The Formation of a Unique 2D Isonicotinate Polymer Driven by Cu(II) Aerobic Oxidation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and General Methods
2.1.1. Synthesis of [Cu(ina)2(4-acpy]n (1)
2.1.2. X-ray Crystallographic Data
3. Results and Discussion
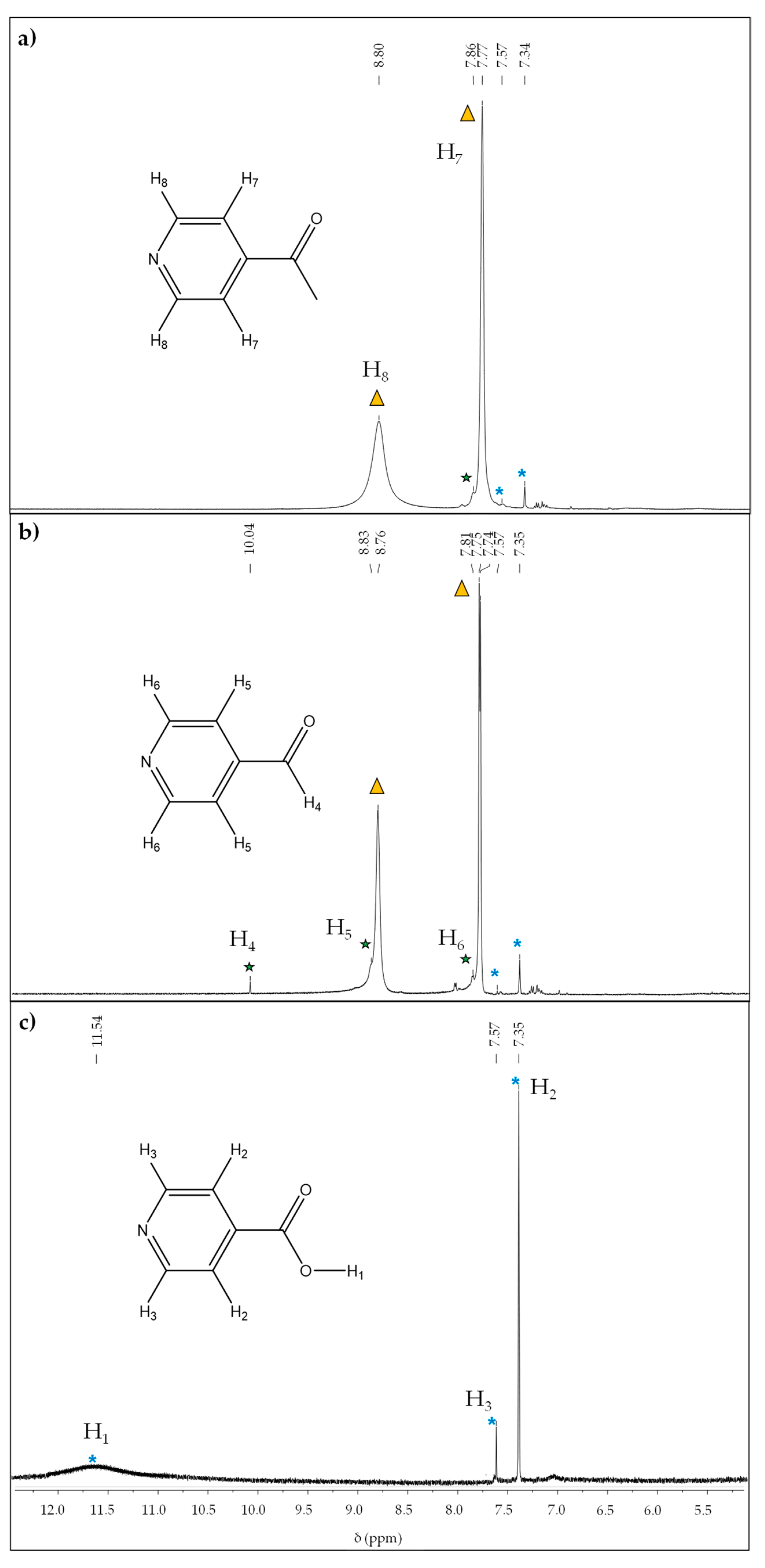
3.1. Synthesis and General Characterization
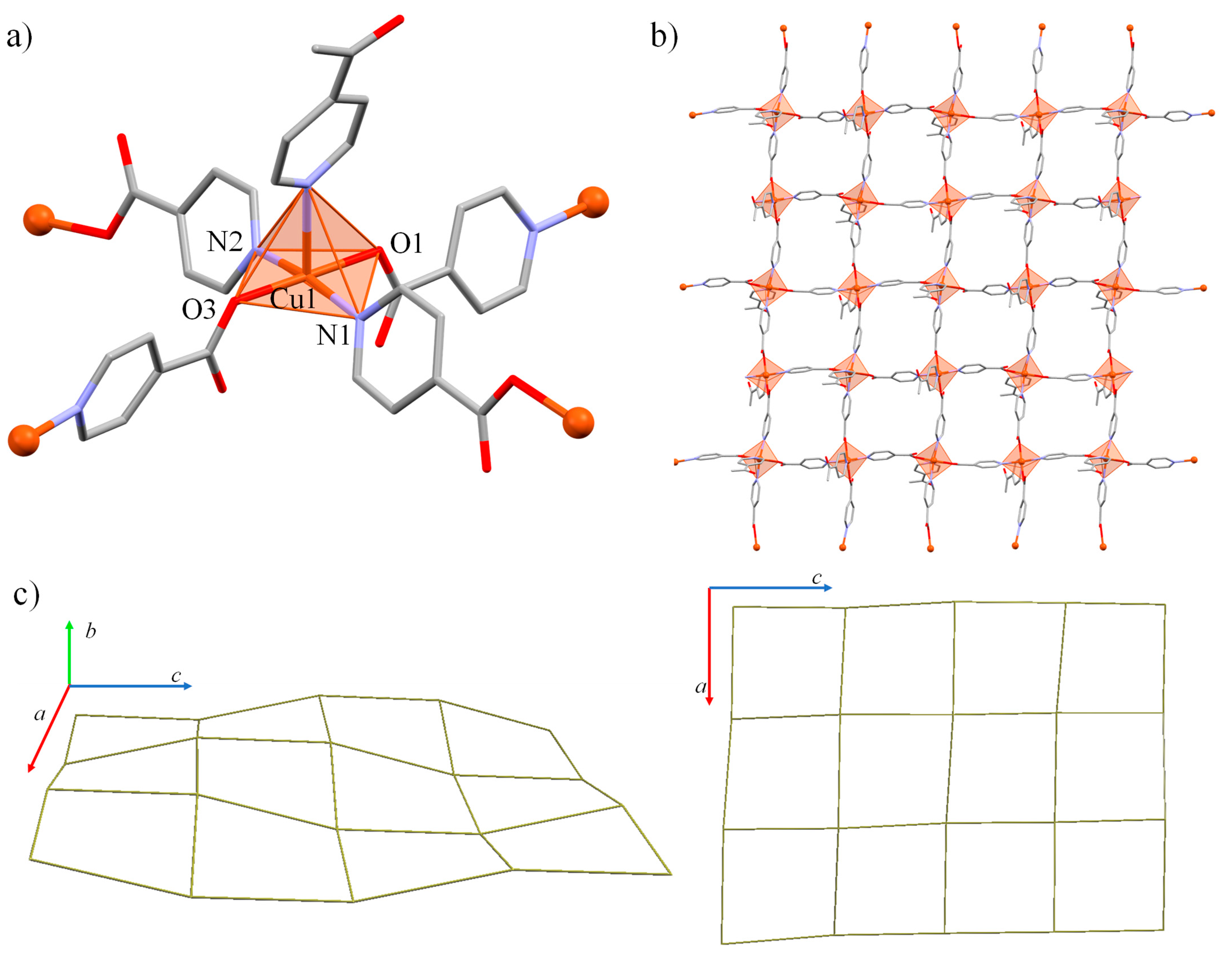
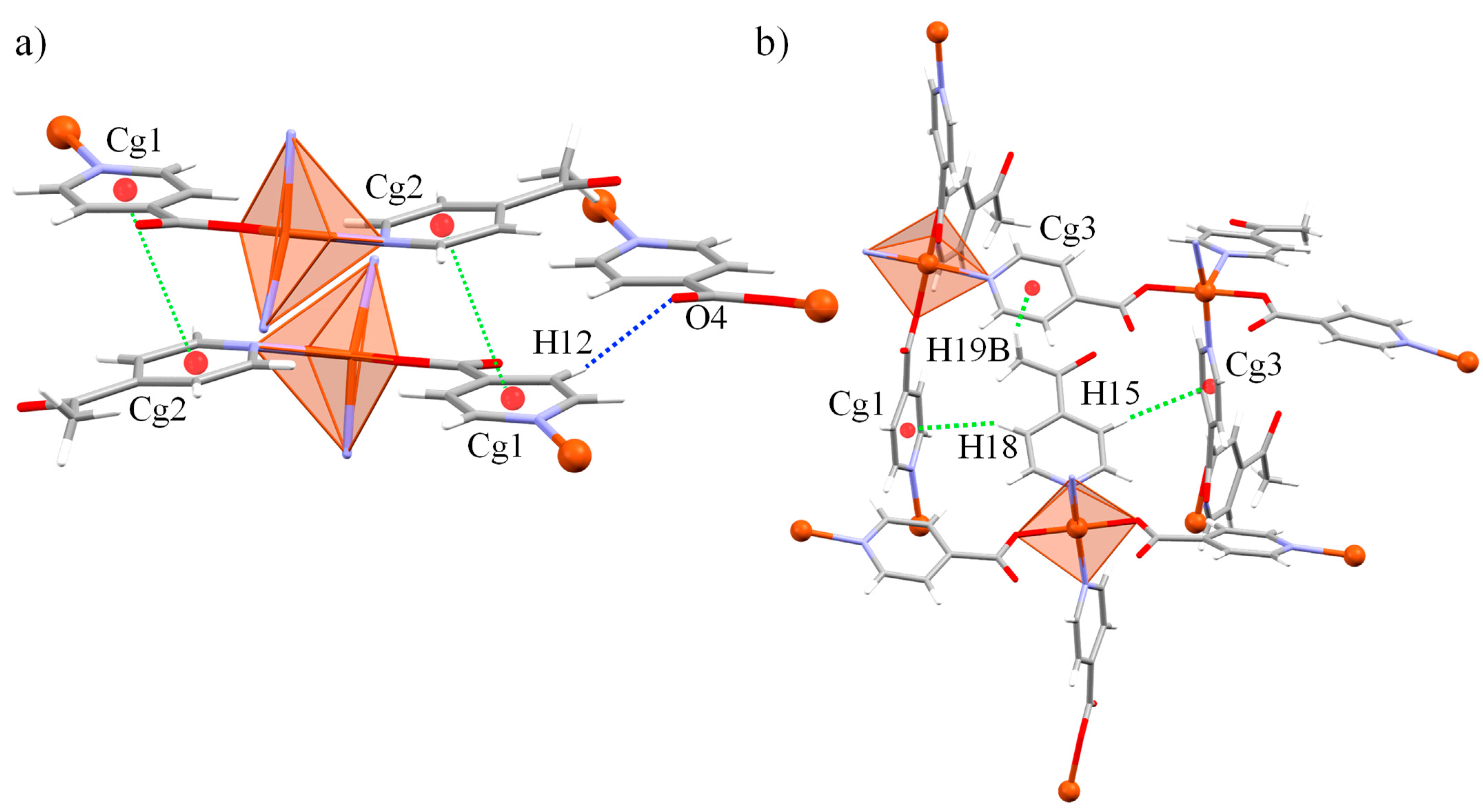
3.2. Crystal Structure of [Cu(ina)2(4-acpy)]n (1)
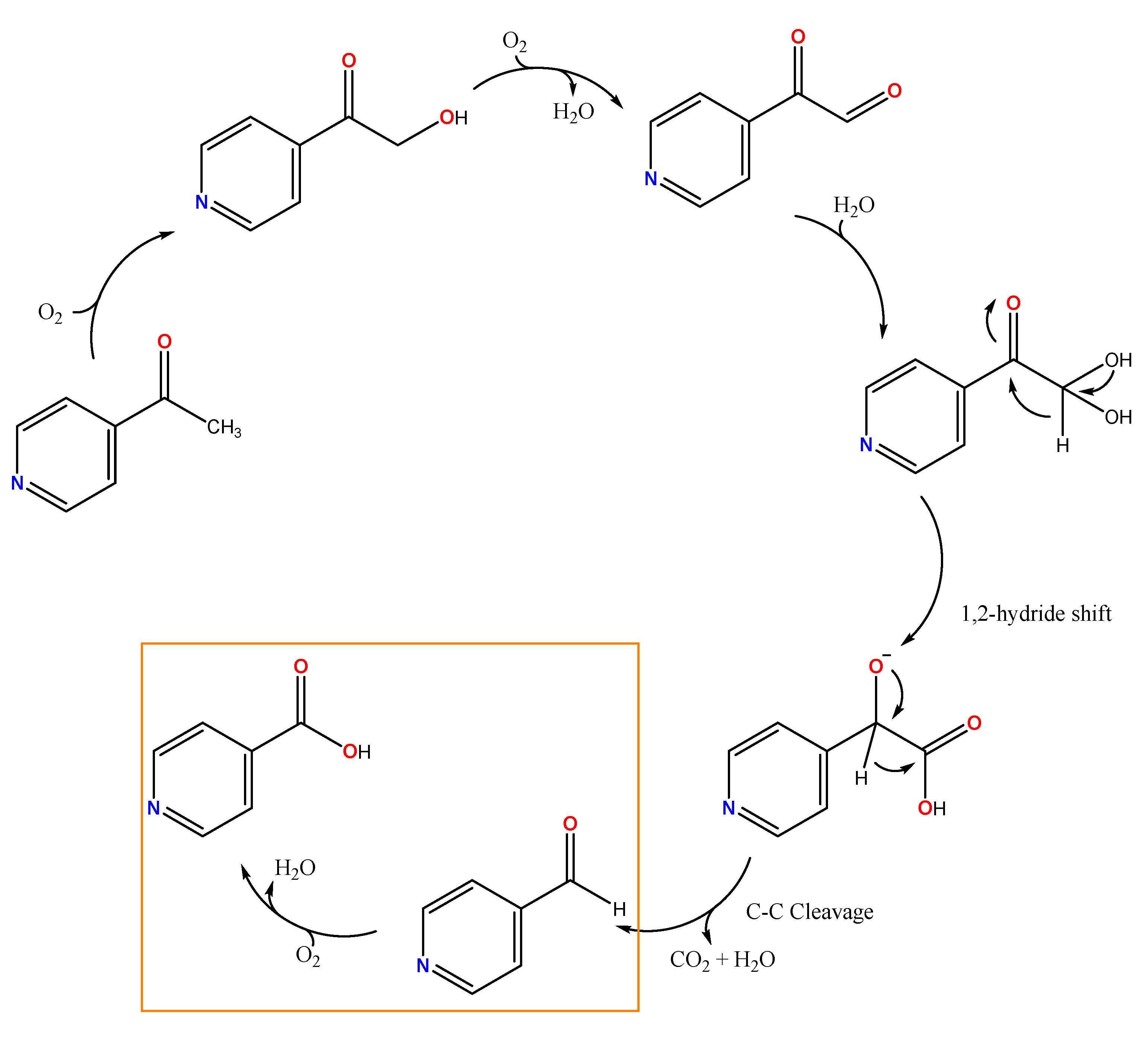
3.3. Catalytic Conversion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, X.; Wang, H.; Chen, J.; Xu, W.; He, Q.; Wang, H.; Zhan, F.; Chen, S.; Chen, L. Emerging 2D Copper-Based Materials for Energy Storage and Conversion: A Review and Perspective. Small 2023, 19, 2204121. [Google Scholar] [CrossRef] [PubMed]
- Soldevila-Sanmartín, J.; Ayllón, J.A.; Calvet, T.; Font-Bardia, M.; Pons, J. Mononuclear and Binuclear Copper(II) Bis(1,3-Benzodioxole-5-Carboxylate) Adducts with Bulky Pyridines. Polyhedron 2017, 126, 184–194. [Google Scholar] [CrossRef]
- Sánchez-Férez, F.; Guerrero, M.; Ayllón, J.A.; Calvet, T.; Font-Bardia, M.; Planas, J.G.; Pons, J. Reactivity of Homoleptic and Heteroleptic Core Paddle Wheel Cu(II) Compounds. Inorg. Chim. Acta 2019, 487, 295–306. [Google Scholar] [CrossRef]
- Sánchez-Férez, F.; Ejarque, D.; Calvet, T.; Font-Bardia, M.; Pons, J. Isonicotinamide-Based Compounds: From Cocrystal to Polymer. Molecules 2019, 24, 4169. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Lu, J.Y.; Babb, A.M. An Extremely Stable Open-Framework Metal–Organic Polymer with Expandable Structure and Selective Adsorption Capability. Chem. Commun. 2002, 2, 1340–1341. [Google Scholar] [CrossRef]
- Geetha, K.; Tiwary, S.K.; Chakravarty, A.R.; Ananthakrishna, G. Synthesis, Crystal Structure and Magnetic Properties of a Polymeric Copper(II) Schiff-Base Complex Having Binuclear Units Covalently Linked by Isonicotinate Ligands. J. Chem. Soc. Dalt. Trans. 1999, 1, 4463–4467. [Google Scholar] [CrossRef]
- Chen, Z.N.; Liu, S.X.; Qiu, J.; Wang, Z.M.; Huang, J.L.; Tang, W.X. Structure and Magnetic Studies of a Two-Dimensional Sheet-like Copper(II) Complex with Bridging Pyridine-4-Carboxylate and Trans-Oxamidate Ligands. J. Chem. Soc. Dalt. Trans. 1994, 20, 2989–2993. [Google Scholar] [CrossRef]
- Abuhmaiera, R.G.; El-mehdawi, R.M.; Ben Younes, M.M.; Treish, F.A.; El-kaheli, M.N.; Daniels, J.; Beck, J. A New Two-Dimensional Coordination Polymer: Poly[[Bis(µ-Chlorido) Tris (µ-Iso-Nicotinate-K4 N:N’:O,O’) Copper(II)] Tris(µ-Iso-Nicotinate-K3O:N:O) (Diaqua) Copper(II)]. GJRA 2015, 4, 400–403. [Google Scholar]
- Zhao, D.-C.; Hu, Y.-Y.; Ding, H.; Guo, H.-Y.; Cui, X.-B.; Zhang, X.; Huo, Q.-S.; Xu, J.-Q. Polyoxometalate-Based Organic–Inorganic Hybrid Compounds Containing Transition Metal Mixed-Organic-Ligand Complexes of N-Containing and Pyridinecarboxylate Ligands. Dalton Trans. 2015, 44, 8971–8983. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y. Crystal Engineering of Cu-Containing Metal-Organic Coordination Polymers under Hydrothermal Conditions. Coord. Chem. Rev. 2003, 246, 327–347. [Google Scholar] [CrossRef]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef]
- Wang, F.; Yang, G.; Zhang, W.; Wu, W.; Xu, J. Copper and Manganese: Two Concordant Partners in the Catalytic Oxidation of p-Cresol to p-Hydroxybenzaldehyde. Chem. Commun. 2003, 3, 1172–1173. [Google Scholar] [CrossRef] [PubMed]
- Bolm, C.; Schlingloff, G.; Bienewald, F. Copper- and Vanadium-Catalyzed Asymmetric Oxidations. J. Mol. Catal. A Chem. 1997, 117, 347–350. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Bochkova, M.M.; Nizova, G.V. Aerobic Oxidation of Saturated Hydrocarbons into Alkyl Hydroperoxides Induced by Visible Light and Catalysed by a ‘Quinone–Copper Acetate’ System. J. Chem. Soc. Perkin Trans. 2 1995, 7, 1465–1469. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Hess, M.; Flörke, U.; Wieghardt, K. From Structural Models of Galactose Oxidase to Homogeneous Catalysis: Efficient Aerobic Oxidation of Alcohols. Angew. Chem. Int. Ed. 1998, 37, 2217–2220. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Hess, M.; Müller, J.; Hildenbrand, K.; Bill, E.; Weyhermüller, T.; Wieghardt, K. Aerobic Oxidation of Primary Alcohols (Including Methanol) by Copper(II)− and Zinc(II)−Phenoxyl Radical Catalysts. J. Am. Chem. Soc. 1999, 121, 9599–9610. [Google Scholar] [CrossRef]
- Wang, Y.; DuBois, J.L.; Hedman, B.; Hodgson, K.O.; Stack, T.D.P. Catalytic Galactose Oxidase Models: Biomimetic Cu(II)-Phenoxyl-Radical Reactivity. Science 1998, 279, 537–540. [Google Scholar] [CrossRef]
- Markó, I.E.; Giles, P.R.; Tsukazaki, M.; Brown, S.M.; Urch, C.J. Copper-Catalyzed Oxidation of Alcohols to Aldehydes and Ketones: An Efficient, Aerobic Alternative. Science 1996, 274, 2044–2046. [Google Scholar] [CrossRef]
- Markó, I.E.; Tsukazaki, M.; Giles, P.R.; Brown, S.M.; Urch, C.J. Anaerobic Copper-Catalyzed Oxidation of Alcohols to Aldehydes and Ketones. Angew. Chem.-Int. Ed. 1997, 36, 2208–2210. [Google Scholar] [CrossRef]
- Esguerra, K.V.N.; Fall, Y.; Petitjean, L.; Lumb, J.-P. Controlling the Catalytic Aerobic Oxidation of Phenols. J. Am. Chem. Soc. 2014, 136, 7662–7668. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Hartigan, E.M.; Feula, G.; Huang, Z.; Lumb, J.-P.; Arndtsen, B.A. Simple Copper Catalysts for the Aerobic Oxidation of Amines: Selectivity Control by the Counterion. Angew. Chem. 2016, 128, 16034–16038. [Google Scholar] [CrossRef]
- Kelly, D.R.; Knowles, C.J.; Mahdi, J.G.; Taylor, I.N.; Wright, M.A. Mapping of the Functional Active Site of Baeyer–Villigerases by Substrate Engineering. J. Chem. Soc. Chem. Commun. 1995, 7, 729–730. [Google Scholar] [CrossRef]
- Sayre, L.M.; Jin, S.J. Mechanism of Copper-Catalyzed Oxygenation of Ketones. J. Org. Chem. 1984, 49, 3498–3503. [Google Scholar] [CrossRef]
- Zhang, L.; Bi, X.; Guan, X.; Li, X.; Liu, Q.; Barry, B.-D.; Liao, P. Chemoselective Oxidative C(CO)-(Methyl) Bond Cleavage of Methyl Ketones to Aldehydes Catalyzed by CuI with Molecular Oxygen. Angew. Chem. 2013, 125, 11513–11517. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Zou, M.; Song, S.; Tang, C.; Yuan, Y.; Jiao, N. From Ketones to Esters by a Cu-Catalyzed Highly Selective C(CO)–C(Alkyl) Bond Cleavage: Aerobic Oxidation and Oxygenation with Air. J. Am. Chem. Soc. 2014, 136, 14858–14865. [Google Scholar] [CrossRef]
- Liu, H.; Wang, M.; Li, H.; Luo, N.; Xu, S.; Wang, F. New Protocol of Copper-Catalyzed Oxidative C(CO) C Bond Cleavage of Aryl and Aliphatic Ketones to Organic Acids Using O2 as the Terminal Oxidant. J. Catal. 2017, 346, 170–179. [Google Scholar] [CrossRef]
- Wang, M.; Lu, J.; Li, L.; Li, H.; Liu, H.; Wang, F. Oxidative C(OH) C Bond Cleavage of Secondary Alcohols to Acids over a Copper Catalyst with Molecular Oxygen as the Oxidant. J. Catal. 2017, 348, 160–167. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Persistence of Vision (TM) Raytracer; Persistence of Vision Pty. Ltd.: Williamstown, Australia, 2004; Available online: https://www.povray.org/ (accessed on 28 March 2023).
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE. Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. 5. The Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef]
- Deacon, G.B. Relationships between the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; Wiley Interscience: Hoboken, NJ, USA, 2009. [Google Scholar]
- Williams, D.H.; Fleming, I. Spectroscopic Methods in Organic Chemistry, 7th ed.; Springer Nature: Cham, Switzerland, 2008. [Google Scholar]
- Avarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape Maps and Polyhedral Interconversion Paths in Transition Metal Chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Reinen, D.; Friebel, C. Cu2+ in 5-Coordination: A Case of a Second-Order Jahn-Teller Effect. 2. CuCl53− and Other CuIIL5 Complexes: Trigonal Bipyramid or Square Pyramid? Inorg. Chem. 1984, 23, 791–798. [Google Scholar] [CrossRef]
- Soldevila-Sanmartín, J.; Calvet, T.; Font-Bardia, M.; Domingo, C.; Ayllón, J.A.; Pons, J. Modulating p-Hydroxycinnamate Behavior as a Ditopic Linker or Photoacid in Copper(II) Complexes with an Auxiliary Pyridine Ligand. Dalton Trans. 2018, 47, 6479–6493. [Google Scholar] [CrossRef] [PubMed]
- Handy, J.V.; Ayala, G.; Pike, R.D. Structural Comparison of Copper(II) Thiocyanate Pyridine Complexes. Inorg. Chim. Acta 2017, 456, 64–75. [Google Scholar] [CrossRef]
- Youngme, S.; Cheansirisomboon, A.; Danvirutai, C.; Pakawatchai, C.; Chaichit, N.; Engkagul, C.; van Albada, G.A.; Costa, J.S.; Reedijk, J. Three New Polynuclear Tetracarboxylato-Bridged Copper(II) Complexes: Syntheses, X-ray Structure and Magnetic Properties. Polyhedron 2008, 27, 1875–1882. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Sabbatini, A.; Drew, M.G.B.; Martins, L.M.D.R.S.; Pettinari, C.; Pombeiro, A.J.L. Cooperative Metal–Ligand Assisted E/Z Isomerization and Cyano Activation at CuII and CoII Complexes of Arylhydrazones of Active Methylene Nitriles. Inorg. Chem. 2014, 53, 9946–9958. [Google Scholar] [CrossRef]
- López-Periago, A.; Vallcorba, O.; Domingo, C.; Ayllón, J.A. Hollow Microcrystals of Copper Hexafluoroacetylacetonate-Pyridine Derivative Adducts via Supercritical CO2 Recrystallization. Cryst. Growth Des. 2016, 16, 1725–1736. [Google Scholar] [CrossRef]
- Crespi, A.F.; Zomero, P.N.; Sánchez, V.M.; Pérez, A.L.; Brondino, C.D.; Vega, D.; Rodríguez-Castellón, E.; Lázaro-Martínez, J.M. Solid-State Characterization of Acetylpyridine Copper Complexes for the Activation of H2O2 in Advanced Oxidation Processes. Chempluschem 2022, 87, e202200169. [Google Scholar] [CrossRef]

| Sample | 1 |
|---|---|
| Empirical Formula | C19H15CuN3O5 |
| Formula weight | 428.88 |
| T (K) | 254 (2) |
| Wavelength (Å) | 0.71073 |
| System, space group | Orthorhombic, Pbca |
| Unit cell dimensions | |
| a (Å) | 12.6673 (11) |
| b (Å) | 11.7649 (9) |
| c (Å) | 24.118 (2) |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 90 |
| V (Å3) | 3594.4 (5) |
| Z | 8 |
| Dcalc (mg/m3) | 1.585 |
| µ (mm−1) | 1.253 |
| F (000) | 1752 |
| Crystal size (mm−3) | 0.180 × 0.173 × 0.076 |
| hkl ranges | −16 ≤ h ≤ 16 −14 ≤ k ≤ 14 −30 ≤ l ≤ 30 |
| θ range (°) | 2.332 to 26.747 |
| Reflections collected/unique/[Rint] | 27,042/3808/0.0405 |
| Completeness to θ (%) | 99.6 |
| Absorption correction | Semi-empirical from equivalents |
| Refinement method | Full-matrix least-squares on |F|2 |
| Data/restraints/parameters | 3808/1/229 |
| Goodness-of-fit on F2 | 1.034 |
| Final R indices [I>2σ(I)] | R1 = 0.0525, wR2 = 0.1293 |
| R indices (all data) | R1 = 0.0662, wR2 = 0.1397 |
| Extinction coefficient | n/a |
| Largest diff-peak and hole (e. Å−3) | 1.037 and −0.914 |
| Bond lengths | |||||
| Cu(1)-O(1) | 1.949(3) | Cu(1)-N(1)#2 | 2.038(3) | ||
| Cu(1)-O(3) | 1.957(2) | Cu(1)-N(3) | 2.351(3) | ||
| Cu(1)-N(2)#1 | 2.036(3) | ||||
| Bond Angles | |||||
| O(1)-Cu(1)-O(3) | 179.76(12) | N(2)#1-Cu(1)-N(1)#2 | 167.80(13) | ||
| O(1)-Cu(1)-N(2)#1 | 88.25(12) | O(1)-Cu(1)-N(3) | 90.49(12) | ||
| O(3)-Cu(1)-N(2)#1 | 91.92(11) | O(3)-Cu(1)-N(3) | 89.32(11) | ||
| O(1)-Cu(1)-N(1)#2 | 89.66(12) | N(2)#1-Cu(1)-N(3) | 99.33(12) | ||
| O(3)-Cu(1)-N(1)#2 | 90.21(12) | N(1)#2-Cu(1)-N(3) | 92.71(13) | ||
| π···π interactions | |||||
| Cg(I)···Cg(J) | Cg···Cg a | A b | β, γ c | Cg(I)_Perp, Cg(J)_Perp d | Slippage e |
| Cg(1)···Cg(2) | 3.868(2) | 6.7(2) | 18.3, 24.6 | 3.5170(18), 3.6727(17) | 1.213 |
| C-H···π interactions | |||||
| C-H···Cg(J) | H···Cg(J) f | H-Perp g | Γ c | X···Cg(J) h | X-H, π i |
| C18-H18···Cg(1) | 2.98 | 2.87 | 15.70 | 3.849(5) | 74 |
| C15-H15···Cg(3) | 2.96 | 2.70 | 23.86 | 3.777(5) | 68 |
| C19-H19B···Cg(3) | 2.92 | 2.87 | 10.47 | 3.702(7) | 59 |

| Precursor | Quantity (mmol) | Solvent | O2 Pressure (bar) | Product |
|---|---|---|---|---|
| Cu(NO3)2·3H2O | 24.16 mg (0.1) | DMF | atm | n.r. |
| ACN | atm | 4-fopy + ina | ||
| DMF | 2.1 | n.r. | ||
| ACN | 2.1 | ina | ||
| Cu(OAc)2·H2O | 19.96 mg (0.1) | DMF | 2.1 | n.r. |
| ACN | 2.1 | 4-fopy + ina |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Férez, F.; Calvet, T.; Font-Bardia, M.; Pons, J. The Formation of a Unique 2D Isonicotinate Polymer Driven by Cu(II) Aerobic Oxidation. Materials 2023, 16, 3724. https://doi.org/10.3390/ma16103724
Sánchez-Férez F, Calvet T, Font-Bardia M, Pons J. The Formation of a Unique 2D Isonicotinate Polymer Driven by Cu(II) Aerobic Oxidation. Materials. 2023; 16(10):3724. https://doi.org/10.3390/ma16103724
Chicago/Turabian StyleSánchez-Férez, Francisco, Teresa Calvet, Mercè Font-Bardia, and Josefina Pons. 2023. "The Formation of a Unique 2D Isonicotinate Polymer Driven by Cu(II) Aerobic Oxidation" Materials 16, no. 10: 3724. https://doi.org/10.3390/ma16103724
APA StyleSánchez-Férez, F., Calvet, T., Font-Bardia, M., & Pons, J. (2023). The Formation of a Unique 2D Isonicotinate Polymer Driven by Cu(II) Aerobic Oxidation. Materials, 16(10), 3724. https://doi.org/10.3390/ma16103724






