, J.D.; Wells, P.P.; Russell, A.E.; Kashtiban, R.J.; Sloan, J.; Walton, R.I. Water-Splitting Electrocatalysis in Acid Conditions Using Ruthenate-Iridate Pyrochlores. Angew. Chem. Int. Ed. 2014, 126, 11140–11144. [Google Scholar] [CrossRef]
- Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; Van Der Vliet, D.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 2013, 5, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.; Liu, J.; Guo, C. Engineering transition metal-based nanomaterials for high-performance electrocatalysis. Mater. Rep. Energy 2021, 1, 100006. [Google Scholar] [CrossRef]
- Feng, Y.; Duan, Y.; Zou, H.; Ma, J.; Zhou, K.; Zhou, X. Research Status of Single Atom Catalyst in Hydrogen Production by Photocatalytic Water Splitting. Chin. J. Rare Met. 2021, 45, 551–568. [Google Scholar]
- Zhao, X.; Chang, Y.; He, X.; Zhang, H.; Jia, J.; Jia, M. Understanding ultra-dispersed CeOx modified iridium clusters as bifunction electrocatalyst for high-efficiency water splitting in acid electrolytes. J. Rare Earths 2023, 41, 208–214. [Google Scholar] [CrossRef]
- Skúlason, E.; Karlberg, G.S.; Rossmeisl, J.; Bligaard, T.; Greeley, J.; Jónsson, H.; Nørskov, J.K. Density functional theory calculations for the hydrogen evolution reaction in an electrochemical double layer on the Pt(111) electrode. Phys. Chem. Chem. Phys. 2007, 9, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, Q.; Shi, X.; Asiri, A.M.; Luo, Y.; Sun, X. Superior alkaline hydrogen evolution electrocatalysis enabled by an ultrafine PtNi nanoparticle-decorated Ni nanoarray with ultralow Pt loading. Inorg. Chem. Front. 2018, 5, 1365–1369. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2019, 120, 851–918. [Google Scholar] [CrossRef]
- Xie, Y.; Cai, J.; Wu, Y.; Zang, Y.; Zheng, X.; Ye, J.; Cui, P.; Niu, S.; Liu, Y.; Zhu, J. Boosting Water Dissociation Kinetics on Pt-Ni Nanowires by N-Induced Orbital Tuning. Adv. Mater. 2019, 31, 1807780. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, X.; Luo, Y.; Wang, L.; Cui, G.; Xie, F.; Wang, H.; Xie, Y.; Sun, X. An ultrafine platinum–cobalt alloy decorated cobalt nanowire array with superb activity toward alkaline hydrogen evolution. Nanoscale 2018, 10, 12302–12307. [Google Scholar] [CrossRef]
- Li, Q.; Wu, L.; Wu, G.; Su, D.; Lv, H.; Zhang, S.; Zhu, W.; Casimir, A.; Zhu, H.; Mendoza-Garcia, A. New Approach to Fully Ordered fct-FePt Nanoparticles for Much Enhanced Electrocatalysis in Acid. Nano Lett. 2015, 15, 2468–2473. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Wang, A.J.; Zhang, L.; Fang, K.-M.; Wu, L.-J.; Feng, J.J. Melamine-assisted solvothermal synthesis of PtNi nanodentrites as highly efficient and durable electrocatalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2018, 531, 578–584. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, H.; Gao, W.; Xue, W.; Liu, Z.; Huang, J.; Pan, X.; Huang, Y. Surface-Engineered PtNi-O Nanostructure with Record-High Performance for Electrocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 9046–9050. [Google Scholar] [CrossRef]
- Levy, R.; Boudart, M. Platinum-like behavior of tungsten carbide in surface catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Trends in the chemical properties of early transition metal carbide surfaces: A density functional study. Catal. Today 2005, 105, 66–73. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef]
- Xiao, P.; Yan, Y.; Ge, X.; Liu, Z.; Wang, J.-Y.; Wang, X. Investigation of molybdenum carbide nano-rod as an efficient and durable electrocatalyst for hydrogen evolution in acidic and alkaline media. Appl. Catal. B 2014, 154, 232–237. [Google Scholar] [CrossRef]
- Gao, W.; Shi, Y.; Zhang, Y.; Zuo, L.; Lu, H.; Huang, Y.; Fan, W.; Liu, T. Molybdenum Carbide Anchored on Graphene Nanoribbons as Highly Efficient All-pH Hydrogen Evolution Reaction Electrocatalyst. ACS Sustain. Chem. Eng. 2016, 4, 6313–6321. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A. Catalysts for Hydrogen Evolution from the [NiFe] Hydrogenase to the Ni2P(001) Surface: The Importance of Ensemble Effect. J. Am. Chem. Soc. 2005, 127, 14871–14878. [Google Scholar] [CrossRef] [PubMed]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Yu, S.; Wen, T.; Zhu, X.; Yang, F.; Sun, X.; Wang, X.; Hu, W. Ternary NiCo2Px Nanowires as pH-Universal Electrocatalysts for Highly Efficient Hydrogen Evolution Reaction. Adv. Mater. 2017, 29, 1605502. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Faber, M.S.; Dziedzic, R.; Lukowski, M.A.; Kaiser, N.S.; Ding, Q.; Jin, S. High-Performance Electrocatalysis Using Metallic Cobalt Pyrite (CoS2) Micro- and Nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [PubMed]
- Chen, Z.; Wei, W.; Ni, B.-J. Cost-effective catalysts for renewable hydrogen production via electrochemical water splitting: Recent advances. Curr. Opin. Green Sustain. Chem. 2021, 27, 100398. [Google Scholar]
- Zhao, D.; Zhuang, Z.; Cao, X.; Zhang, C.; Peng, Q.; Chen, C.; Li, Y. Atomic site electrocatalysts for water splitting, oxygen reduction and selective oxidation. Chem. Soc. Rev. 2020, 49, 2215–2264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, W.; Duan, X.; Sun, H.; Liu, S.; Wang, S. Catalysis of a single transition metal site for water oxidation: From mono nuclear molecules to single atoms. Adv. Mater. 2019, 32, 1904037. [Google Scholar] [CrossRef]
- Liu, J. Single-atom catalysis for a sustainable and greener future. Curr. Opin. Green Sustain. Chem. 2020, 22, 54–64. [Google Scholar] [CrossRef]
- Pu, Z.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Mu, S.; Zhao, W.; Su, F.; Zhang, G.; Liao, S.; et al. Single-atom catalysts for electrochemical hydrogen evolution reaction: Recent advances and future perspectives. Nano-Micro Lett. 2020, 12, 21. [Google Scholar] [CrossRef]
- Sultan, S.; Tiwari, J.N.; Singh, A.N.; Zhumagali, S.; Ha, M.; Myung, C.W.; Thangavel, P.; Kim, K.S. Single atoms and clusters based nanomaterials for hydrogen evolution, oxygen evolution re actions, and full water splitting. Adv. Energy Mater. 2019, 9, 1900624. [Google Scholar] [CrossRef]
- Qi, K.; Cui, X.; Gu, L.; Yu, S.; Fan, X.; Luo, M.; Xu, S.; Li, N.; Zheng, L.; Zhang, Q.; et al. Single-atom cobalt array bound to distorted 1T MoS2 with ensemble effect for hydrogen evolution catalysis. Nat. Commun. 2019, 10, 5231. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.-D.; Xu, R.; Chai, G.-L.; Zhang, T.; Zang, K.; Nan, B.; Lin, H.; Liang, Y.-L.; Lv, J.; Luo, J.; et al. Cobalt single-atoms anchored on porphyrinic triazine-based frameworks as bifunctional electrocatalysts for oxygen reduction and hydrogen evolution reactions. J. Mater. Chem. A 2019, 7, 1252–1259. [Google Scholar] [CrossRef]
- Du, J.; Wang, L.; Bai, L.; Dang, S.; Su, L.; Qin, X.; Shao, G. Datura-like Ni-HG-rGO as highly efficient electrocatalyst for hydrogen evolution reaction in alkaline conditions. J. Colloid Interface Sci. 2019, 535, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Liu, X.; Chen, S.; Vasileff, A.; Li, L.; Jiao, Y.; Song, L.; Zheng, Y.; Qiao, S.-Z. Heteroatom-doped transition metal electrocatalysts for hydrogen evolution reaction. ACS Energy Lett. 2019, 4, 805–810. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Ni, B.-J. Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J. Mater. Chem. A 2019, 7, 14971–15005. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Huang, C.-L.; Chen, Y.-A.; Lu, S.-Y. NiFeMo alloy inverse opals on Ni foam as outstanding bifunctional catalysts for electrolytic water splitting of ultra-low cell voltages at high current densities. Appl. Catal. B Environ. 2020, 267, 118376. [Google Scholar] [CrossRef]
- Gao, D.; Liu, R.; Biskupek, J.; Kaiser, U.; Song, Y.F.; Streb, C. Modular design of noble-metal-free mixed metal oxide electro catalysts for complete water splitting. Angew. Chem. Int. Ed. 2019, 58, 4644–4648. [Google Scholar] [CrossRef]
- Sun, H.; Li, J.-G.; Lv, L.; Li, Z.; Ao, X.; Xu, C.; Xue, X.; Hong, G.; Wang, C. Engineering hierarchical CoSe/NiFe layered-double hydroxide nanoarrays as high efficient bifunctional electro catalyst for overall water splitting. J. Power Sources 2019, 425, 138–146. [Google Scholar] [CrossRef]
- Cai, Z.; Bu, X.; Wang, P.; Su, W.; Wei, R.; Ho, J.C.; Yang, J.; Wang, X. Simple and cost effective fabrication of 3D porous core–shell Ni nanochains@NiFe layered double hydroxide nanosheet bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2019, 7, 21722–21729. [Google Scholar] [CrossRef]
- Hu, Q.; Li, G.; Han, Z.; Wang, Z.; Huang, X.; Yang, H.; Zhang, Q.; Liu, J.; He, C. Recent progress in the hybrids of transition metals/carbon for electrochemical water splitting. J. Mater. Chem. A 2019, 7, 14380–14390. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, H.; Liu, D.; Liu, X.; Xin, J.; Xie, J.; Zhao, M.; Song, L.; Dai, L.; Liu, H. Promotion of overall water splitting activity over a wide pH range by interfacial electrical effects of metallic NiCo nitrides nanoparticle/NiCo2O4 nanoflake/graphite fibers. Adv. Sci. 2019, 6, 1801829. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, S.; Ma, Y.; Qiu, Y.; An, Y.; Cheng, L. Interfacial en gineering of cobalt nitrides and mesoporous nitrogen-doped carbon: Toward efficient overall water-splitting activity with enhanced charge-transfer efficiency. ACS Energy Lett. 2020, 5, 692–700. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, B.; Wu, S.; Yu, J. A general approach to the syn thesis of transition metal phosphide nanoarrays on MXene nanosheets for pH-universal hydrogen evolution and alkaline overall water splitting. J. Mater. Chem. A 2020, 8, 14234–14242. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Cui, X.; Chen, B.; Zhu, Y.; Gong, Y.; Saleem, F.; Xi, S.; Du, Y.; Borgna, A.; et al. Crystal Phase and Architecture Engineering of Lotus-Thalamus-Shaped Pt-Ni Anisotropic Superstructures for Highly Efficient Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1801741. [Google Scholar] [CrossRef]
- Koo, B.; Chu, J.; Seo, J.; Jung, G.; Baek, S.H.; Nam, S.W.; Duah, C.; Lee, Y.K.; Jung, W.C.; Shin, B. Drop-casted Platinum Nanocube Catalysts for Hydrogen Evolution Reaction with Ultrahigh Mass Activity. ChemSusChem 2021, 14, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, J. A Review on Particle Size Effect in Metal-Catalyzed Heterogeneous Reactions. Chin. J. Chem. 2020, 38, 1422–1444. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Yu, P.-W.; Elmas, S.; Roman, T.; Pan, X.; Yin, Y.; Gibson, C.T.; Andersson, G.G.; Andersson, M.R. Highly Active Platinum Single-Atom Catalyst Grafted onto 3D Carbon Cloth Support for the Electrocatalytic Hydrogen Evolution Reaction. Appl. Surf. Sci. 2022, 595, 153480. [Google Scholar] [CrossRef]
- Elmas, S.; Beelders, W.; Bradley, S.J.; Kroon, R.; Laufersky, G.; Andersson, M.; Nann, T. Platinum Terpyridine Metallopolymer Electrode as Cost-Effective Replacement for Bulk Platinum Catalysts in Oxygen Reduction Reaction and Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2017, 5, 10206–10214. [Google Scholar]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.R.; Xiao, Y.Y.; Yin, Y.C.; Huang, W.M.; Huang, Z.C.; Zheng, Y.Z.; Mu, F.Y.; Huang, R.; Shi, G.Y.; et al. Electronic metal–support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nat. Commun. 2021, 12, 3021. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, M.; Holmberg, N.; Kronberg, R.; Jiang, H.; Sainio, J.; Kauppinen, E.I.; Kallio, T.; Laasonen, K.; Tavakkoli, M.; Holmberg, N.; et al. Electrochemical activation of single-walled carbon nanotubes with pseudo atomic-scale platinum for hydrogen evolution reaction. ACS Catal. 2017, 7, 3121–3130. [Google Scholar] [CrossRef]
- Fang, S.; Zhu, X.; Liu, X.; Gu, J.; Liu, W.; Wang, D.; Zhang, W.; Lin, Y.; Lu, J.; Wei, S.; et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 2020, 11, 1029. [Google Scholar] [CrossRef]
- Xiong, B.Y.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Palladium. Adv. Mater. 2007, 19, 3385–3391. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, S.; Liu, J.; Park, J.; Huang, C.Z.; Xia, Y. Shape-Controlled Synthesis of Palladium Nanocrystals: A Mechanistic Understanding of the Evolution from Octahedrons to Tetrahedrons. Nano Lett. 2013, 13, 2276–2281. [Google Scholar] [CrossRef]
- Janssen, A.; Shi, Y.; Xia, Y. Separating Growth from Nucleation for Facile Control over the Size and Shape of Palladium Nanocrystals. Chem. A Eur. J. 2020, 26, 13890–13895. [Google Scholar] [CrossRef]
- Zalineeva, A.; Baranton, S.; Coutanceau, C.; Jerkiewicz, G. Octahedral palladium nanoparticles as excellent hosts for electrochemically adsorbed and absorbed hydrogen. Sci. Adv. 2017, 3, e1600542. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Guo, S.-X.; Zhang, J.; Ma, J. PdCu@Pd Nanocube with Pt-like Activity for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 8151–8160. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Sui, Y.; Wang, M.; Qiao, L.; Du, F.; Zou, B. Palladium structure engineering induced by electrochemical H intercalation boosts hydrogen evolution catalysis. J. Mater. Chem. A 2019, 7, 14876–14881. [Google Scholar] [CrossRef]
- Liang, Z.; Ahn, H.S.; Bard, A.J. A Study of the Mechanism of the Hydrogen Evolution Reaction on Nickel by Surface Interrogation Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2017, 139, 4854–4858. [Google Scholar] [CrossRef] [PubMed]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.G.; Yoon, T.; Kim, K.S. Nickel-based electrocatalysts for energy-related applications: Oxygen reduction, oxygen evolution, and hydrogen evolution reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Wen, Y.; Gao, Y.; Xing, X.; Wei, Z.; Sun, H.; Zhang, Y.W.; Song, W. Mesoporous Hollow Cu-Ni Alloy Nanocage from Core-Shell Cu@Ni Nanocube for Efficient Hydrogen Evolution Reaction. ACS Catal. 2019, 9, 5084–5095. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, Q.; Sun, Y.; Ning, G.; Fan, X.; Wang, H.; Lu, H.; Zhang, Y.; Wang, H. Ni–Fe nanocubes embedded with Pt nanoparticles for hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 20832–20842. [Google Scholar] [CrossRef]
- Hong, Y.; Choi, C.H.; Choi, S.-I. Catalytic Surface Specificity of Ni(OH)2-Decorated Pt Nanocubes for the Hydrogen Evolution Reaction in an Alkaline Electrolyte. ChemSusChem 2019, 12, 4021–4028. [Google Scholar] [CrossRef]
- Kavian, R.; Choi, S.-I.; Park, J.; Liu, T.; Peng, H.C.; Lu, N.; Wang, J.; Kim, M.J.; Xia, Y.; Lee, S.W. Pt-Ni octahedral nanocrystals as a class of highly active electrocatalysts toward the hydrogen evolution reaction in an alkaline electrolyte. J. Mater. Chem. A 2016, 4, 12392–12397. [Google Scholar] [CrossRef]
- Seo, B.; Baek, D.S.; Sa, Y.J.; Joo, S.H. Shape effects of nickel phosphide nanocrystals on hydrogen evolution reaction. CrystEngComm 2016, 18, 6083–6089. [Google Scholar] [CrossRef]
- Xiang, D.; Zhang, B.; Zhang, H.; Shen, L. One-Step Synthesis of Bifunctional Nickel Phosphide Nanowires as Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. Front. Chem. 2021, 9, 773018. [Google Scholar] [CrossRef]
- Fiorio, J.L.; Gothe, M.L.; Kohlrausch, E.C.; Zardo, M.L.; Tanaka, A.A.; de Lima, R.B.; da Silva, A.G.M.; Garcia, M.A.S.; Vidinha, P.; Machado, G. Nanoengineering of Catalysts for Enhanced Hydrogen Production. Hydrogen 2022, 3, 218–254. [Google Scholar] [CrossRef]
- Li, J.; Zhou, P.; Li, F.; Ren, R.; Liu, Y.; Niu, J.; Ma, J.; Zhang, X.; Tian, M.; Jin, J.; et al. Ni@Pd/PEI–rGO stack structures with controllable Pd shell thickness as advanced electrodes for efficient hydrogen evolution. J. Mater. Chem. A 2015, 3, 11261–11268. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Lin, X.; Li, X.; Fang, Y.; Jiao, L.; An, X.; Fu, Y.; Jin, J.; Li, R. Designed Synthesis of Multi-Walled Carbon Nanotubes@Cu@MoS2 Hybrid as Advanced Electrocatalyst for Highly Efficient Hydrogen Evolution Reaction. J. Power Sources 2015, 300, 301–308. [Google Scholar] [CrossRef]
- Ren, H.; Xu, W.; Zhu, S.; Cui, Z.; Yang, X.; Inoue, A. Synthesis and Properties of Nanoporous Ag2S/CuS Catalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2016, 190, 221–228. [Google Scholar] [CrossRef]
- Seo, B.; Jeong, H.Y.; Hong, S.Y.; Zak, A.; Joo, S.H. Impact of a conductive oxide core in tungsten sulfide-based nanostructures on the hydrogen evolution reaction. Chem. Commun. 2015, 51, 8334–8337. [Google Scholar] [CrossRef]
- Niu, X.; Tang, Q.; He, B.; Yang, P. Robust and Stable Ruthenium Alloy Electrocatalysts for Hydrogen Evolution by Seawater Splitting. Electrochim. Acta 2016, 208, 180–187. [Google Scholar] [CrossRef]
- Yoon, D.; Seo, B.; Lee, J.; Nam, K.S.; Kim, B.; Park, S.; Baik, H.; Joo, S.H.; Lee, K. Facet-Controlled Hollow Rh2S3 Hexagonal Nanoprisms as Highly Active and Structurally Robust Catalysts Toward Hydrogen Evolution Reaction. Energy Environ. Sci. 2016, 9, 850–856. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Gong, M.; Chou, H.-L.; Pan, C.-J.; Chen, H.-A.; Wu, Y.; Lin, M.-C.; Guan, M.; Yang, J.; Chen, C.-W. Highly Active and Stable Hybrid Catalyst of Cobalt-Doped FeS2 Nanosheets–Carbon Nanotubes for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 1587–1592. [Google Scholar] [CrossRef]
- Seo, B.; Jung, G.Y.; Sa, Y.J.; Jeong, H.Y.; Cheon, J.Y.; Lee, J.H.; Kim, H.Y.; Kim, J.C.; Shin, H.S.; Kwak, S.K. Monolayer-Precision Synthesis of Molybdenum Sulfide Nanoparticles and Their Nanoscale Size Effects in the Hydrogen Evolution Reaction. ACS Nano 2015, 9, 3728–3739. [Google Scholar] [CrossRef]
- Jiao, L.; Li, F.; Li, X.; Ren, R.; Li, J.; Zhou, X.; Jin, J.; Li, R. Ultrathin PdTe Nanowires Anchoring Reduced Graphene Oxide Cathodes for Efficient Hydrogen Evolution Reaction. Nanoscale 2015, 7, 18441–18445. [Google Scholar] [CrossRef]
- Lv, H.; Xi, Z.; Chen, Z.; Guo, S.; Yu, Y.; Zhu, W.; Li, Q.; Zhang, X.; Pan, M.; Lu, G. A New Core/Shell NiAu/Au Nanoparticle Catalyst with Pt-like Activity for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 5859–5862. [Google Scholar] [CrossRef]
- Mondal, S.; Mohanty, B.; Nurhuda, M.; Dalapati, S.; Jana, R.; Addicoat, M.; Datta, A.; Jena, B.K.; Bhaumik, A. A thiadiazole-based covalent organic framework: A metal-free electrocatalyst toward oxygen evolution reaction. ACS Catal. 2020, 10, 5623–5630. [Google Scholar] [CrossRef]
- Lei, C.; Zheng, Q.; Cheng, F.; Hou, Y.; Yang, B.; Li, Z.; Wen, Z.; Lei, L.; Chai, G.; Feng, X. High-performance metal-free nanosheets array electrocatalyst for oxygen evolution reaction in acid. Adv. Funct. Mater. 2020, 30, 2003000. [Google Scholar] [CrossRef]
- Paul, R.; Zhu, L.; Chen, H.; Qu, J.; Dai, L. Recent advances in carbonbased metal-free electrocatalysts. Adv. Mater. 2019, 31, 1806403. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, M.; Jiang, G.; Li, G.; Zhu, J.; Xiao, M.; Zhu, Y.; Gao, R.; Yu, A.; Feng, M.; et al. Graphene quantum dots-based advanced electrode materials: Design, synthesis and their applications in electrochemical energy storage and electrocatalysis. Adv. Energy Mater. 2020, 10, 2001275. [Google Scholar] [CrossRef]
- Li, B.; Lai, C.; Zhang, M.; Zeng, G.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Yi, H.; Xu, F.; et al. Graphdiyne: A rising star of electrocatalyst support for energy conversion. Adv. Energy Mater. 2020, 10, 2000177. [Google Scholar] [CrossRef]
- Wang, X.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Electronic and structural engineering of carbon-based metal-free electrocatalysts for water splitting. Adv. Mater. 2019, 31, 1803625. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Chhetri, M. Borocarbonitrides as metal-free catalysts for the hydrogen evolution reaction. Adv. Mater. 2019, 31, 1803668. [Google Scholar] [CrossRef]
- Liu, Z.; Ai, J.; Sun, M.; Han, F.; Li, Z.; Peng, Q.; Wang, Q.D.; Liu, J.; Liu, L. Phosphorous-doped graphite layers with outstanding electrocatalytic activities for the oxygen and hydrogen evolutionreactions in water electrolysis. Adv. Funct. Mater. 2020, 30, 1910741. [Google Scholar] [CrossRef]
- Dinh, K.N.; Zhang, Y.; Zhu, J.; Sun, W. Phosphorene-based electrocatalysts. Chem. Eur. J. 2020, 26, 6437–6446. [Google Scholar] [CrossRef]
- Xia, X.; Liu, L.; Li, X.; Gao, S.; Yang, T. Highly efficient electrocatalytic hydrogen evolution over edge-modified phosphorene quantum dot/prussian blue skeleton structure. J. Catal. 2019, 374, 401–408. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Liu, D.; Yang, N.; Huang, H.; Jin, S.; Wang, J.; Chu, P.K.; Yu, X.F. Modulation of phosphorene for optimal hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2019, 11, 37787–37795. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Muthu, M.; Sivanesan, I. A Comprehensive Compilation of Graphene/Fullerene Polymer Nanocomposites for Electrochemical Energy Storage. Polymers 2023, 15, 701. [Google Scholar] [CrossRef]
- Jawhari, A.H.; Hasan, N.; Radini, I.A.; Malik, M.A.; Narasimharao, K. Pt-Ag/Ag3PO4-WO3 nanocomposites for photocatalytic H2 production from bioethanol. Fuel 2023, 344, 127998. [Google Scholar] [CrossRef]
- Khan, H.; Rigamonti, M.G.; Boffito, D.C. Enhanced photocatalytic activity of Pt-TiO2/WO3 hybrid material with energy storage ability. Appl. Catal. B Environ. 2019, 252, 77–85. [Google Scholar] [CrossRef]
- Kovács, G.; Baia, L.; Vulpoi, A.; Radu, T.; Karácsonyi, É.; Dombi, A.; Hernádi, K.; Danciu, V.; Simon, S.; Pap, Z. TiO2/WO3/Au nanoarchitectures’ photocatalytic activity, “from degradation intermediates to catalysts’ structural peculiarities”, Part I: Aeroxide P25 based composites. Appl. Catal. B Environ. 2014, 147, 508–517. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z.; Sun, C. Highly efficient Z-Scheme Ag3PO4/Ag/WO3−x photocatalyst for its enhanced photocatalytic performance. Appl. Catal. B Environ. 2015, 179, 363–371. [Google Scholar] [CrossRef]
- Shi, H.; Yang, S.; Han, C.; Niu, Z.; Li, H.; Huang, X.; Ma, J. Fabrication of Ag/Ag3PO4/WO3 ternary nanoparticles as superior photocatalyst for phenol degradation under visible light irradiation. Solid State Sci. 2019, 96, 105967. [Google Scholar] [CrossRef]
- Jawhari, A.H.; Hasan, N.; Radini, I.A.; Narasimharao, K.; Malik, M.A. Noble Metals Deposited LaMnO3 Nanocomposites for Photocatalytic H2 Production. Nanomaterials 2022, 12, 2985. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawhari, A.H.; Hasan, N. Nanocomposite Electrocatalysts for Hydrogen Evolution Reactions (HERs) for Sustainable and Efficient Hydrogen Energy—Future Prospects. Materials 2023, 16, 3760. https://doi.org/10.3390/ma16103760
Jawhari AH, Hasan N. Nanocomposite Electrocatalysts for Hydrogen Evolution Reactions (HERs) for Sustainable and Efficient Hydrogen Energy—Future Prospects. Materials. 2023; 16(10):3760. https://doi.org/10.3390/ma16103760
Chicago/Turabian StyleJawhari, Ahmed Hussain, and Nazim Hasan. 2023. "Nanocomposite Electrocatalysts for Hydrogen Evolution Reactions (HERs) for Sustainable and Efficient Hydrogen Energy—Future Prospects" Materials 16, no. 10: 3760. https://doi.org/10.3390/ma16103760
APA StyleJawhari, A. H., & Hasan, N. (2023). Nanocomposite Electrocatalysts for Hydrogen Evolution Reactions (HERs) for Sustainable and Efficient Hydrogen Energy—Future Prospects. Materials, 16(10), 3760. https://doi.org/10.3390/ma16103760






