An Engineering Method of Analyzing the Dynamics of Mass Transfer during Concrete Corrosion Processes in Offshore Structures
Abstract
1. Introduction
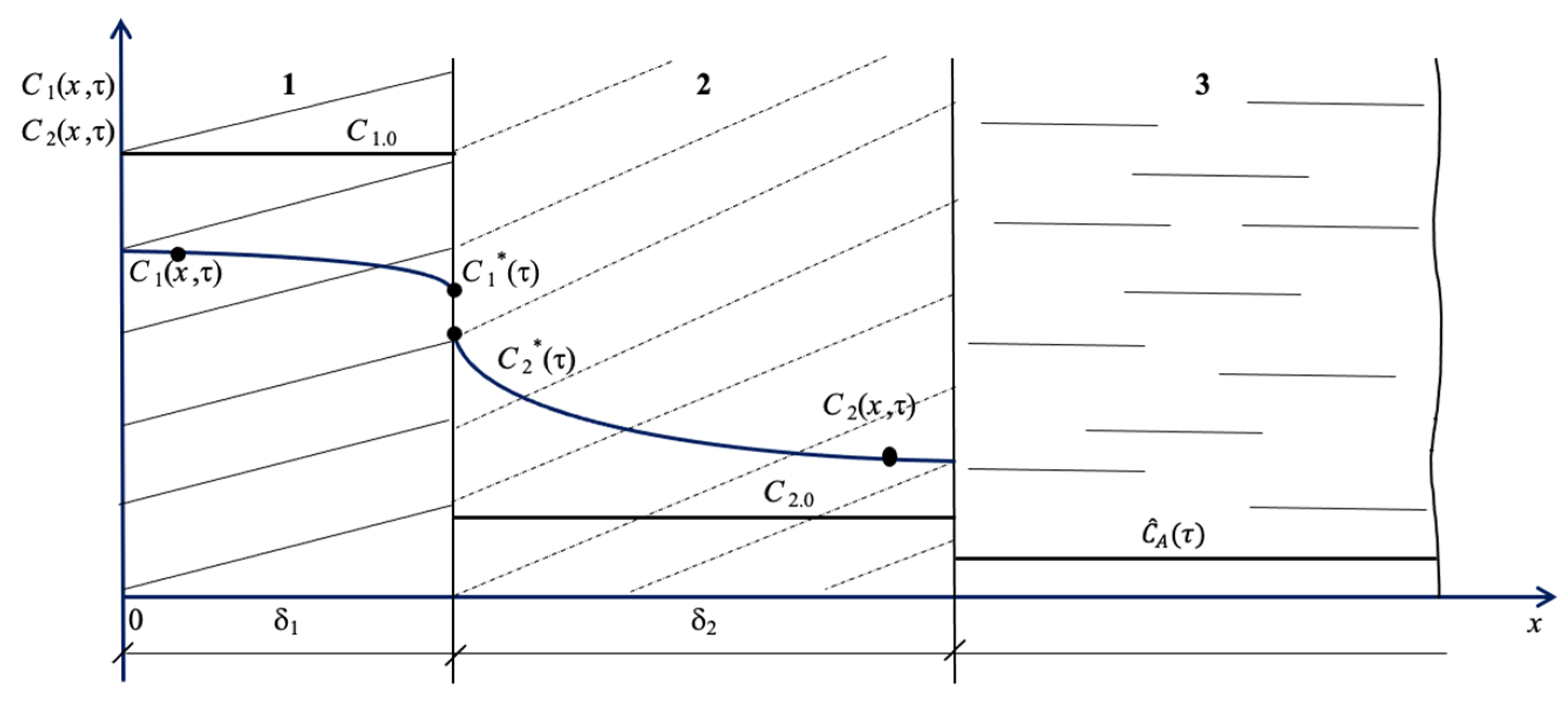
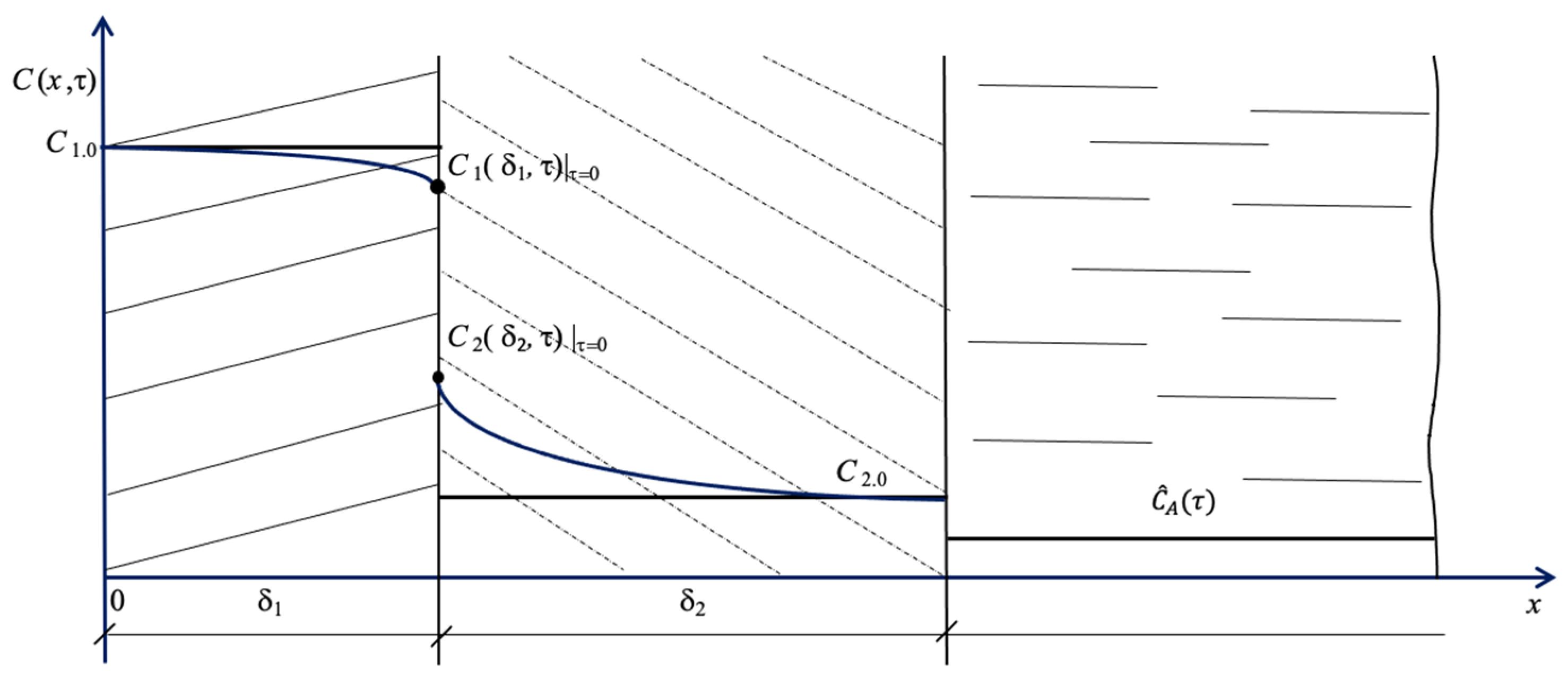
2. Materials and Methods
- -
- for an enclosing structure:
- -
- for a soil layer:
- Initial values of concentrations.
- Values of Henry constants “m” as the equilibrium in the “structure–soil” system.
- Values of Henry constants “mA” as the equilibrium in the “soil–water area” system.
- Value of coefficient “β” that shows mass transfer from the soil to the water area.
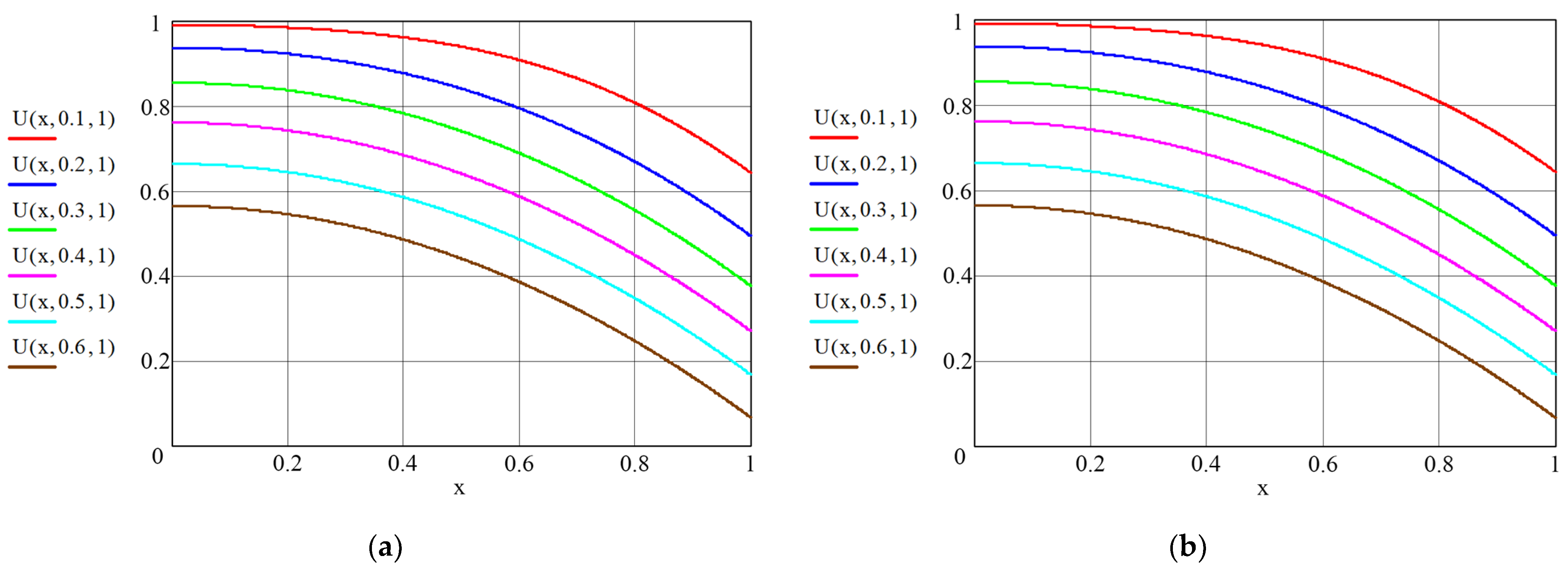
3. Results and Discussion
- Uniform initial distribution;
- Constancy of Kirpichev criteria values.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinitsin, D.A.; Khalikov, R.M.; Bulatov, B.G.; Galitskov, K.S.; Nedoseko, I.V. Technological approaches to directed structure formation of building nanocomposites having increased corrosion resistance. Nanotechnol. Constr. Sci. Online J. 2019, 11, 153–164. [Google Scholar]
- Wang, L.; Zheng, D.; Zhang, S.; Cui, H.; Li, D. Effect of nano-SiO2 on the hydration and microstructure of Portland cement. Nanomaterials 2016, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.V.; Stepanova, V.F.; Rumyantseva, V.E.; Kotlov, V.G.; Stepanov, A.Y.; Konovalova, V.S. Corrosion of Building Materials: Problems, Solutions; ASV: Moscow, Russia, 2022; p. 400. ISBN 978-5-4323-0435-3. [Google Scholar]
- Bonen, D.; Cohen, M.D. Magnesium Sulfate Attack on Portland Cement Paste. I. Microstructural Analysis; II. Chemical and Mineralogical Analysis. Cem. Concr. Res. 1992, 22, 169–180. [Google Scholar] [CrossRef]
- Snehal, K.; Das, B.; Akanksha, M. Early age, hydration, mechanical and microstructure properties of nano-silica blended cementitious composites. Constr. Build. Mater. 2020, 233, 16. [Google Scholar] [CrossRef]
- Carnot, A. Corrosion mechanisms of steel concrete moulds in the presence of a demoulding agent. J. Appl. Electrochem. 2002, 32, 865–869. [Google Scholar] [CrossRef]
- Böhni, H. Corrosion in Reinforced Concrete Structures; A Volume in Woodhead Publishing Series in Civil and Structural Engineering; Elsevier: Amsterdam, The Netherlands, 2005; p. 264. [Google Scholar]
- Leenderste, W.; Oud, H.J.C.; Mehta, K. The Dutch Experience with Construction and Repair of Marine Structures, Proceedings Gerwick Symposium on Durability of Concrete in Marine Medium; Department of Civil Engineering, University of California at Berkeley: Berkeley, CA, USA, 1989; pp. 147–172. [Google Scholar]
- Gerwick, B.C.; Mehta, K. Pressing Needs and Future Opportunities in Durability of Concrete in Marine Medium, Proceedings Gerwick Symposium on Durability of Concrete in Marine Medium; Department of Civil Engineering, University of California at Berkeley: Berkeley, CA, USA, 1989; pp. 1–5. [Google Scholar]
- Mehta, K. Concrete in the Marine Medium; Taylor & Francis: London, UK, 2003; p. 224. [Google Scholar]
- Fedosov, S.V.; Aleksandrova, O.V.; Fedoseev, V.N.; Loginova, S.A.; Quang, N.D.V. Physico-Mechanical Foundations of Theoretical and Engineering Investigation on the Development to Corrosion-Resistant Materials in Buried Structures of Nearshore zones. Solid State Phenom. 2022, 334, 209–216. [Google Scholar] [CrossRef]
- Fedosov, S.; Bulgakov, B.; Ngo, H.X.; Aleksandrova, O.; Solovev, V. Theoretical and Experimental Models to Evaluate the Possibility of Corrosion Resistant Concrete for Coastal Offshore Structures. Materials 2022, 15, 4697. [Google Scholar] [CrossRef]
- Lei, M.; Peng, L.; Shi, C.; Wang, S. Experimental study on the damage mechanism of tunnel structure suffering from sulfate attack. Tunn. Undergr. Space Technol. 2013, 36, 5–13. [Google Scholar] [CrossRef]
- Ryazanova, V.A. Features of sulfate corrosion of concrete under conditions of directional moisture transfer. Bashkir. Chem. J. 2016, 23, 45–52. [Google Scholar]
- Brown, W.; Taylor, H.F.W.; Marchand, J.; Skalny, J. (Eds.) The role of ettringite in external sulfate attack. In Materials Science of Concrete Special Volume: Sulfate Attack Mechanisms; The American Ceramic Society: Westerville, OH, USA, 1999; pp. 73–98. [Google Scholar]
- Lehner, P.; Konečný, P.; Ponikiewski, T. Comparison of Material Properties of SCC Concrete with Steel Fibres Related to Ingress of Chlorides. Crystals 2020, 10, 220. [Google Scholar] [CrossRef]
- Stepanov, S.N. Forecasting the durability of reinforced concrete structures operating in aggressive mediums, taking into account the corrosion wear of working fittings. N. Novgorod 2005, 213. [Google Scholar]
- Li, C.; Chen, Q.; Wang, R.; Wu, M.; Jiang, Z. Corrosion assessment of reinforced concrete structures exposed to chloride mediums in underground tunnels: Theoretical insights and practical data interpretations. Cem. Concr. Compos. 2020, 112, 103652. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Rumyantseva, V.E.; Konovalova, S.V.; Karavaev, I.V. The rate of penetration of chloride ions to the surface of steel reinforcement in hydrophobized concrete. Mod. Sci.-Intensive Technol. 2018, 56, 93–99. [Google Scholar]
- Kayumov, R.A.; Fedosov, S.V.; Rumyanceva, V.E.; Khrunov, V.A.; Manokhina, Y.V.; Krasilnikov, I.V. Mathematical modeling of the corrosion mass transfer of the heterogeneous system “liquid aggressive medium-cement concrete”. Special cases of the solution. Izv. KGASU 2013, 4, 343–348. [Google Scholar]
- Smirnova, N.N.; Krasil’nikov, I.V. An effect of the nature of immobilized components on the adsorption and mass transfer properties of ultrafiltration membranes based on sulfonate-containing copolyamide. Russ. J. Appl. Chem. 2019, 92, 1570–1580. [Google Scholar] [CrossRef]
- Rumyantseva, V.E.; Krasilnikov, I.V.; Krasilnikova, I.A.; Novikova, U.A.; Kasyanenko, N.S. Study of temperature effect on the intensity of mass transfer during corrosion of the first type of cement concrete. Mod. Probl. Civ. Prot. 2022, 42, 24–31. [Google Scholar]
- Rumyantseva, V.E.; Krasilnikov, I.V.; Krasilnikova, I.A.; Strokin, K.B.; Novikova, U.A. Determination of the influence of the viscosity of the saturating fluid on the physical and mechanical characteristics of the cement stone of different porosity. Mod. Probl. Civ. Prot. 2022, 143–152. [Google Scholar]
- Guzmán-Torres José, A.; Domínguez-Mota Francisco, J.; Alonso-Guzmán Elia, M. A multi-layer approach to classify the risk of corrosion in concrete specimens that contain different additives. Case Stud. Constr. Mater. 2021, 15, 00719. [Google Scholar] [CrossRef]
- Gusev, B.V.; Fayvusovich, A.S. Calculated dependencies for predicting the technical condition of reinforced concrete structures. Ind. Civ. Constr. 2021, 6, 4–12. [Google Scholar] [CrossRef]
- Petropavlovskaya, V.; Sulman, M.; Novichenkova, T.; Sidorov, A.; Petropavlovskii, K. Ultra-Dispersed Ash Filler for Dispersed Binding Systems. Chem. Eng. Trans. 2021, 88, 1003–1008. [Google Scholar] [CrossRef]
- Dai, L.; Long, D.; Wang, L. Meso-scale modeling of concrete cracking induced by 3D corrosion expansion of helical. Comput. Struct. 2021, 254, 106615. [Google Scholar] [CrossRef]
- Wang, Z.; Maekawa, K.; Takeda, H.; Gong, F. Numerical simulation and experiment on the coupled effects of macro-cell corrosion and multi-ion equilibrium with pseudo structural concrete. Cem. Concr. Compos. 2021, 123, 104181. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Luan, Y. Modeling of Hydration, Strength Development, and Optimum Combinations of Cement-Slag-Limestone Ternary Concrete. Int. J. Concr. Struct. Mater. 2018, 12, 12. [Google Scholar] [CrossRef]
- Bazhenov Yu Garkina, I.; Korolev, E.; Danilov, A. System Analysis in Construction Materials Science; MGSU: Moscow, Russia, 2012; p. 432. ISBN 978-5-7264-0683-1. [Google Scholar]
- Krasilnikov, I.V. Determination of parameters of nonisothermal mass transfer process in liquid corrosion of concrete. Bulletin of Perm National Research Polytechnic University. Appl. Ecol. Urban 2022, 45, 99–109. [Google Scholar]
- Fu, J.; Kamali-Bernard, S.; Bernard, F.; Cornen, M. Comparison of Mechanical Properties of C–S–H and Portlandite between Nano-Indentation Experiments and a Modeling Approach Using Various Simulation Techniques. Compos. Part B Eng. 2018, 151, 127–138. [Google Scholar] [CrossRef]
- Wailes, C. Phase Equilibria in Chemical Engineering; Butterworth-Heinemann: Oxford, UK, 1989; p. 304. ISBN 5-03-001105-6. [Google Scholar]
- Fedosov, S.V.; Rumyantseva, V.E.; Krasilnikov, I.V. Methods of Mathematical Physics in Applications to Problems of Concrete Corrosion in Liquid Aggressive Media; ASV: Moscow, Russia, 2021; p. 244. ISBN 978-5-4323-0399-8. [Google Scholar]
- Rudobashta, S.P.; Dorniak, O.R.; Dmitriev, V.M. Calculation of the drying kinetics of a plate taking into account its shrinkage. Theor. Bases Chem. Technol. 2021, 55, 612–617. [Google Scholar] [CrossRef]
- Rudobashta, S.P.; Kartashov, É.M.; Zueva, G.A. Influence of the topology of a solid body on its mass conductivity. J. Eng. Phys. Thermophys. 2019, 92, 899–906. [Google Scholar] [CrossRef]
- Rudobashta, S.P.; Kosheleva, M.K. The determination of mass transfer and mass conductivity coefficients from the kinetic curves. Text. Ind. Technol. 2015, 360, 175–180. [Google Scholar]
- Rudobashta, S.P.; Kartashov, E.M. Diffusion in Chemical Engineering Processes; KolosS: Moscow, Russia, 2010; 478p. [Google Scholar]
- Fedosov, S.V. Heat-Mass Transfer in Technological Processes of the Construction Industry; IPK “PresSto”: Ivanovo, Russia, 2010; p. 363. [Google Scholar]
- Rudobashta, S.P. Mass Transfer in Systems with a Solid Phase; Chemistry: Moscow, Russia, 1980; p. 248. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedosov, S.V.; Aleksandrova, O.V.; Lapidus, A.A.; Kuzmina, T.K.; Topchiy, D.V. An Engineering Method of Analyzing the Dynamics of Mass Transfer during Concrete Corrosion Processes in Offshore Structures. Materials 2023, 16, 3705. https://doi.org/10.3390/ma16103705
Fedosov SV, Aleksandrova OV, Lapidus AA, Kuzmina TK, Topchiy DV. An Engineering Method of Analyzing the Dynamics of Mass Transfer during Concrete Corrosion Processes in Offshore Structures. Materials. 2023; 16(10):3705. https://doi.org/10.3390/ma16103705
Chicago/Turabian StyleFedosov, Sergey Viktorovich, Olga Vladimirovna Aleksandrova, Azariy Abramovich Lapidus, Tatiana Konstantinovna Kuzmina, and Dmitriy Vladimirovich Topchiy. 2023. "An Engineering Method of Analyzing the Dynamics of Mass Transfer during Concrete Corrosion Processes in Offshore Structures" Materials 16, no. 10: 3705. https://doi.org/10.3390/ma16103705
APA StyleFedosov, S. V., Aleksandrova, O. V., Lapidus, A. A., Kuzmina, T. K., & Topchiy, D. V. (2023). An Engineering Method of Analyzing the Dynamics of Mass Transfer during Concrete Corrosion Processes in Offshore Structures. Materials, 16(10), 3705. https://doi.org/10.3390/ma16103705






