New Benzotrithiophene-Based Molecules as Organic P-Type Semiconductor for Small-Molecule Organic Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis
2.1.1. Synthesis of 2,2′:3′,2″-Terthiophene (1)
2.1.2. Synthesis of Benzo[1,2-b:3,4-b′:6,5-b″]trithiophene (BTT) (2)
2.1.3. Synthesis of 5-Bromo-2-dicyanovinylthiophene (3)
2.1.4. Synthesis of 2,5,8-Tris[5-(2,2-dicyanovinyl)-2-thienyl]-benzo[1,2-b:3,4-b′:6,5-b″]trithiophene (DCVT-BTT)
2.2. DCVT-BTT Compound Characterization
2.3. Computational Methods
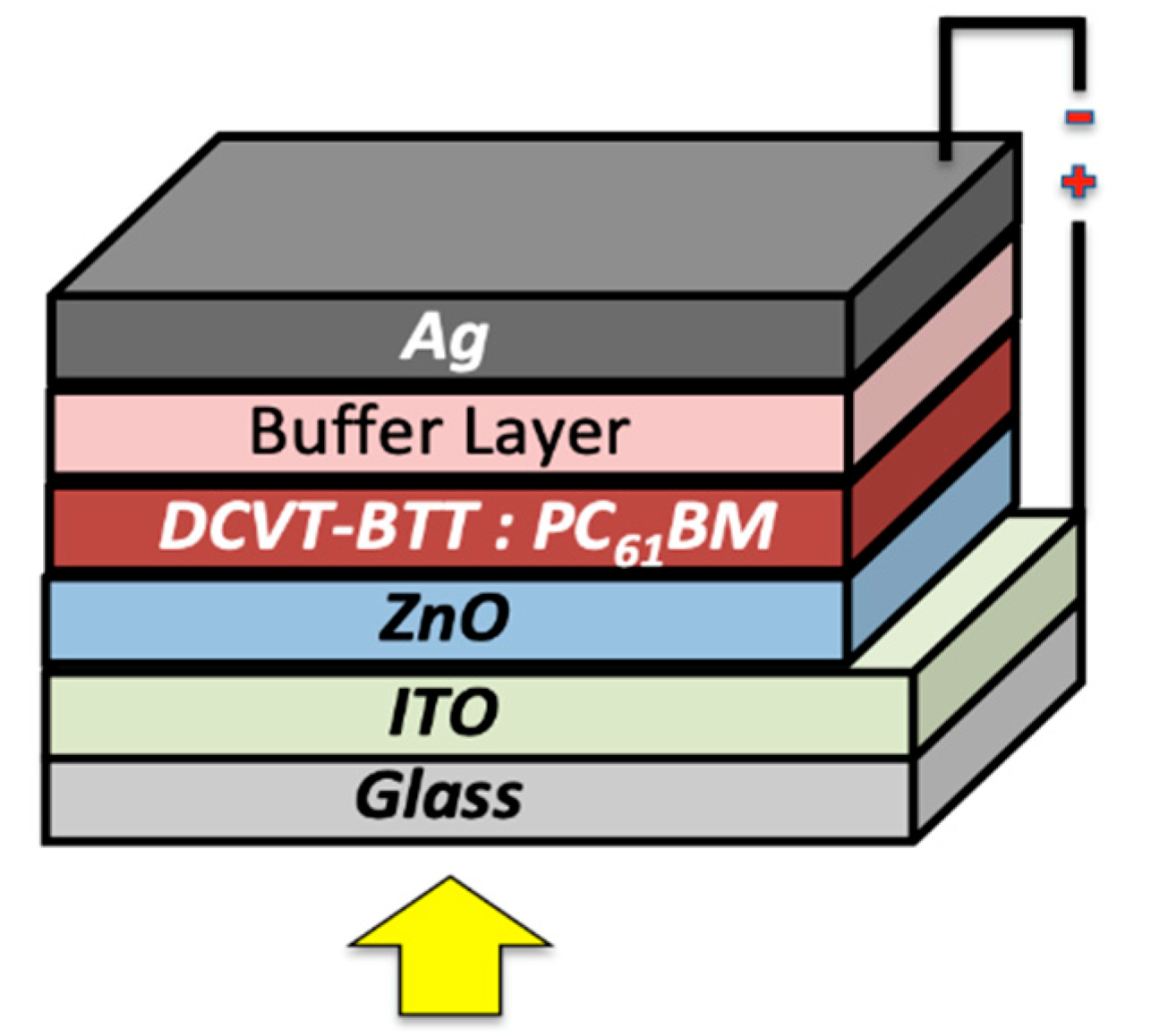
2.4. Organic Solar Cells Fabrication
2.5. Photovoltaic Characterization of Solar Cells
3. Results and Discussions
3.1. Molecular Design and Synthesis of Compounds
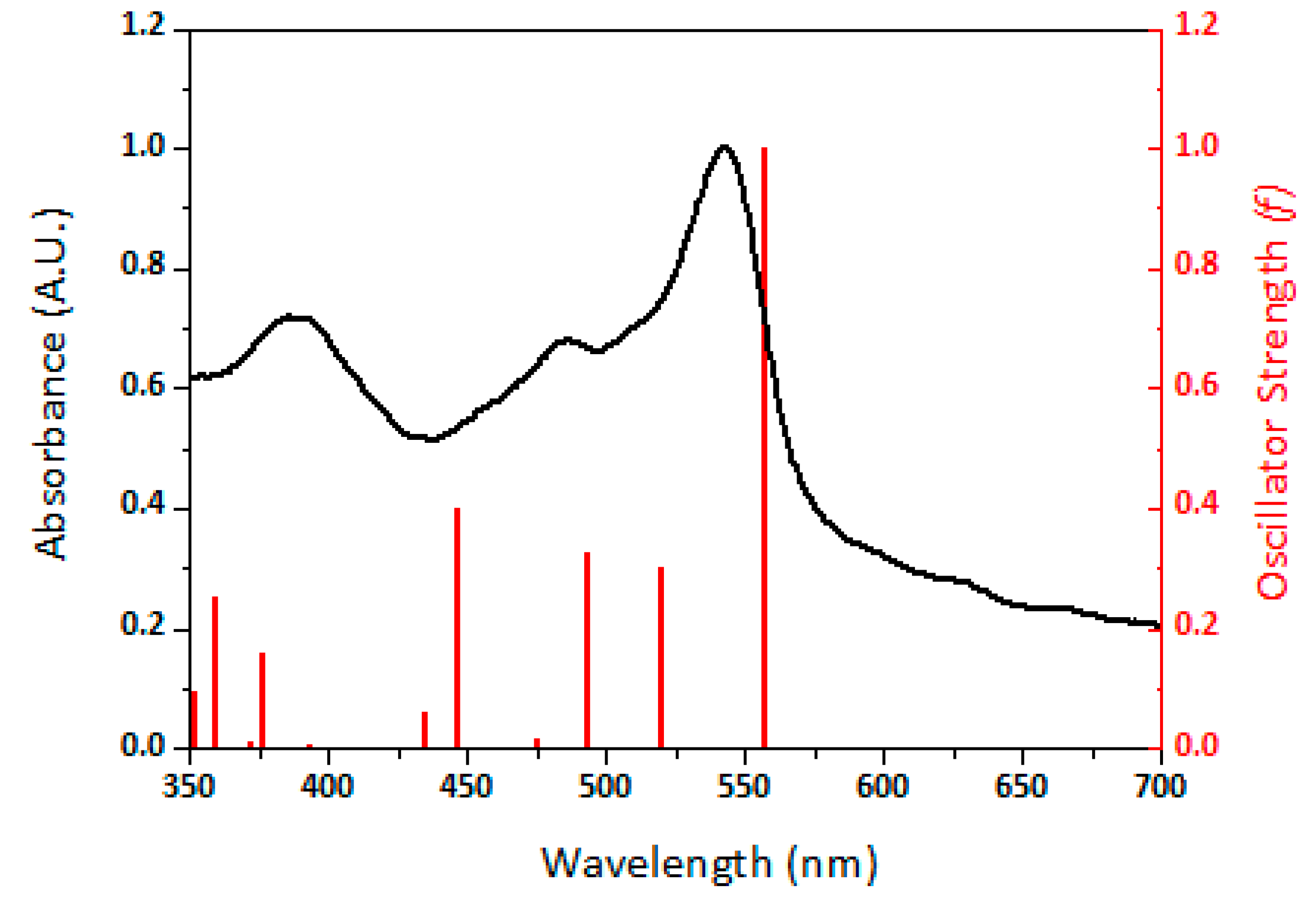
3.2. Experimental UV–Visible Spectrum and Theoretical UV–Visible Electronic Transitions for DCVT-BTT
3.3. Electronic Excitation Properties of DCVT-BTT
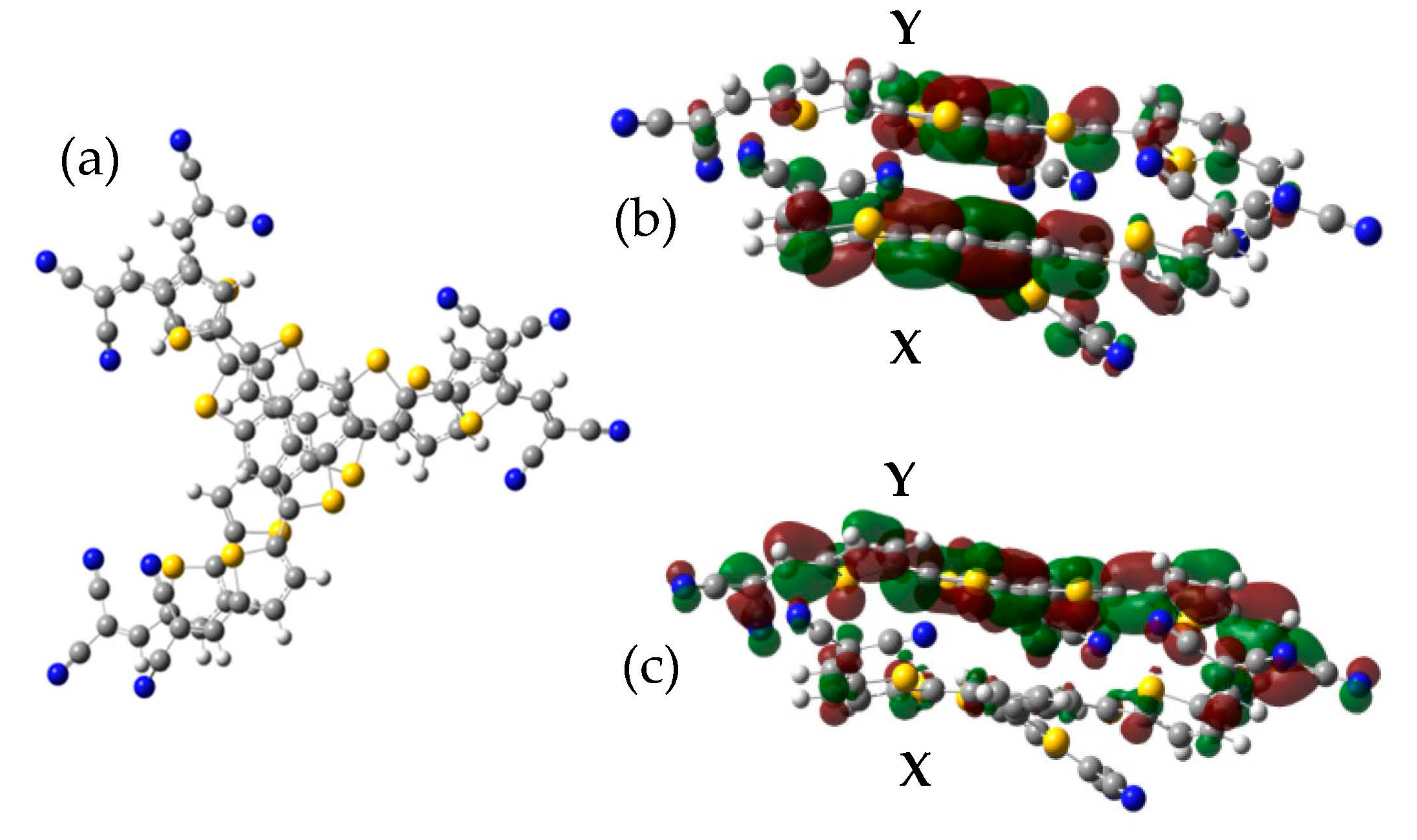
3.4. Calculation of Charge Transport Properties for DCVT-BTT
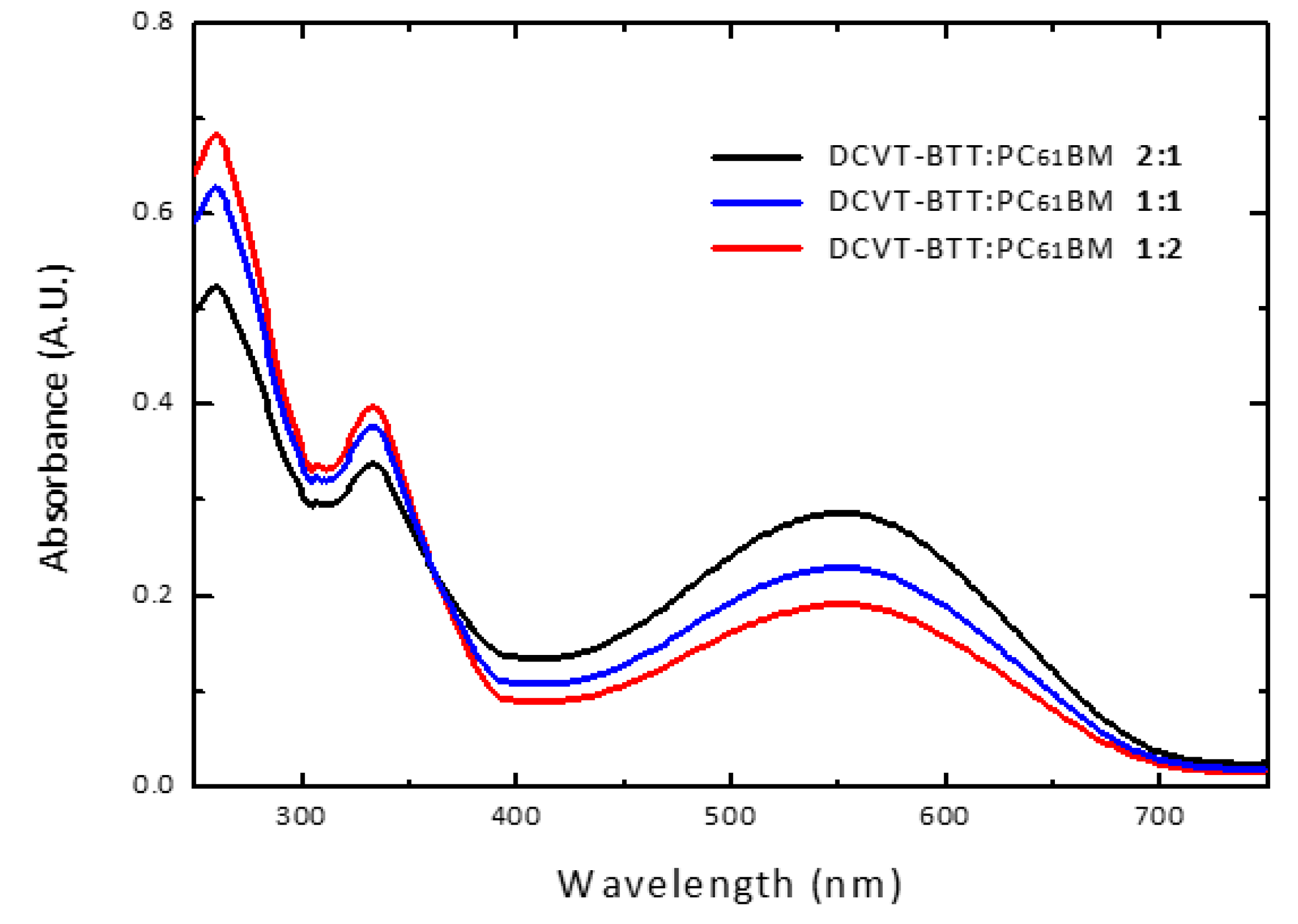
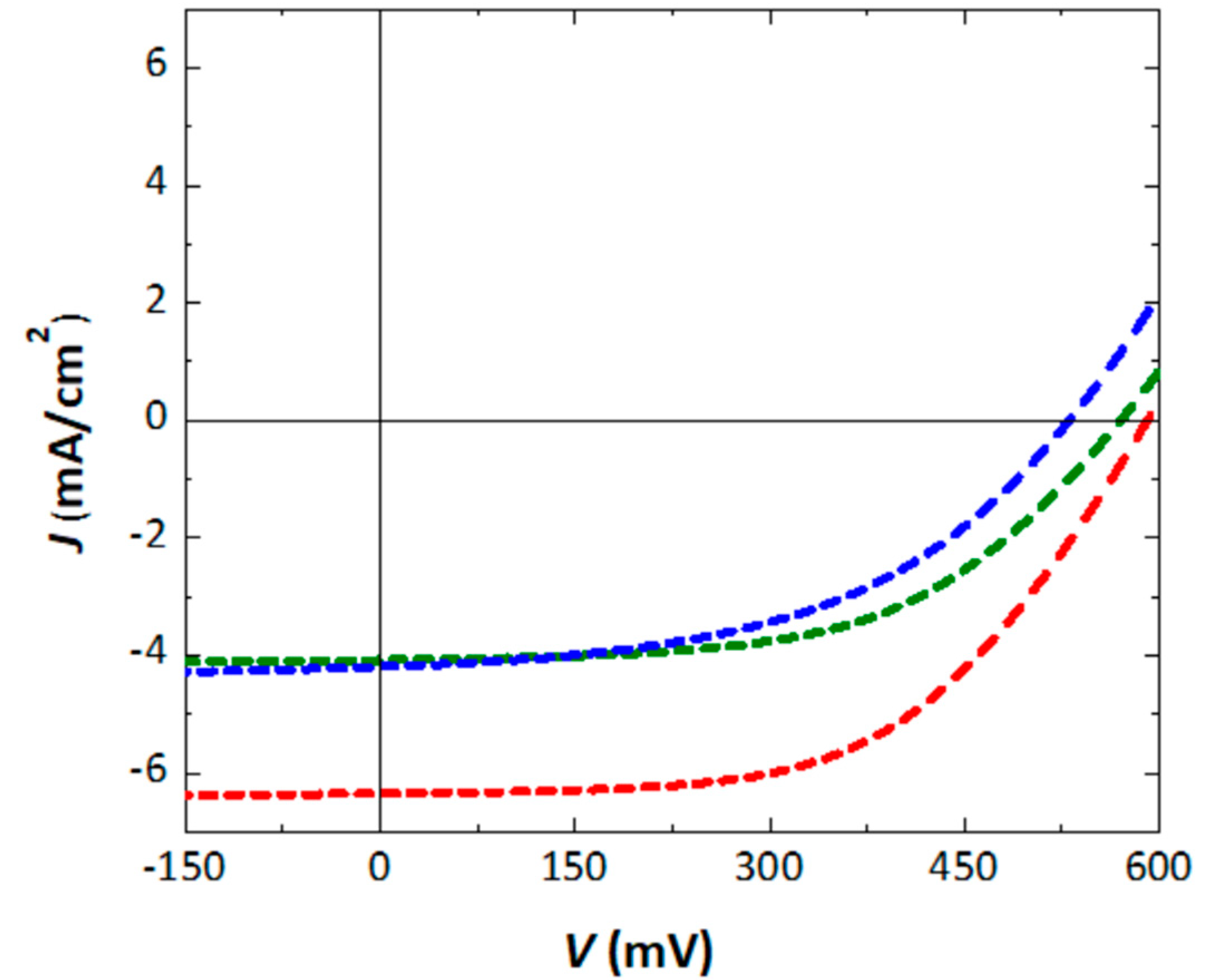
3.5. SM-OSCs Performance and Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rappaport, P. The photovoltaic effect and its utilization. Sol. Energy 1959, 3, 8–18. [Google Scholar] [CrossRef]
- Saga, T. Advances in crystalline silicon solar cell technology for industrial mass production. NPG Asia Mater. 2010, 2, 96–102. [Google Scholar] [CrossRef]
- De Wolf, S.; Descoeudres, A.; Holman, Z.; Ballif, C. High-efficiency Silicon Heterojunction Solar Cells: A Review. Green 2012, 2, 7–24. [Google Scholar] [CrossRef]
- Miles, R.; Zoppi, G.; Forbes, I. Inorganic photovoltaic cells. Mater. Today 2007, 10, 20. [Google Scholar] [CrossRef]
- Le Donne, A.; Scaccabarozzi, A.; Tombolato, S.; Marchionna, S.; Garattini, P.; Vodopivec, B.; Acciarri, M.; Binetti, S. State of the Art and Perspectives of Inorganic Photovoltaics. ISRN Ren. Energy 2013, 2013, 830731. [Google Scholar] [CrossRef]
- Tamai, Y. What’s Next for Organic Solar Cells? The Frontiers and Challenges. Adv. Energy Sustain. Res. 2022, 4, 2200149. [Google Scholar] [CrossRef]
- Shangfei, Y.; Linji, Y.; Shasha, S.; Yang, Z.; Min, L.; Wenhao, Z.; Songlin, C.; Ciyuan, H.; Tao, L.; Bingsuo, Z. A Two-in-One Annealing Enables Dopant Free Block Copolymer Based Organic Solar Cells with over 16% Efficiency. Chin. J. Chem. 2023, 41, 672–678. [Google Scholar] [CrossRef]
- Kim, S.; Jahandar, M.; Jeong, J.; Lim, D. Recent Progress in Solar Cell Technology for Low-Light Indoor Applications. Curr. Altern. Energy 2019, 28, 3–17. [Google Scholar] [CrossRef]
- Ma, L.-K.; Chen, Y.; Chow, P.C.; Zhang, G.; Huang, J.; Ma, C.; Zhang, J.; Yin, H.; Cheung, A.M.H.; Wong, K.S.; et al. High-Efficiency Indoor Organic Photovoltaics with a Band-Aligned Interlayer. Joule 2020, 4, 1486–1500. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Luo, X.; Chen, H.; Wei, Q.; Yuan, J.; Zou, Y. An Overview of High-Performance Indoor Organic Photovoltaics. ChemSusChem 2021, 14, 3428. [Google Scholar] [CrossRef]
- Xie, L.; Song, W.; Ge, J.; Tang, B.; Zhang, X.; Wu, T.; Ge, Z. Recent progress of organic photovoltaics for indoor energy harvesting. Nano Energy 2021, 82, 105770. [Google Scholar] [CrossRef]
- Zheng, H.; Li, D.; Ran, C.; Zhong, Q.; Song, L.; Chen, Y.; Müller-Buschbaum, P.; Huang, W. Emerging Organic/Hybrid Photovoltaic Cells for Indoor Applications: Recent Advances and Perspectives. Sol. RRL 2021, 5, 2100042. [Google Scholar] [CrossRef]
- Jahandar, M.; Kim, S.; Lim, D. Indoor Organic Photovoltaics for Self-Sustaining IoT Devices: Progress, Challenges and Practicalization. ChemSusChem 2021, 14, 3449. [Google Scholar] [CrossRef] [PubMed]
- Burwell, G.; Sandberg, O.; Li, W.; Meredith, P.; Carnie, M.; Armin, A. Scaling Considerations for Organic Photovoltaics for Indoor Applications. Sol. RRL 2022, 6, 2200315. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fang, L.; Luo, G. Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136. [Google Scholar] [CrossRef]
- Roy-Mayhew, J.; Aksay, I. Graphene Materials and Their Use in Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 6323–6348. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhao, Z.; Yang, K.; Niu, L.; Ma, X.; Zhou, Z.; Zhang, X.; Zhang, F. Recent progress in all-small-molecule organic photovoltaics. J. Mater. Chem. A 2022, 10, 6291–6329. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Scharber, M.; Sariciftci, N. Efficiency of bulk-heterojunction organic solar cells. Prog. Polym. Sci. 2013, 38, 1929–1940. [Google Scholar] [CrossRef]
- Mishra, A.; Bauerle, P. Small Molecule Organic Semiconductors on the Move: Promises for Future Solar Energy Technology. Ang. Chem. Int. Ed. 2012, 51, 2020. [Google Scholar] [CrossRef]
- Zang, Y.; Huang, J.; Li, H.; Yu, J.; Jiang, Y. Effect of Molybdenum Oxide Anode Buffer Layer on the Performance of Inverted Small Molecular Organic Solar Cells. Energy Procedia 2011, 12, 513. [Google Scholar] [CrossRef]
- Guo, X.; Wang, S.; Enkelmann, V.; Baumgarten, M.; Müllen, K. Making Benzotrithiophene a Stronger Electron Donor. Org. Lett. 2011, 13, 6062–6065. [Google Scholar] [CrossRef]
- Patra, D.; Chiang, C.; Chen, W.; Wei, K.; Wu, M.; Chu, C. Solution-processed benzotrithiophene-based donor molecules for efficient bulk heterojunction solar cells. J. Mat. Chem. A 2013, 1, 7767. [Google Scholar] [CrossRef]
- Santi, S.; Rossi, S. Molecular design of star-shaped benzotrithiophene materials for organic electronics. Tetrahedron Lett. 2019, 60, 151021. [Google Scholar] [CrossRef]
- Dang, D.; Zhou, P.; Wu, Y.; Xu, Y.; Zhi, Y.; Zhu, W. Isomeric organic semiconductors containing fused-thiophene cores: Molecular packing and charge transport. Phys. Chem. Chem. Phys. 2018, 20, 13171–13177. [Google Scholar] [CrossRef] [PubMed]
- Termine, R.; Golemme, A. Charge Mobility in Discotic Liquid Crystals. Int. J. Mol. Sci. 2021, 22, 877. [Google Scholar] [CrossRef]
- García-Benito, I.; Zimmermann, I.; Urieta-Mora, J.; Aragó, J.; Molina-Ontoria, A.; Ortí, E.; Martín, N.; Nazeeruddin, M.K. Isomerism effect on the photovoltaic properties of benzotrithiophene-based hole-transporting materials. J. Mat. Chem. A 2017, 5, 8317–8324. [Google Scholar] [CrossRef]
- Budiawan, W.; Lai, K.W.; Karuppuswamy, P.; Jadhav, T.S.; Lu, Y.A.; Ho, K.C.; Wang, P.C.; Chang, C.C.; Chu, C.W. Asymmetric Benzotrithiophene-Based Hole Transporting Materials Provide High-Efficiency Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Sena, E.L.; Peel, J.H.; Wesenberg, D.; Nathan, S.; Wallis, M.; Giammona, M.J.; Adalsteinsson, T.; McNelis, B.J.; Barber, R.P., Jr. Transport and spectroscopic studies of the effects of fullerene structure on the efficiency and lifetime of polythiophene-based solar cells. Sol. Energy Mater Sol. Cells 2012, 100, 192–198. [Google Scholar] [CrossRef]
- Lanzi, M.; Paganin, L.; Caretti, D.; Setti, L.; Errani, F. Synthesis of new methoxy-functionalized polythiophenes for charge transport in organic solar cells. React. Funct. Polym. 2011, 71, 745–755. [Google Scholar] [CrossRef]
- Meyers, F.; Brédas, J. Electronic structure and nonlinear optical properties of push-pull conjugated molecules. Int. J. Quantum Chem. 1992, 42, 1595–1614. [Google Scholar] [CrossRef]
- Malytskyi, V.; Gadenne, V.; Ksari, Y.; Patrone, L.; Raimundo, J. Synthesis and characterization of thiophene-based push-pull chromophores for tuning the electrical and optical properties of surfaces with controlled SAM formation. Tetrahedron 2017, 73, 5738–5744. [Google Scholar] [CrossRef]
- Fitzner, R.; Reinold, E.; Mishra, A.; Mena-Osteritz, E.; Ziehlke, H.; Körner, C.; Leo, K.; Riede, M.; Weil, M.; Tsaryova, O.; et al. Dicyanovinyl-Substituted Oligothiophenes: Structure-Property Relationships and Application in Vacuum-Processed Small Molecule Organic Solar Cells. Adv. Funct. Mater. 2011, 21, 897–910. [Google Scholar] [CrossRef]
- Fitzner, R.; Elschner, C.; Weil, M.; Uhrich, C.; Körner, C.; Riede, M.; Leo, K.; Pfeiffer, M.; Reinold, E.; Mena-Osteritz, E.; et al. Interrelation between Crystal Packing and Small-Molecule Organic Solar Cell Performance. Adv. Mater. 2012, 24, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Nitti, A.; Po, R.; Bianchi, G.; Pasini, D. Direct Arylation Strategies in the Synthesis of π-Extended Monomers for Organic Polymeric Solar Cells. Molecules 2016, 22, 21. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef]
- White, M.; Olson, D.; Shaheen, S.; Kopidakis, N.; Ginley, D. Inverted bulk-heterojunction organic photovoltaic device using a solution-derived ZnO underlayer. App. Phys. Lett. 2006, 89, 143517. [Google Scholar] [CrossRef]
- Yafei, W.; Zhong, Z.; Jianqiu, W.; Xiaoyu, L.; Junzhen, R.; Cunbin, A.; Shaoqing, Z.; Jianhui, H. New Method for Preparing ZnO Layer for Efficient and Stable Organic Solar Cells. Adv. Mater. 2023, 35, 2208305. [Google Scholar] [CrossRef]
- Zisheng, S.; Lidan, W.; Yantao, L.; Guang, Z.; Haifeng, Z.; Haigui, Y.; Yuejia, M.; Bei, C.; Wenlian, L. Surface Plasmon Enhanced Organic Solar Cells with a MoO3 Buffer Layer. ACS Appl. Mater. Interfaces 2013, 5, 12847–12853. [Google Scholar] [CrossRef]
- Yoosuf-Ameen, M.; Pradhan, S.; Remyth-Suresh, M.; Reddy, V. MoO3 anode buffer layer for efficient and stable small molecular organic solar cells. Opt. Mater. 2015, 39, 134–139. [Google Scholar] [CrossRef]
- Kudrjasova, J.; Kesters, J.; Verstappen, P.; Brebels, J.; Vangerven, T.; Cardinaletti, I.; Drijkoningen, J.; Penxten, H.; Manca, J.; Lutsen, L.; et al. A direct arylation approach towards efficient small molecule organic solar cells. J. Mater. Chem. A 2016, 4, 791–795. [Google Scholar] [CrossRef]
- Wu, W.; Xin, H.; Ge, C.; Gao, X. Application of direct (hetero)arylation in constructing conjugated small molecules and polymers for organic optoelectronic devices. Tetrahedron Lett. 2017, 58, 175–184. [Google Scholar] [CrossRef]
- Newman, C.; Frisbie, C.; da Silva Filho, D.; Brédas, J.; Ewbank, P.; Mann, K. Introduction to Organic Thin Film Transistors and Design of n-Channel Organic Semiconductors. Chem. Mater. 2004, 16, 4436–4451. [Google Scholar] [CrossRef]
- García, G.; Moral, M.; Granadino-Roldán, J.; Garzón, A.; Navarro, A.; Fernández-Gómez, M. Theoretical Approach to the Study of Thiophene-Based Discotic Systems As Organic Semiconductors. J. Phys. Chem. C 2012, 117, 15–22. [Google Scholar] [CrossRef]
- Demenev, A.; Eichhorn, S.H.; Taerum, T.; Perepichka, D.F.; Patwardhan, S.; Grozema, F.C.; Siebbeles, L.D.A.; Klenkler, R. Quasi Temperature Independent Electron Mobility in Hexagonal Columnar Mesophases of an H-Bonded Benzotristhiophene Derivative. Chem. Mater. 2010, 22, 1420–1428. [Google Scholar] [CrossRef]
- Tober, N.; Winter, J.; Jochem, M.; Lehmann, M.; Detert, H. Tris(5-aryl-1,3,4-oxadiazolyl)benzotrithiophenes–Discotic Liquid Crystals with Enormous Mesophase Ranges. Eur. J. Org. Chem. 2021, 2021, 798–809. [Google Scholar] [CrossRef]
- Sanders, A.; Kale, T.; Katz, H.; Tovar, J. Solid-Phase Synthesis of Self-Assembling Multivalent π-Conjugated Peptides. ACS Omega 2017, 2, 409–419. [Google Scholar] [CrossRef]
- Casellas, N.M.; Urbanaviciute, I.; Cornelissen, T.D.; Berrocal, J.A.; Torres, T.; Kemerink, M.; García-Iglesias, M. Resistive switching in an organic supramolecular semiconducting ferroelectric. Chem. Commun. 2019, 55, 8828–8831. [Google Scholar] [CrossRef]
- Gogoi, G.; Bhattacharya, L.; Rahman, S.; Sarma, N.S.; Sahu, S.; Rajbongshi, B.K.; Sharma, S. New donor-acceptor-donor type of organic semiconductors based on the regioisomers of diketopyrrolopyrroles: A DFT study. Mater. Today Commun. 2020, 25, 101364. [Google Scholar] [CrossRef]
- Ahmed, S.; Dutta, R.; Kalita, D. Strategical designing of diketopyrrolopyrrole-thiophene based donor-acceptor type organic oligomers and study their transport properties: A DFT/TD-DFT perspective. Chem. Phys. Lett. 2019, 730, 14–25. [Google Scholar] [CrossRef]
- Jebasingh-Kores, J.; Danish, A.; Sasitha, T.; Gershom-Stuart, J.; Pushpam, J.; Jebaraj, W. Spectral, NBO, NLO, NCI, aromaticity and charge transfer analyses of anthracene-9,10-dicarboxaldehyde by DFT. Heliyon 2021, 7, e08377. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hussain, R.; Sattar, A.; Assiri, M.A.; Imran, M.; Hussain, A.; Yawer, M.A.; Mehboob, M.Y.; Sumrra, S.H.; Khalid, M.; et al. Correction to: Quantum chemical designing of novel fullerene-free acceptor molecules for organic solar cell applications. J. Mol. Model. 2022, 28, 91. [Google Scholar] [CrossRef]
- Khalid, M.; Khan, M.U.; Ahmed, S.; Shafiq, Z.; Alam, M.M.; Imran, M.; Braga, A.A.C.; Akram, M.S. Exploration of promising optical and electronic properties of (non-polymer) small donor molecules for organic solar cells. Sci. Rep. 2021, 11, 21540. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Ahmed, R.; Shafiq, I.; Arshad, M.; Asghar, M.A.; Munawar, K.S.; Imran, M.; Braga, A.A.C. First theoretical framework for highly efficient photovoltaic parameters by structural modification with benzothiophene-incorporated acceptors in dithiophene based chromophores. Sci. Rep. 2022, 12, 20148. [Google Scholar] [CrossRef] [PubMed]
- Guido, C.; Cortona, P.; Mennucci, B.; Adamo, C. On the Metric of Charge Transfer Molecular Excitations: A Simple Chemical Descriptor. J. Chem. Theory Comput. 2013, 9, 3118–3126. [Google Scholar] [CrossRef]
- Le Bahers, T.; Adamo, C.; Ciofini, I. A Qualitative Index of Spatial Extent in Charge-Transfer Excitations. J. Chem. Theory Comput. 2011, 7, 2498–2506. [Google Scholar] [CrossRef]
- Kraner, S.; Scholz, R.; Plasser, F.; Koerner, C.; Leo, K. Exciton size and binding energy limitations in one-dimensional organic materials. J. Chem. Phys. 2015, 143, 244905. [Google Scholar] [CrossRef]
- Kraner, S.; Prampolini, G.; Cuniberti, G. Exciton Binding Energy in Molecular Triads. J. Phys. Chem. C 2017, 121, 17088–17095. [Google Scholar] [CrossRef]
- Peach, M.; Benfield, P.; Helgaker, T.; Tozer, D. Excitation energies in density functional theory: An evaluation and a diagnostic test. J. Chem. Phys. 2008, 128, 044118. [Google Scholar] [CrossRef]
- Brédas, J.; Beljonne, D.; Coropceanu, V.; Cornil, J. Charge-Transfer and Energy-Transfer Processes in π-Conjugated Oligomers and Polymers: A Molecular Picture. Chem. Rev. 2004, 104, 4971–5004. [Google Scholar] [CrossRef]
- Wang, L.; Nan, G.; Yang, X.; Peng, Q.; Li, Q.; Shuai, Z. Computational methods for design of organic materials with high charge mobility. Chem. Soc. Rev. 2010, 39, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, X.; He, F. Electron transfer in poly(p-phenylene) oligomers: Effect of external electric field and application of Koopmans theorem. Chem. Phys. 1999, 248, 137–146. [Google Scholar] [CrossRef]
- Lan, Y.; Huang, C. A Theoretical Study of the Charge Transfer Behavior of the Highly Regioregular Poly-3-hexylthiophene in the Ordered State. J. Phys. Chem. B 2008, 112, 14857–14862. [Google Scholar] [CrossRef]
- Bao, Z.; Locklin, J. Organic Field-Effect Transistors; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- McMahon, D.; Troisi, A. Evaluation of the External Reorganization Energy of Polyacenes. J. Phys. Chem. Lett. 2010, 1, 941–946. [Google Scholar] [CrossRef]
- Lemaur, V.; Filho, D.A.D.S.; Coropceanu, V.; Lehmann, M.; Geerts, Y.; Piris, J.; Debije, M.G.; van de Craats, A.M.; Senthilkumar, K.; Siebbeles, L.D.; et al. Charge Transport Properties in Discotic Liquid Crystals: A Quantum-Chemical Insight into Structure−Property Relationships. J. Am. Chem. Soc. 2004, 126, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Pope, P.; Swenberg, C. Electronic Processes in Organic Crystals and Polymers, 2nd ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Atahan-Evrenk, Ş.; Aspuru-Guzik, A. Prediction and Theoretical Characterization of p-Type Organic Semiconductor Crystals for Field-Effect Transistor Applications. Predict. Calc. Cryst. Struct. 2014, 345, 95–138. [Google Scholar] [CrossRef]
- Yavuz, I.; Martin, B.; Park, J.; Houk, K. Theoretical Study of the Molecular Ordering, Paracrystallinity and Charge Mobilities of Oligomers in Different Crystalline Phases. J. Am. Chem. Soc. 2015, 137, 2856–2866. [Google Scholar] [CrossRef]
- Yin, Z.; Wei, J.; Zheng, Q. Interfacial Materials for Organic Solar Cells: Recent Advances and Perspectives. Adv. Sci. 2016, 3, 1500362. [Google Scholar] [CrossRef]
- Namkoong, G.; Kong, J.; Samson, M.; Hwang, I.; Lee, K. Active layer thickness effect on the recombination process of PCDTBT:PC71BM organic solar cells. Org. Electron. 2013, 14, 74–79. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, S.; Singh-Negi, C. Influence of active layer thickness on photovoltaic performance of PTB7:PC70BM bulk heterojunction solar cell. Superlattices Microstruct. 2019, 135, 106278. [Google Scholar] [CrossRef]



| Frontier Molecular Orbital | Theoretical Energy with M06-2X/B3LYP Functionals (eV) | Experimental Energy (eV) |
|---|---|---|
| HOMO | −7.12/−6.04 | −5.06 |
| LUMO | −3.05/−3.61 | −3.21 |
| DCVT-BTT | Trans | Δr(Å) | D(Å) | t(Å) | H(Å) | Ecoulomb (eV) | Sr (a.u.) | Λ(Å) | HDI | EDI | LE(%) | CT(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monomer | S0→S1 | 1.95 | 1.68 | −2.30 | 6.25 | 2.75 | 0.708 | 0.698 | 4.53 | 4.44 | 27.6 | 72.4 |
| Dimer | 2.13 | 2.11 | −0.320 | 6.27 | 2.29 | 0.580 | 0.570 | 3.56 | 3.52 | 38.8 | 61.2 |
| [t HOMO] (eV) | λh (eV) | [t LUMO] (eV) | λe (eV) | l (cm) | kET h (seg−1) | kET e (seg−1) | µh (cm2/V∗s) | µe (cm2/V∗s) |
|---|---|---|---|---|---|---|---|---|
| 0.0200 | 0.4650 | 0.0030 | 0.5820 | 3.32 × 108 | 1.07 × 1010 | 7.34 × 108 | 4.60 × 10−3 | 3.16 × 10−5 |
| Compounds (Weight Ratio) | JSC (mA cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| DCVT-BTT:PC61BM (2:1) | 4.07 | 0.570 | 47.15 | 1.09 |
| DCVT-BTT:PC61BM (1:1) | 6.33 | 0.591 | 54.64 | 2.04 |
| DCVT-BTT:PC61BM (1:2) | 4.21 | 0.530 | 45.96 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, C.; Aracena, A.; Ballesteros, L.; Neculqueo, G.; Gence, L.; Quero, F. New Benzotrithiophene-Based Molecules as Organic P-Type Semiconductor for Small-Molecule Organic Solar Cells. Materials 2023, 16, 3759. https://doi.org/10.3390/ma16103759
Castillo C, Aracena A, Ballesteros L, Neculqueo G, Gence L, Quero F. New Benzotrithiophene-Based Molecules as Organic P-Type Semiconductor for Small-Molecule Organic Solar Cells. Materials. 2023; 16(10):3759. https://doi.org/10.3390/ma16103759
Chicago/Turabian StyleCastillo, Cristian, Andrés Aracena, Luis Ballesteros, Gloria Neculqueo, Loik Gence, and Franck Quero. 2023. "New Benzotrithiophene-Based Molecules as Organic P-Type Semiconductor for Small-Molecule Organic Solar Cells" Materials 16, no. 10: 3759. https://doi.org/10.3390/ma16103759
APA StyleCastillo, C., Aracena, A., Ballesteros, L., Neculqueo, G., Gence, L., & Quero, F. (2023). New Benzotrithiophene-Based Molecules as Organic P-Type Semiconductor for Small-Molecule Organic Solar Cells. Materials, 16(10), 3759. https://doi.org/10.3390/ma16103759






