Fabrication of Electrospun PLA-nHAp Nanocomposite for Sustained Drug Release in Dental and Orthopedic Applications
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Nano-Hydroxyapatite (nHAp) Synthesis
2.3. Preparation of PLA Fiber
2.4. Fabrication of PLA-nHAp Nanocomposite by Electrospinning
2.5. Characterization Techniques
2.6. Degradation Study
2.7. Loading of Gentamicin Sulfate
2.8. In Vitro Analysis
2.9. Cytotoxicity Test
3. Results and Discussion
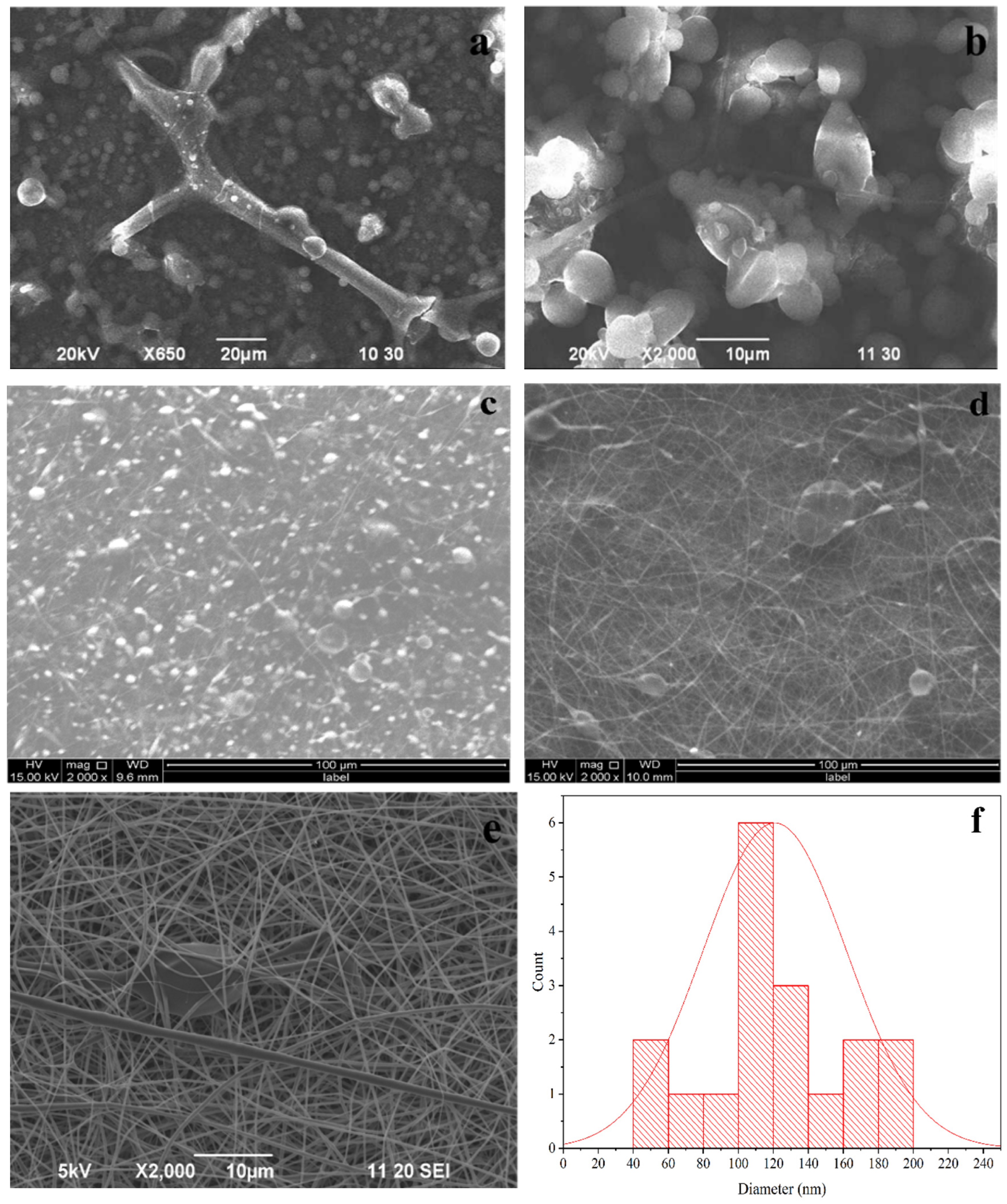
3.1. Morphology and Crystal Structure of nHAp
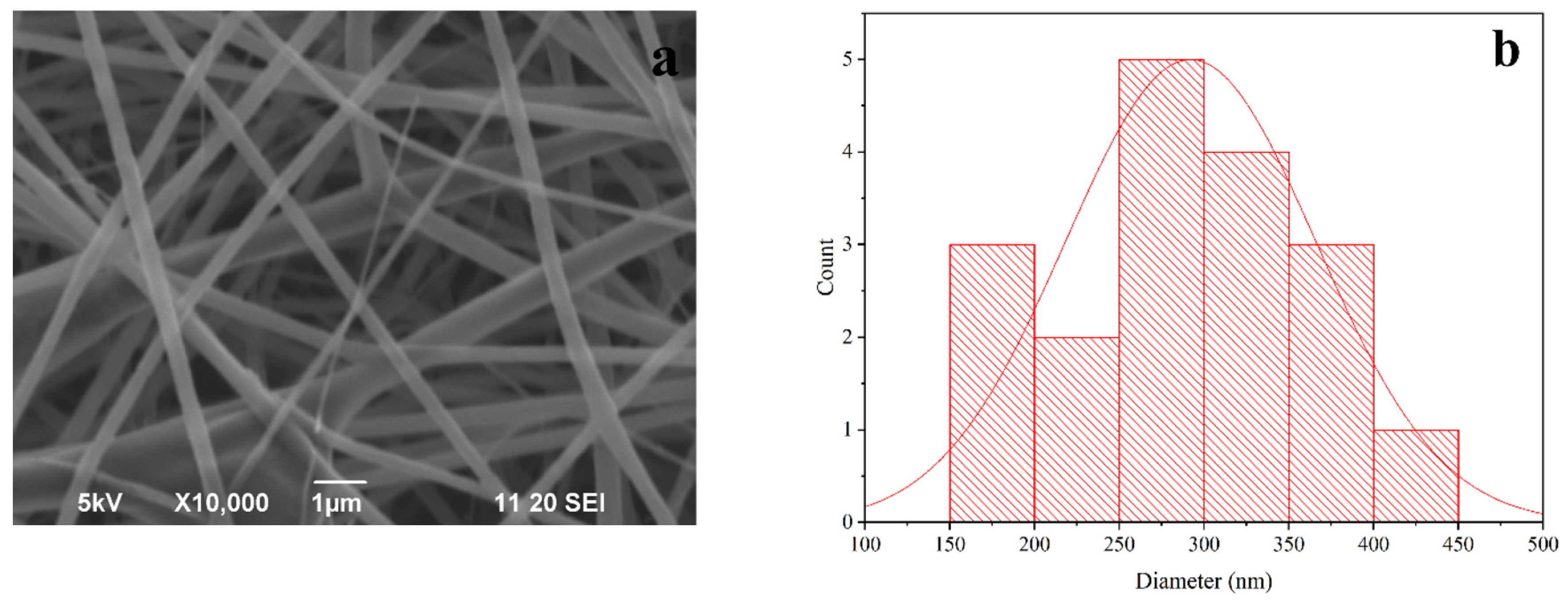
3.2. Morphology of PLA Fiber
3.3. Morphology of PLA-nHAp Nanocomposite
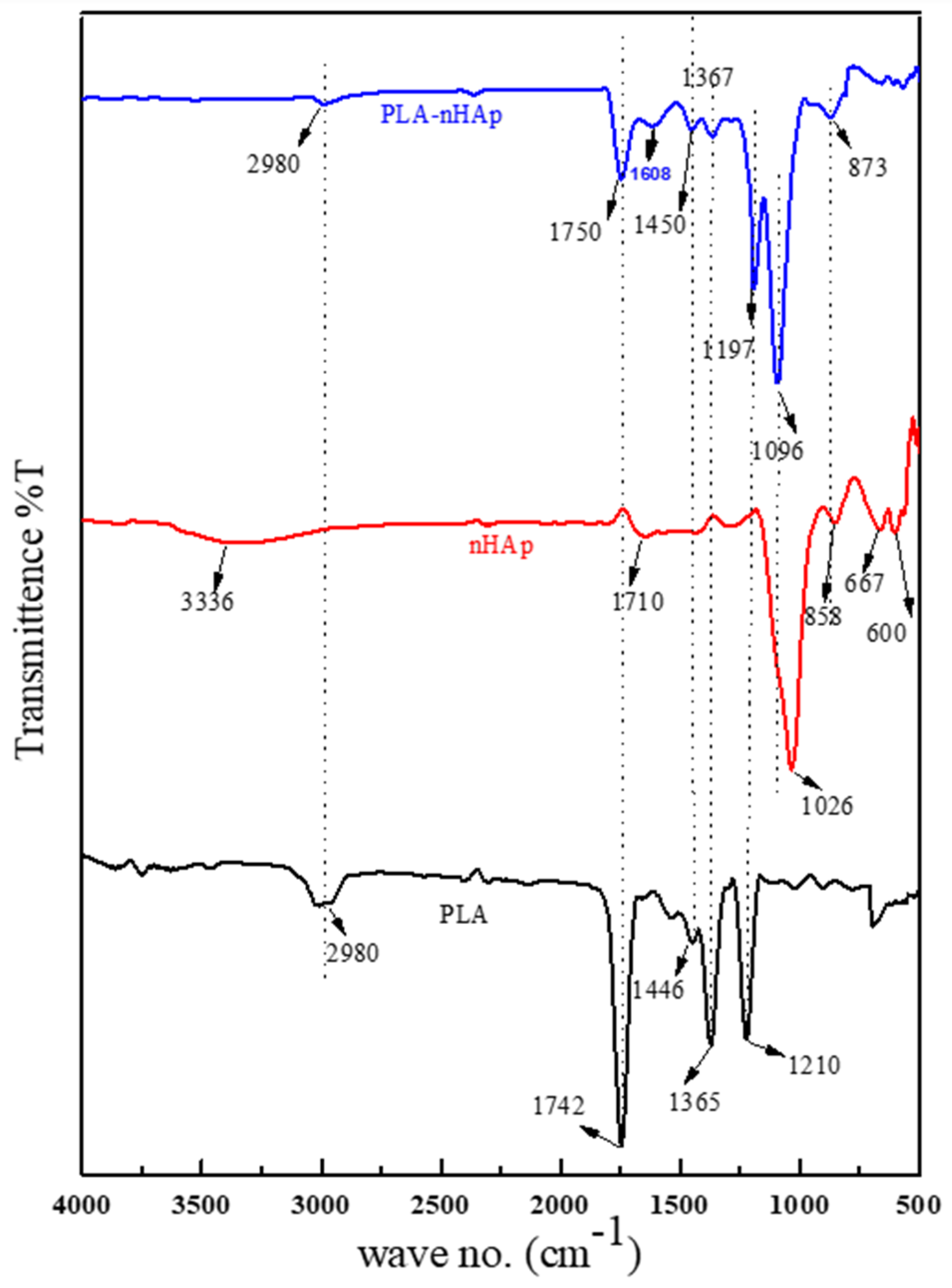
3.4. FT-IR Analysis of PLA-nHAp Nanocomposite
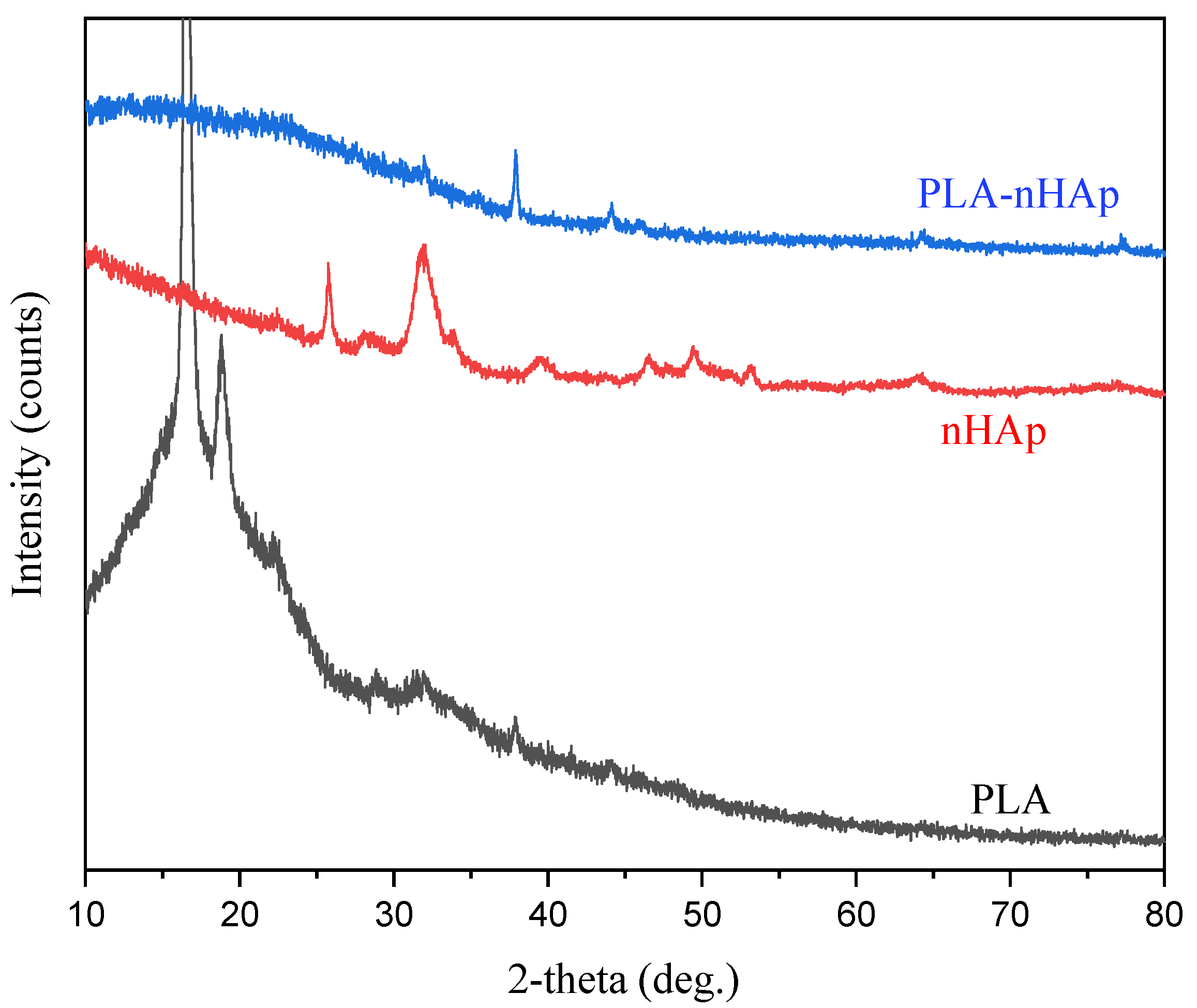
3.5. XRD Studies of PLA-nHAp Nanocomposite
3.6. Thermogravimetric Analysis of PLA-nHAp Nanocomposite
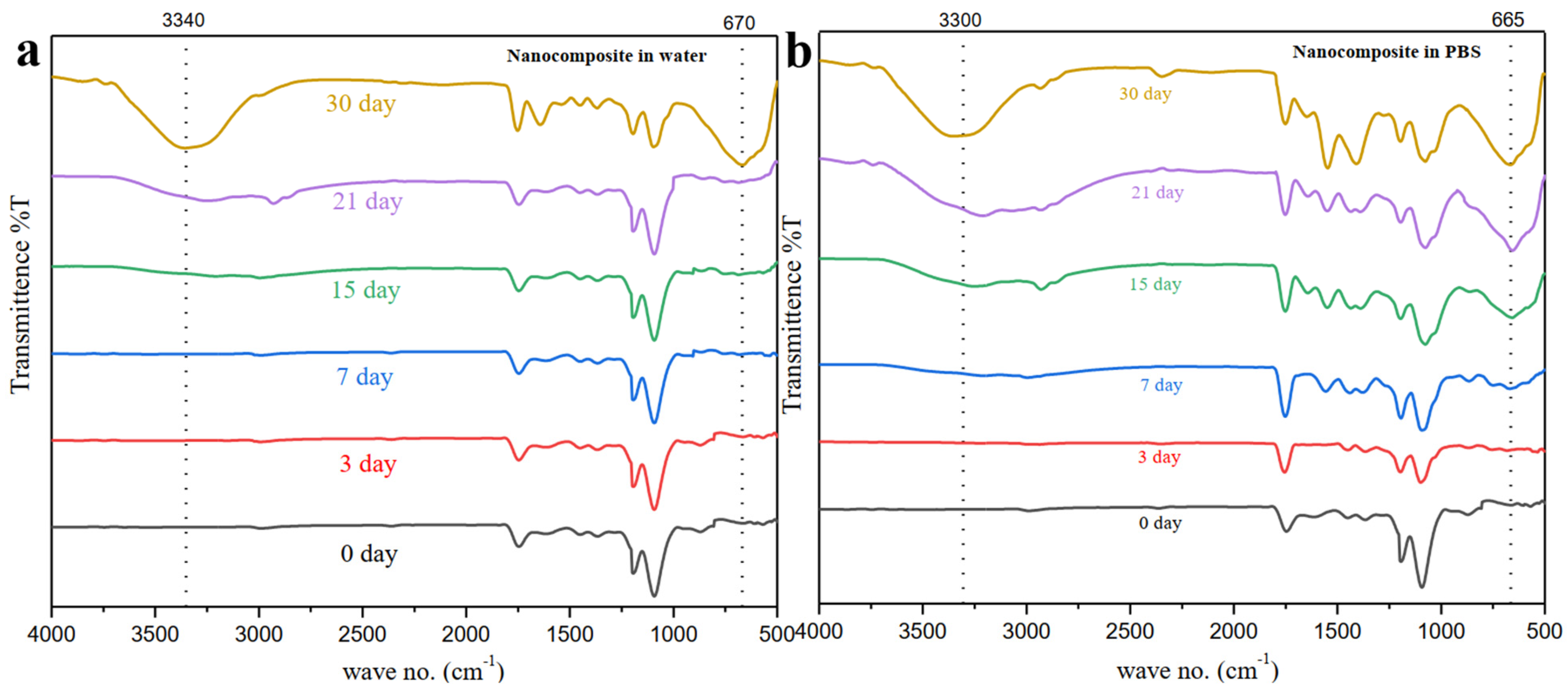
3.7. Degradation Study of PLA-nHAp Nanocomposite
3.8. In-Vitro Cytotoxicity Analysis
3.9. Drug Loading and In-Vitro Release Profile
3.9.1. Standard Curve of Gentamicin Sulfate
3.9.2. FT-IR Spectra of Drug-Loaded Nanocomposite
3.9.3. SEM of Drug Loaded Nanocomposite
3.9.4. In Vitro Drug Release Profile
3.9.5. Mechanism of Drug Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.; Asyraf, M.; Razman, M. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-U. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Farkas, N.-I.; Marincaș, L.; Barabás, R.; Bizo, L.; Ilea, A.; Turdean, G.L.; Toșa, M.; Cadar, O.; Barbu-Tudoran, L. Preparation and Characterization of Doxycycline-Loaded Electrospun PLA/HAP Nanofibers as a Drug Delivery System. Materials 2022, 15, 2105. [Google Scholar] [CrossRef] [PubMed]
- Pisani, S.; Dorati, R.; Chiesa, E.; Genta, I.; Modena, T.; Bruni, G.; Grisoli, P.; Conti, B. Release profile of gentamicin sulfate from polylactide-co-polycaprolactone electrospun nanofiber matrices. Pharmaceutics 2019, 11, 161. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Koumentakou, I.; Samiotaki, C.; Meimaroglou, D.; Varytimidou, D.; Karatza, A.; Kalantzis, Z.; Roussou, M.; Bikiaris, R.D.; Papageorgiou, G.Z. Recent Advances in the Investigation of Poly (lactic acid)(PLA) Nanocomposites: Incorporation of Various Nanofillers and their Properties and Applications. Polymers 2023, 15, 1196. [Google Scholar] [CrossRef]
- Tanaka, K.; Shiga, T.; Katayama, T. Fabrication of hydroxyapatite/PLA composite nanofiber by electrospinning. WIT Trans. Built. Environ. 2016, 166, 371–379. [Google Scholar]
- Habibah, T.U.; Amlani, D.V.; Brizuela, M. Hydroxyapatite Dental Material; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zhou, D.; Qi, C.; Chen, Y.-X.; Zhu, Y.-J.; Sun, T.-W.; Chen, F.; Zhang, C.-Q. Comparative study of porous hydroxyapatite/chitosan and whitlockite/chitosan scaffolds for bone regeneration in calvarial defects. Int. J. Nanomed. 2017, 12, 2673. [Google Scholar] [CrossRef]
- Fu, Z.; Cui, J.; Zhao, B.; Shen, S.G.; Lin, K. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J. Orthop. Transl. 2021, 28, 118–130. [Google Scholar] [CrossRef]
- Prasanna, A.; Venkatasubbu, G.D. Sustained release of amoxicillin from hydroxyapatite nanocomposite for bone infections. Prog. Biomater. 2018, 7, 289–296. [Google Scholar] [CrossRef]
- Slyamova, G.; Gusmanov, A.; Batpenov, A.; Kaliev, N.; Viderman, D. Risk Factors for Postoperative Osteomyelitis among Patients after Bone Fracture: A Matched Case–Control Study. J. Clin. Med. 2022, 11, 6072. [Google Scholar] [CrossRef]
- Kyriacou, H.; Kamaraj, A.; Khan, W.S. Developments in antibiotic-eluting scaffolds for the treatment of osteomyelitis. Appl. Sci. 2020, 10, 2244. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Casettari, L.; Bonacucina, G.; Cespi, M.; Palmieri, G.; Illum, L. Folic acid conjugated chitosan nanoparticles for tumor targeting of therapeutic and imaging agents. Pharm. Nanotechnol. 2013, 1, 184. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci 2013, 8, 1639–1645. [Google Scholar]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef]
- Sonseca, A.; Peponi, L.; Sahuquillo, O.; Kenny, J.M.; Giménez, E. Electrospinning of biodegradable polylactide/hydroxyapatite nanofibers: Study on the morphology, crystallinity structure and thermal stability. Polym. Degrad. Stab. 2012, 97, 2052–2059. [Google Scholar] [CrossRef]
- Thakur, A.; Wanchoo, R.; Singh, P. Hydrogels of poly (acrylamide-co-acrylic acid): In-vitro study on release of gentamicin sulfate. Chem. Biochem. Eng. Q. 2011, 25, 471–482. [Google Scholar]
- Kabir, S.; Ahmed, S.; Mustafa, A.; Ahsan, M.; Islam, S. Synthesis and characterization of Fe-doped hydroxyapatite. Bangladesh J. Sci. Ind. Res. 2012, 47, 1–8. [Google Scholar] [CrossRef]
- Ojha, S. Structure–property relationship of electrospun fibers. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–253. [Google Scholar]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly (lactic acid)/poly (ethylene glycol) polymer nanocomposites: Effects of graphene nanoplatelets. Polymers 2014, 6, 93–104. [Google Scholar] [CrossRef]
- Macha, I.J.; Karacan, I.; Ben-Nissan, B.; Cazalbou, S.; Müller, W.H. Development of antimicrobial composite coatings for drug release in dental, orthopaedic and neural prostheses applications. SN Appl. Sci. 2019, 1, 68. [Google Scholar] [CrossRef]
- Kabira, S.F.; Ahmed, S.; Ahsanb, M.; Mustafa, A.I. Investigation of Sintering Temperature and Concentration Effects on Sodium Doped Hydroxyapatite. Trends Biomater. Artif. Organs 2012, 26, 56–63. [Google Scholar]
- Nejati, E.; Mirzadeh, H.; Zandi, M. Synthesis and characterization of nano-hydroxyapatite rods/poly (l-lactide acid) composite scaffolds for bone tissue engineering. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1589–1596. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.P.; Feldmann, M. Effects of surface modification on dispersion, mechanical, thermal and dynamic mechanical properties of injection molded PLA-hydroxyapatite composites. Compos. Part A Appl. Sci. Manuf. 2017, 103, 96–105. [Google Scholar] [CrossRef]
- Liao, S.; Cui, F.; Zhang, W.; Feng, Q. Hierarchically biomimetic bone scaffold materials: Nano-HA/collagen/PLA composite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69, 158–165. [Google Scholar] [CrossRef]
- Nair, K.M.; Thomas, S.; Groeninckx, G. Thermal and dynamic mechanical analysis of polystyrene composites reinforced with short sisal fibres. Compos. Sci. Technol. 2001, 61, 2519–2529. [Google Scholar] [CrossRef]
- Siparsky, G.L.; Voorhees, K.J.; Miao, F. Hydrolysis of polylactic acid (PLA) and polycaprolactone (PCL) in aqueous acetonitrile solutions: Autocatalysis. J. Polym. Environ. 1998, 6, 31–41. [Google Scholar] [CrossRef]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Auras, R.A.; Lim, L.-T.; Selke, S.E.; Tsuji, H. Poly (Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 10. [Google Scholar]
- Rodriguez, E.J.; Marcos, B.; Huneault, M.A. Hydrolysis of polylactide in aqueous media. J. Appl. Polym. Sci. 2016, 133, 44152. [Google Scholar] [CrossRef]
- Warabino, K.; Ueno, S.; Hino, T.; Shimabayashi, S. Preparation and adsorption properties of hydroxyapatite surface-modified by cetylphosphate. Phosphorus Res. Bull. 2006, 20, 129–134. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Liu, A.; Hong, Z.; Jing, X. Electrospun poly (L-lactide)-grafted hydroxyapatite/poly (L-lactide) nanocomposite fibers. Eur. Polym. J. 2007, 43, 3187–3196. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Properties and morphology of poly (L-lactide). II. Hydrolysis in alkaline solution. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 59–66. [Google Scholar] [CrossRef]
- Mehedi Hasan, M.; Nuruzzaman Khan, M.; Haque, P.; Rahman, M.M. Novel alginate-di-aldehyde cross-linked gelatin/nano-hydroxyapatite bioscaffolds for soft tissue regeneration. Int. J. Biol. Macromol. 2018, 117, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Masud, R.A.; Islam, M.S.; Haque, P.; Khan, M.N.I.; Shahruzzaman, M.; Khan, M.; Takafuji, M.; Rahman, M.M. Preparation of novel chitosan/poly (ethylene glycol)/ZnO bionanocomposite for wound healing application: Effect of gentamicin loading. Materialia 2020, 12, 100785. [Google Scholar] [CrossRef]
- Baia, M.; Astilean, S.; Iliescu, T. Raman and SERS Investigations of Pharmaceuticals; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Nabipour, H.; Soltani, B.; Ahmadi Nasab, N. Gentamicin loaded Zn 2 (bdc) 2 (dabco) frameworks as efficient materials for drug delivery and antibacterial activity. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1206–1213. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Ramteke, P.W. Fabrication and assessment of gentamicin loaded electrospun nanofibrous scaffolds as a quick wound healing dressing material. Curr. Nanosci. 2015, 11, 222–228. [Google Scholar] [CrossRef]
- Macha, I.J.; Ben-Nissan, B.; Müller, W. Kinetics and the Theoretical Aspects of Drug Release from PLA/HAp Thin Films. Key Eng. Mater. 2017, 758, 113–119. [Google Scholar] [CrossRef]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Gimeno-Alcañiz, J.V.; Ocio, M.J.; Lagaron, J.M. Controlled delivery of gentamicin antibiotic from bioactive electrospun polylactide-based ultrathin fibers. Adv. Eng. Mater. 2012, 14, B112–B122. [Google Scholar] [CrossRef]
- Zhu, X.; Ni, S.; Xia, T.; Yao, Q.; Li, H.; Wang, B.; Wang, J.; Li, X.; Su, W. Anti-neoplastic cytotoxicity of SN-38-loaded PCL/Gelatin electrospun composite nanofiber scaffolds against human glioblastoma cells in vitro. J. Pharm. Sci. 2015, 104, 4345–4354. [Google Scholar] [CrossRef]
- Onunkwo, G.; Onyishi, I. Kinetics and Mechanisms of Drug Release from Swellable and Non Swellable Matrices: A Review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 97–103. [Google Scholar]




| Sample | Composition (Concentration, %w/v) |
|---|---|
| PF-1 | 5% |
| PF-2 | 10% |
| PF-3 | 15% |
| PF-4 | 20% |
| PF-5 | 25% |
| Duration | pH Value | |
|---|---|---|
| Water | PBS | |
| 0 days | 6.13 | 7.40 |
| 7 days | 6.60 | 7.28 |
| 14 days | 7.40 | 7.13 |
| 21 days | 7.04 | 7.07 |
| 30 days | 6.70 | 6.96 |
| Sample ID | BHK-21 Cell’s Survival Rate | Vero Cell’s Survival Rate | Remarks |
|---|---|---|---|
| Control | 100% | 100% | --- |
| PLA-nHAp nanocomposite | >95% | >95% | No cytotoxicity |
| Correlation Coefficient (R2) | |||
|---|---|---|---|
| Kinetic Models | pH 7.4 | pH 6 | pH 5.5 |
| Zero-order | 0.9369 | 0.6763 | 0.7680 |
| First-order | 0.9606 | 0.8586 | 0.8613 |
| Higuchi | 0.9848 | 0.9198 | 0.9382 |
| Korsmeyer-Peppas | 0.9807 | 0.8260 | 0.8939 |
| Media | Value of n | Drug Transport Mechanism |
|---|---|---|
| pH 7.4 | 0.5 | Fickian diffusion |
| pH 6 | 0.27 | Quasi Fickian |
| pH 5.5 | 0.32 | Quasi Fickian |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanak, N.A.; Shahruzzaman, M.; Islam, M.S.; Takafuji, M.; Rahman, M.M.; Kabir, S.F. Fabrication of Electrospun PLA-nHAp Nanocomposite for Sustained Drug Release in Dental and Orthopedic Applications. Materials 2023, 16, 3691. https://doi.org/10.3390/ma16103691
Kanak NA, Shahruzzaman M, Islam MS, Takafuji M, Rahman MM, Kabir SF. Fabrication of Electrospun PLA-nHAp Nanocomposite for Sustained Drug Release in Dental and Orthopedic Applications. Materials. 2023; 16(10):3691. https://doi.org/10.3390/ma16103691
Chicago/Turabian StyleKanak, Nishat Anzum, Md. Shahruzzaman, Md. Sazedul Islam, Makoto Takafuji, Mohammed Mizanur Rahman, and Sumaya F. Kabir. 2023. "Fabrication of Electrospun PLA-nHAp Nanocomposite for Sustained Drug Release in Dental and Orthopedic Applications" Materials 16, no. 10: 3691. https://doi.org/10.3390/ma16103691
APA StyleKanak, N. A., Shahruzzaman, M., Islam, M. S., Takafuji, M., Rahman, M. M., & Kabir, S. F. (2023). Fabrication of Electrospun PLA-nHAp Nanocomposite for Sustained Drug Release in Dental and Orthopedic Applications. Materials, 16(10), 3691. https://doi.org/10.3390/ma16103691







