Effect of Iron Tailing Powder-Based Ternary Admixture on Acid Corrosion Resistance of Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Proportion and Preparation of Concrete
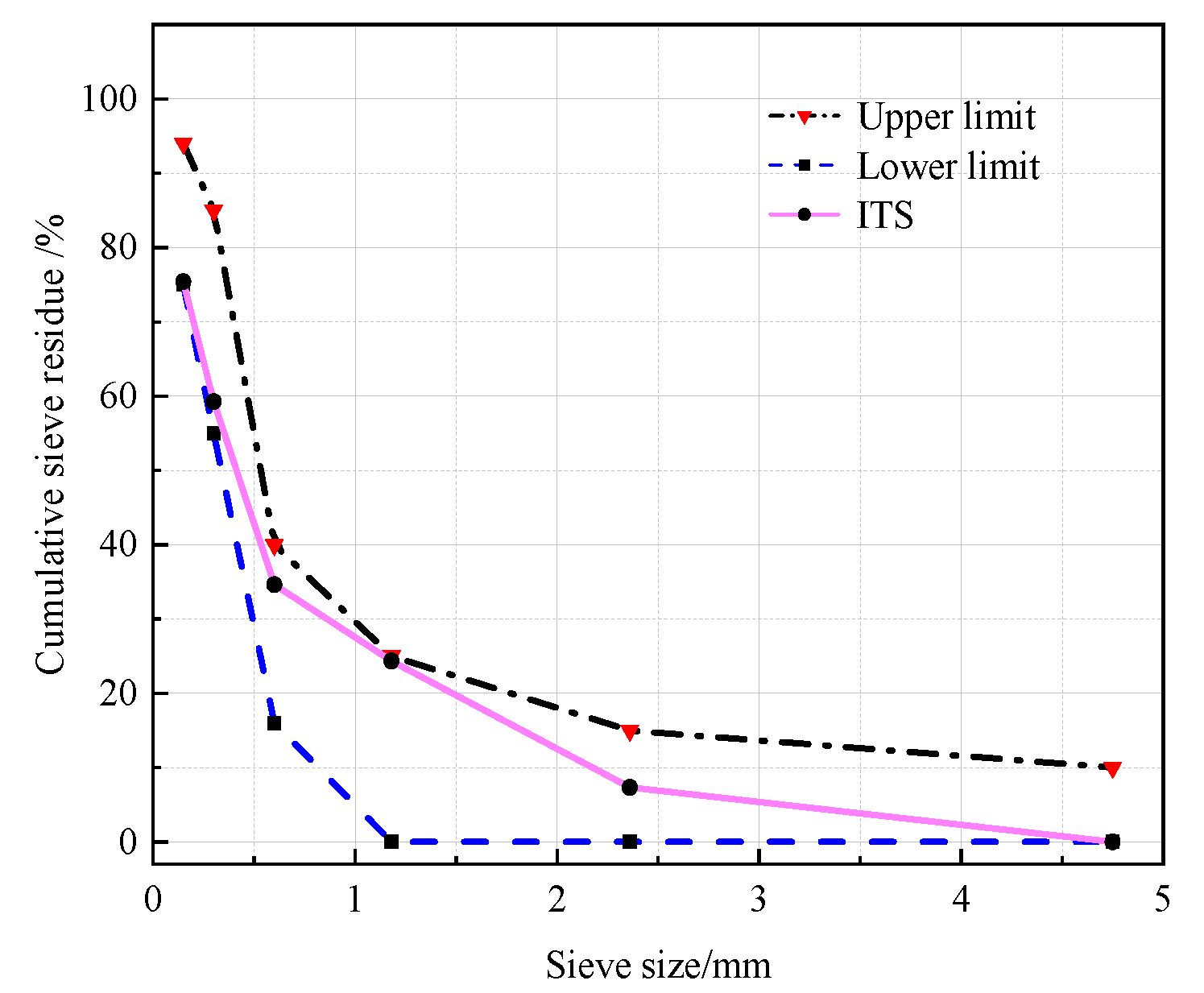
2.3. Test Methods
- (1)
- Acid erosion: The acid erosion test consisted of 99.5% concentrated acetic acid being configured into an acidic solution with pH = 3. During the erosion process, the concentration of the saturated solution was adjusted every seven days to keep the concentration of the solution stable. The saturated solution was replaced once every 30 days, and the soaking cycle was 60 days.
- (2)
- Compressive strength loss: The Shenzhen Universal testing equipment was used to test the concrete cube’s compressive strength in line with Chinese standard GB/T 50081-2019, and the compressive strength loss (CSL) was computed as follows:where ft is the average compressive strength (MPa) of three specimens for the standard curing 28 days and fs is the average compressive strength (MPa) of three specimens for the acid erosion 60 days.
- (3)
- Mass loss: The mass test measures the mass of the specimen after 28 days of the standard curing and the mass of the specimen after 60 days of acid erosion. The mass of the concrete was measured using an electronic scale with a precision of ±1 g. At the measurement time, the specimens were rinsed with tap water, and then dried at 50 ± 2 °C for 48 h. The mass loss (ML) of concrete was then determined as followswhere is the average mass (g) of three specimens for the standard curing 28 days and is the average mass (g) of three specimens for the acid erosion 60 days.
- (4)
- Apparent deterioration: The apparent deterioration test examined soaking concrete specimens in an acetic acid solution that causes apparent changes by recording the apparent changes of acid erosion on concrete specimens in the early, middle, and late stages.
- (5)
- MIP: Taking the concrete specimen, a 15 mm thick slice of concrete was cut out with a cutter before a core was drilled and sampled using an electric drill and a hollow drill bit with an inner diameter of 8–14 mm. The sample contained no aggregate. After sampling, the specimen was immersed in anhydrous ethanol for seven days to terminate hydration. Finally, the specimen was baked for three days at a temperature of 50 ± 2 °C to obtain the sample to be tested. The pore distribution was determined using the AutoPore Iv 9510 high performance automatic mercury injection instrument.
- (6)
- SEM: Taking the concrete specimen, a 3–5 mm thick slice of concrete was cut out with a cutter before using an electric drill to drill a core sample in the slice. After sampling, the specimen was immersed in anhydrous ethanol for seven days to terminate hydration. Then, the sample was placed in an ultrasonic cleaner to clean, and finally put into an oven at a temperature of 50 ± 2 °C for three days to obtain the sample to be tested. The micromorphology was determined by ZEISS Gemini 300 scanning electron microscope.
3. Results and Discussion
3.1. Compressive Strength
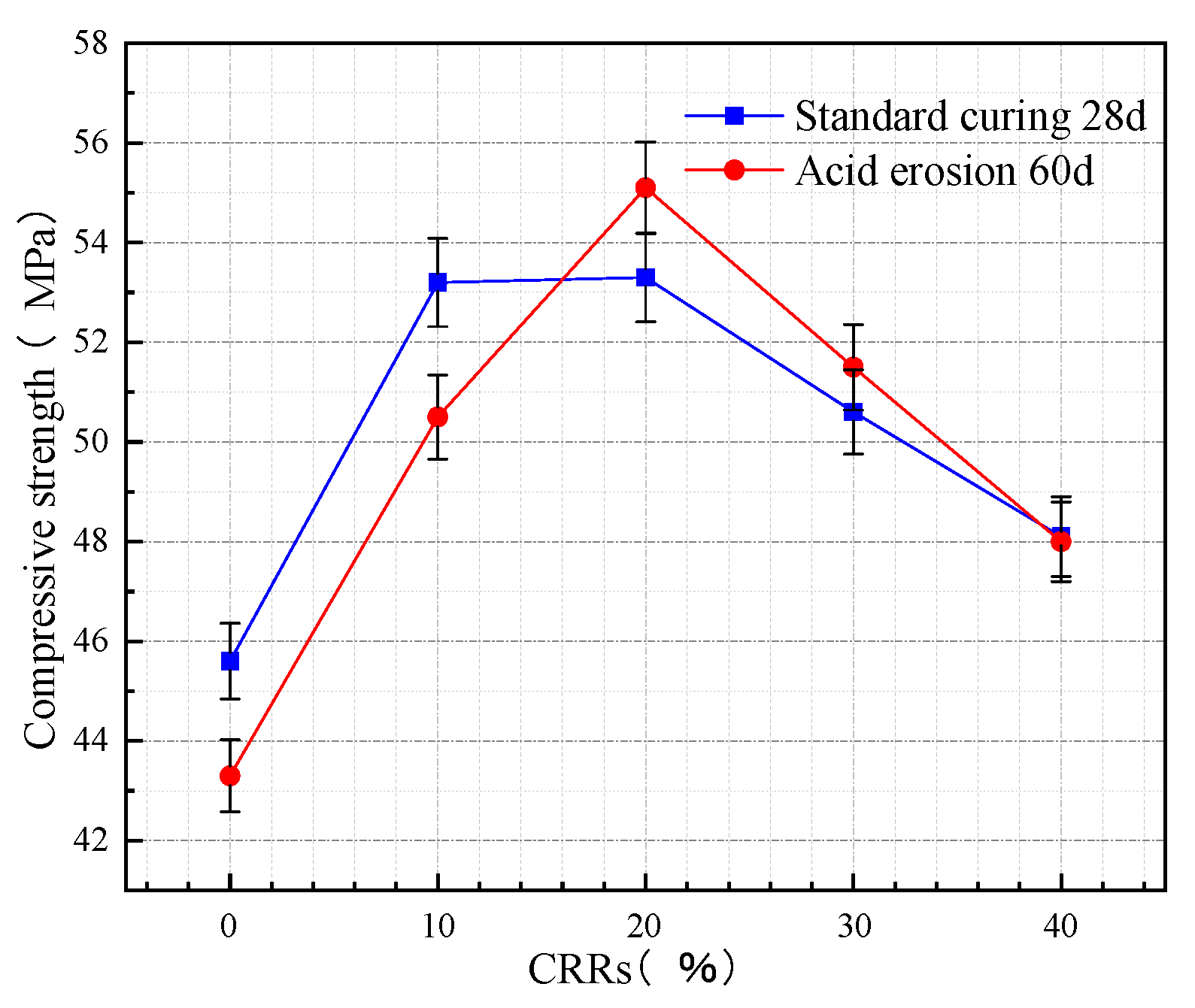
3.1.1. Compressive Strength of Concrete at Different CRRs
3.1.2. Compressive Strength of Concrete at Different w/b
3.2. Mass Loss
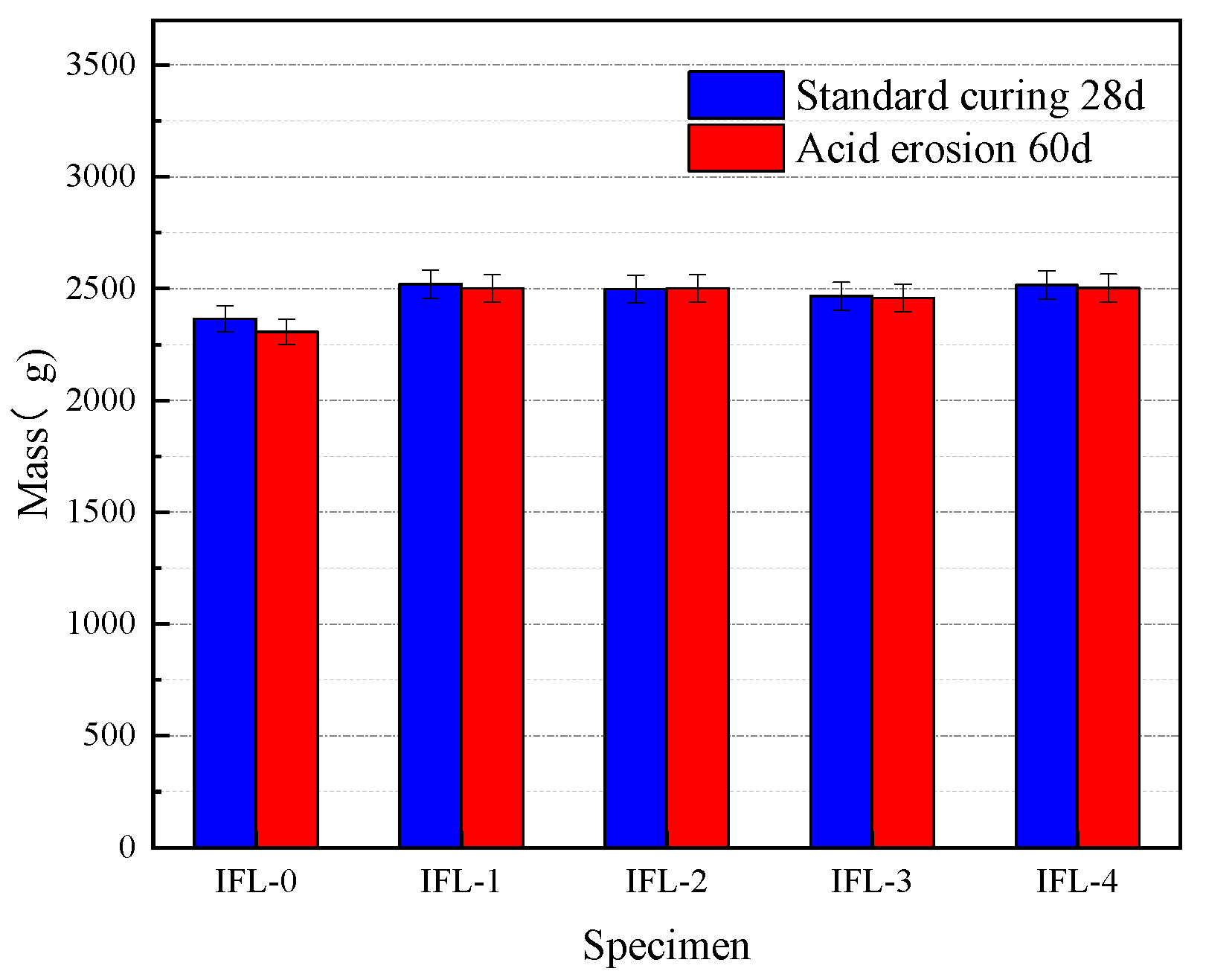
3.2.1. Mass Loss of Concrete at Different CRRs
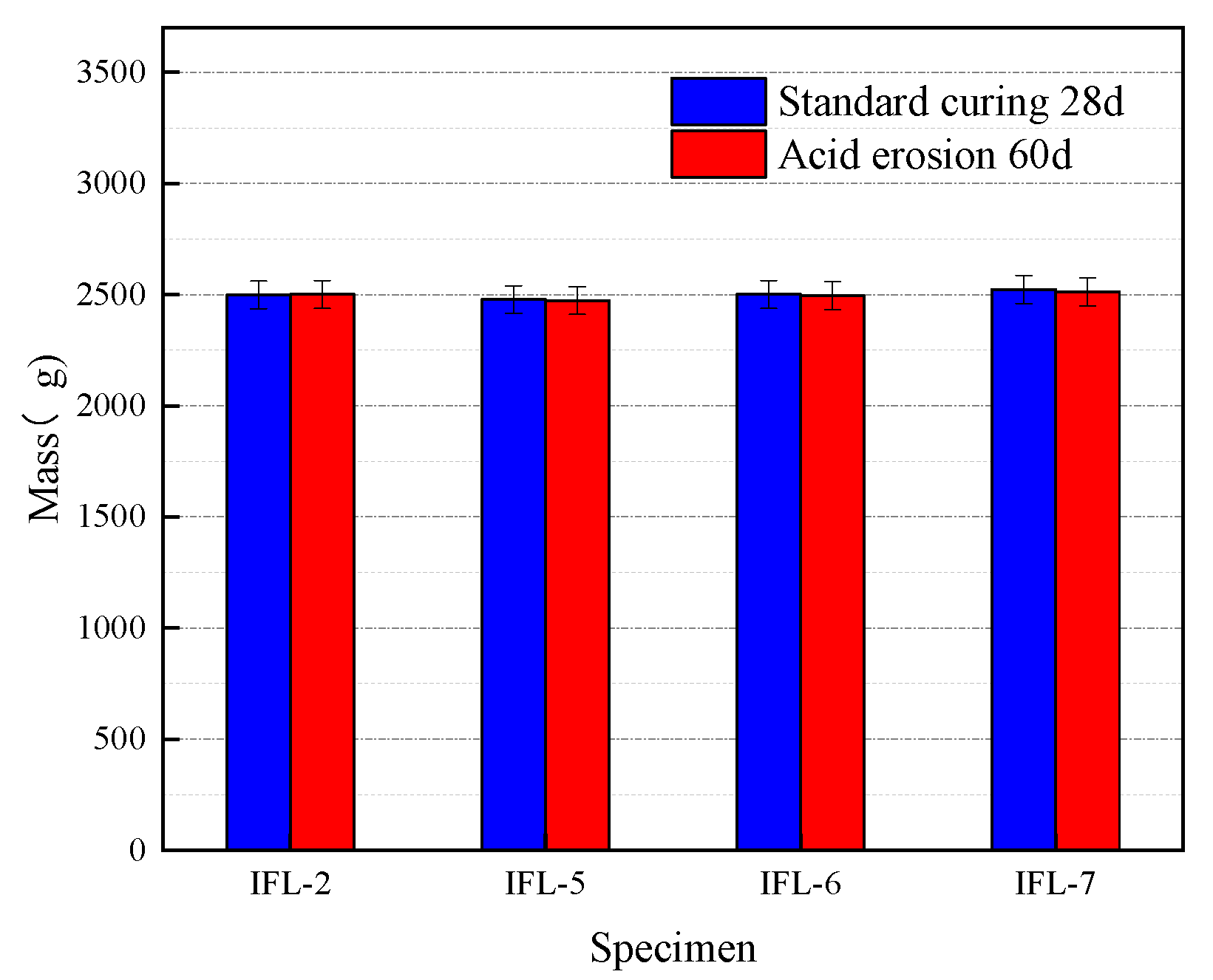
3.2.2. Mass Loss of Concrete at Different w/b
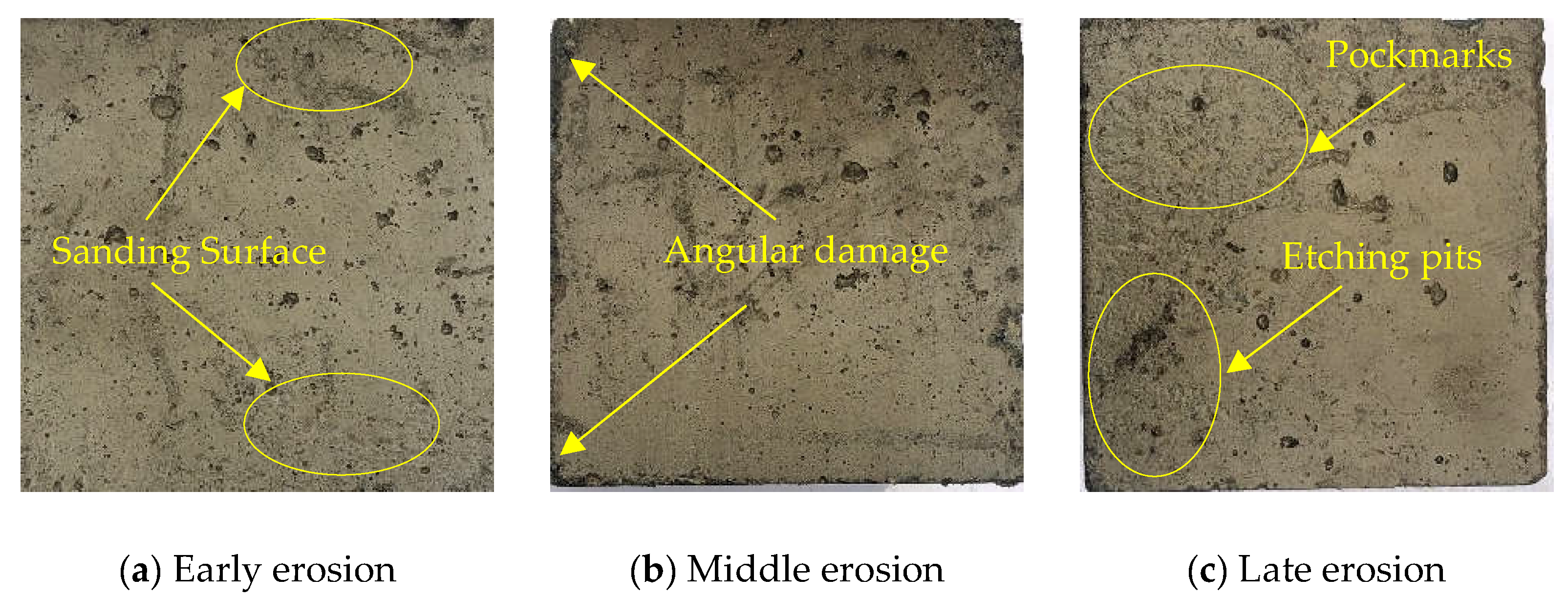
3.3. Apparent Deterioration
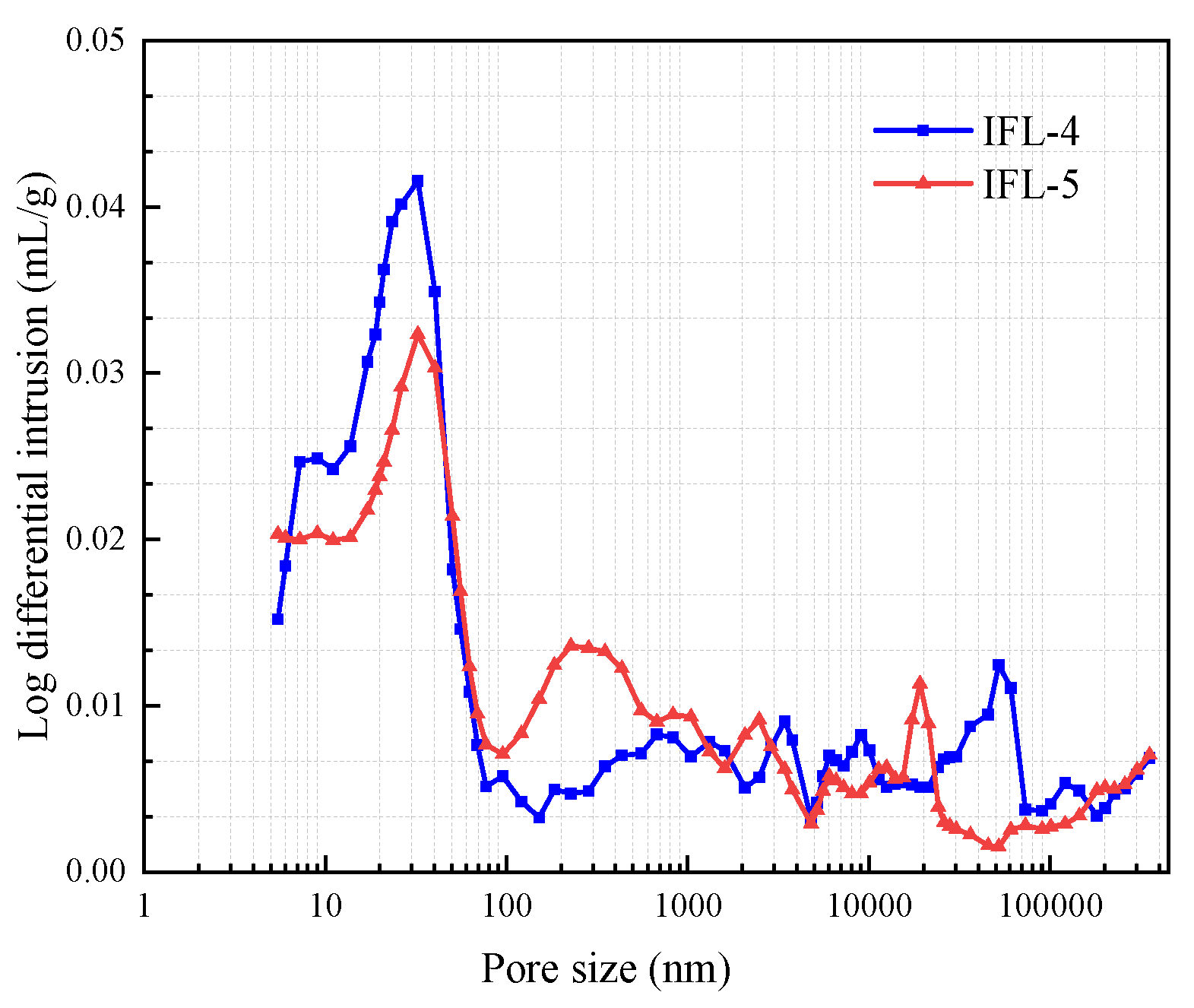
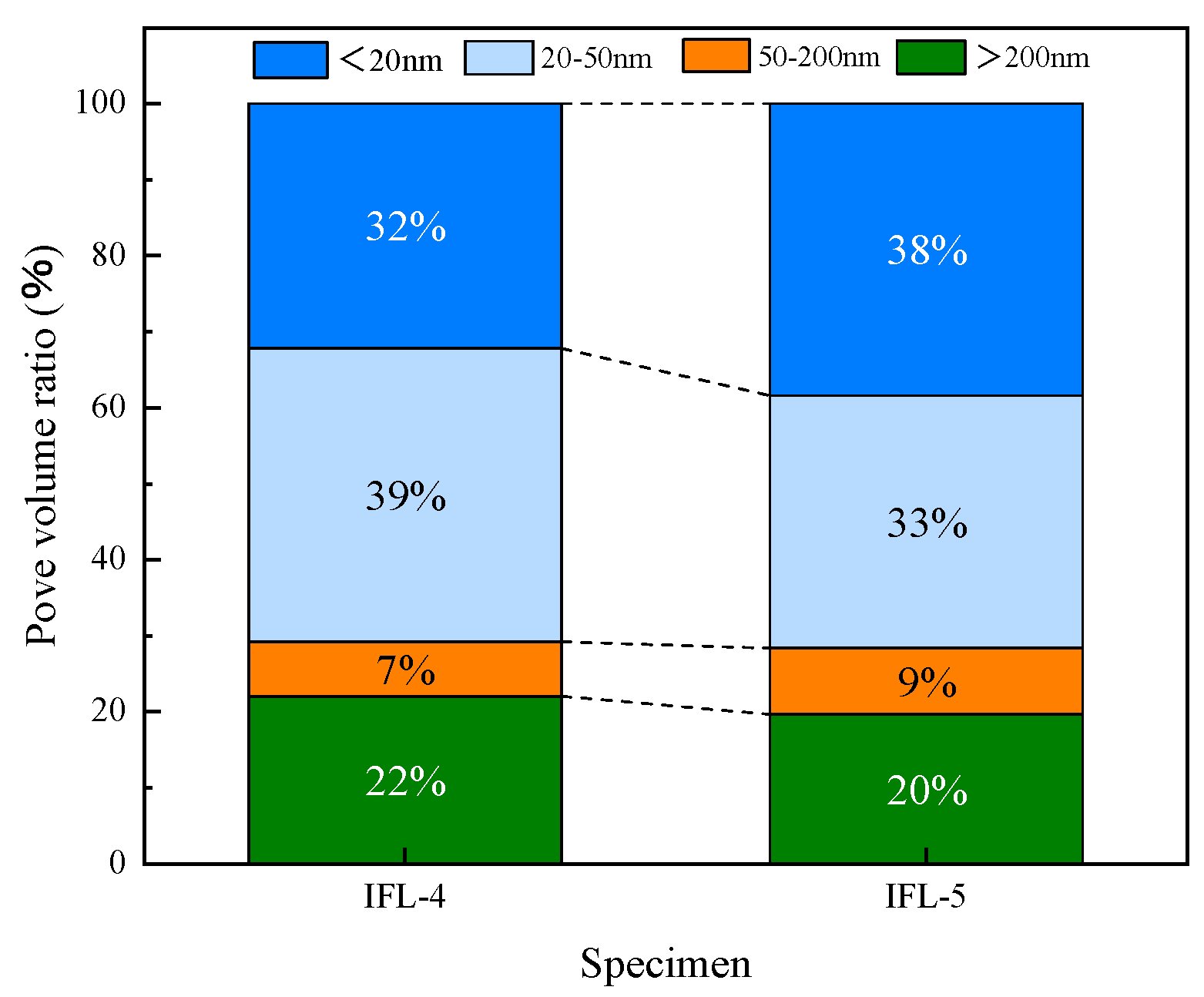
3.4. MIP
3.5. SEM
4. Conclusions
- (1)
- When the CRR is 20%, the standard curing 28 d compressive strength of concrete is the highest, which is higher than OPC concrete. When the w/b is 0.42, the standard curing 28 d compressive strength of concrete is the highest. The activity of ITP is significantly increased after grinding. Appropriate mixing with FA and LS can help with the particle gradation and produce a micro-aggregate effect. A volcanic ash reaction occurs, which has a strong hydration reaction ability and makes the compressive strength of concrete increase.
- (2)
- Concrete eroded with acetic acid solution after 60 days. When the CRR is greater than 16%, especially at 20%, the concrete strength increase rate after acid erosion 60 d was the largest and showed strong acid erosion resistance. When the w/b is less than 0.47, especially at 0.42, the concrete strength increase rate after acid erosion 60 d is the largest and shows strong acid erosion resistance. The good volcanic ash activity of FA and LS improved the pore structure of concrete, and improved the amount of cementation and cementation process. The filling effect increased the compactness of concrete to a certain extent, strengthened the acid penetration resistance of concrete, and hindered the transport of ions in acetic acid solution in the pores of the concrete.
- (3)
- Acidic solution erosion deteriorates the concrete surface. At the same time, there is a loss of mass of concrete, which is minimal when the CRRs is 20% and minimal when the w/b is 0.42; all of them are smaller than the ML of the OPC concrete specimen. The ternary mineral admixture system consisting of ITP, FA, and LS resulted in more delicate pores and a denser structure of concrete, which effectively hindered the erosion of concrete by acetic acid solution.
- (4)
- The mineral doping system reduces the permeability of the erosion medium. To a certain extent, it can alleviate the erosion of acid solution concrete through MIP and SEM, which confirms that the concrete has lower internal porosity and better density. The ITP-FA-LS ternary mineral admixture concrete has better acid erosion resistance than OPC concrete.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fattuhi, N.I.; Hughes, B.P. Ordinary Portland cement mixes with selected admixtures subjected to sulfuric acid attack. Mater. J. 1988, 85, 512–518. [Google Scholar]
- Hobbs, D.W.; Taylor, M.G. Nature of the thaumasite sulfate attack mechanism in field concrete. Cem. Concr. Res. 2000, 30, 529–533. [Google Scholar] [CrossRef]
- Bassuoni, M.T.; Nehdi, M.L. Resistance of self-consolidating concrete to sulfuric acid attack with consecutive pH reduction. Cem. Concr. Res. 2007, 37, 1070–1084. [Google Scholar] [CrossRef]
- Daczko, J.A. Decreasing concrete sewer pipe degradation using admixtures. Mater. Perform. 1997, 36, 51–56. [Google Scholar]
- Ehrich, S.; Helard, L.; Letourneux, R.; Willocq, J.; Bock, E. Biogenic and chemical sulfuric acid corrosion of mortars. J. Mater. Civ. Eng. 1999, 11, 340–344. [Google Scholar] [CrossRef]
- Fattuhi, N.I.; Hughes, B.P. SRPC and modified concretes subjected to severe sulphuric acid attack. Mag. Concr. Res. 1988, 40, 159–166. [Google Scholar] [CrossRef]
- Hewayde, E.; Nehdi, M.L.; Allouche, E.; Nakhla, G. Using concrete admixtures for sulphuric acid resistance. Proc. Inst. Civ. Eng.-Constr. Mater. 2007, 160, 25–35. [Google Scholar] [CrossRef]
- Torii, K.; Kawamura, M. Effects of fly ash and silica fume on the resistance of mortar to sulfuric acid and sulfate attack. Cem. Concr. Res. 1994, 24, 361–370. [Google Scholar] [CrossRef]
- Roy, D.M.; Arjunan, P.; Silsbee, M.R. Effect of silica fume, metakaolin, and low-calcium fly ash on chemical resistance of concrete. Cem. Concr. Res. 2001, 31, 1809–1813. [Google Scholar] [CrossRef]
- Alexander, M.G.; Fourie, C. Performance of sewer pipe concrete mixtures with portland and calcium aluminate cements subject to mineral and biogenic acid attack. Mater. Struct. 2011, 44, 313–330. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Lee, K.M.; Bang, J.W.; Kwon, S.-J. Effect of W/C ratio on durability and porosity in cement mortar with constant cement amount. Adv. Mater. Sci. Eng. 2014, 2014, 273460. [Google Scholar] [CrossRef]
- Wang, W.; Shen, A.; He, Z.; Guo, Y.; Li, D. Mechanism and erosion resistance of internally cured concrete including super absorbent polymers against coupled effects of acid rain and fatigue load. Constr. Build. Mater. 2021, 290, 123252. [Google Scholar] [CrossRef]
- Araghi, H.J.; Nikbin, I.M.; Reskati, S.R.; Rahmani, E.; Allahyari, H. An experimental investigation on the erosion resistance of concrete containing various PET particles percentages against sulfuric acid attack. Constr. Build. Mater. 2015, 77, 461–471. [Google Scholar] [CrossRef]
- De Silva, L.C.G.; Dilrukshi, W.A.N.; Jayasekara, A.; Priyadarshana, T.; Samantha, M.K. Development of Pervious Concrete by Using Bottom Ash as Supplementary Cementious Material; ICSBE 2020; Springer: Singapore, 2022; pp. 409–421. [Google Scholar]
- Reddy, P.N.; Kavyateja, B.V. Durability performance of high strength concrete incorporating supplementary cementitious materials. Mater. Today Proc. 2020, 33, 66–72. [Google Scholar] [CrossRef]
- Fahmy, M.A.; Abu El-Hassan, M.M.; Kamh, G.M.; Bashandy, A.A. Investigation of Using Nano-silica, Silica Fume and Fly Ash in High Strength Concrete. Eng. Res. J. 2020, 43, 211–221. [Google Scholar] [CrossRef]
- Kishan, A.; Prabakar, J. Influence of Mineral and Chemical admixtures in Ordinary Portland Cement on Physical and Mechanical Properties. J. Impact Factor 2017, 3, 26–34. [Google Scholar]
- Puerta-Falla, G.; Balonis, M.; Le Saout, G.; Neithalath, N.; Sant, G. The influence of metakaolin on limestone reactivity in cementitious materials. In Calcined Clays for Sustainable Concrete; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–19. [Google Scholar]
- Keppert, M.; Urbanová, M.; Brus, J.; Čáchová, M.; Fořt, J.; Trník, A.; Scheinherrová, L.; Záleská, M.; Černý, R. Rational design of cement composites containing pozzolanic additions. Constr. Build. Mater. 2017, 148, 411–418. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Pavlík, Z.; Jankovský, O.; Pokorný, J.; Tydlitát, V.; Svora, P.; Černý, R. Physical and chemical characterization of technogenic pozzolans for the application in blended cements. Constr. Build. Mater. 2018, 160, 106–116. [Google Scholar] [CrossRef]
- Juenger, M.C.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, F.; Qi, S.; Li, W.; Liu, R.; Li, G. Durability of concrete incorporated with siliceous iron tailings. Constr. Build. Mater. 2020, 242, 118147. [Google Scholar] [CrossRef]
- Zhai, M.; Zhao, J.; Wang, D.; Wang, Y.; Wang, Q. Hydration properties and kinetic characteristics of blended cement containing lithium slag powder. J. Build. Eng. 2021, 39, 102287. [Google Scholar] [CrossRef]
- Goyal, S.; Kumar, M.; Sidhu, D.S.; Bhattacharjee, B. Resistance of mineral admixture concrete to acid attack. J. Adv. Concr. Technol. 2009, 7, 273–283. [Google Scholar] [CrossRef]
- Monteny, J.; De Belie, N.; Vincke, E.; Verstraete, W.; Taerwe, L. Chemical and microbiological tests to simulate sulfuric acid corrosion of polymer-modified concrete. Cem. Concr. Res. 2001, 31, 1359–1365. [Google Scholar] [CrossRef]
- Vincke, E.; Van Wanseele, E.; Monteny, J.; Beeldens, A.; De Belie, N.; Taerwe, L.; Van Gemert, D.; Verstraete, W. Influence of polymer addition on biogenic sulfuric acid attack of concrete. Int. Biodeterior. Biodegrad. 2002, 49, 283–292. [Google Scholar] [CrossRef]
- Wang, C.; Ni, W.; Zhang, S.; Wang, S.; Gai, G.-S.; Wang, W.-K. Preparation and properties of autoclaved aerated concrete using coal gangue and iron ore tailings. Constr. Build. Mater. 2016, 104, 109–115. [Google Scholar] [CrossRef]
- Cai, L.; Li, X.; Liu, W.; Ma, B.; Lv, Y. The slurry and physical-mechanical performance of autoclaved aerated concrete with high content solid wastes: Effect of grinding process. Constr. Build. Mater. 2019, 218, 28–39. [Google Scholar] [CrossRef]
- Cai, L.; Ma, B.; Li, X.; Lv, Y.; Liu, Z.; Jian, S. Mechanical and hydration characteristics of autoclaved aerated concrete (AAC) containing iron-tailings: Effect of content and fineness. Constr. Build. Mater. 2016, 128, 361–372. [Google Scholar] [CrossRef]
- Khan, S.U.; Nuruddin, M.F.; Ayub, T.; Shafiq, N. Effects of different mineral admixtures on the properties of fresh concrete. Sci. World J. 2014, 2014, 986567. [Google Scholar] [CrossRef]
- Hassan, K.E.; Cabrera, J.G.; Maliehe, R.S. The effect of mineral admixtures on the properties of high-performance concrete. Cem. Concr. Compos. 2000, 22, 267–271. [Google Scholar] [CrossRef]
- Gonen, T.; Yazicioglu, S. The influence of mineral admixtures on the short and long-term performance of concrete. Build. Environ. 2007, 42, 3080–3085. [Google Scholar] [CrossRef]
- Ogawa, K.; Uchikawa, H.; Takemoto, K.; Yasui, I. The mechanism of the hydration in the system C3S-pozzolana. Cem. Concr. Res. 1980, 10, 683–696. [Google Scholar] [CrossRef]
- Mishra, D.P.; Das, S.K. A study of physico-chemical and mineralogical properties of Talcher coal fly ash for stowing in underground coal mines. Mater. Charact. 2010, 61, 1252–1259. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Popovics, S.; Ujhelyi, J. Contribution to the concrete strength versus water-cement ratio relationship. J. Mater. Civ. Eng. 2008, 20, 459–463. [Google Scholar] [CrossRef]
- Monteny, J.; Vincke, E.; Beeldens, A.; De Belie, N.; Taerwe, L.; Van Gemert, D.; Verstraete, W. Chemical, microbiological, and in situ test methods for biogenic sulfuric acid corrosion of concrete. Cem. Concr. Res. 2000, 30, 623–634. [Google Scholar] [CrossRef]
- Fattuhi, N.I.; Hughes, B.P. The performance of cement paste and concrete subjected to sulphuric acid attack. Cem. Concr. Res. 1988, 18, 545–553. [Google Scholar] [CrossRef]
- Hu, L.; He, Z.; Shao, Y.; Cai, X.; Zhang, S. Microstructure and properties of sustainable cement-based materials using combustion treated rice husk ash. Constr. Build. Mater. 2021, 294, 123482. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]







| Materials | Chemical Composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Fe2O3 | MgO | SO3 | K2O | Na2O | TiO2 | MnO | |
| ITP | 62.26 | 4.78 | 7.77 | 14.37 | 6.33 | 0.48 | 1.39 | 1.34 | 0.53 | 0.21 |
| FA | 60.06 | 25.09 | 2.93 | 6.74 | 0.86 | 0.26 | 1.61 | 0.11 | 1.49 | 0.16 |
| LS | 54.55 | 25.38 | 6.44 | 1.41 | 0.60 | 6.05 | 0.70 | 0.10 | 0.03 | 0.07 |
| Materials | ITP | FA | LS |
|---|---|---|---|
| Specific surface area (m2/kg) | 1290 | 1391 | 13,627 |
| Stone Powder Content (%) | Sludge Content (%) | Stacking Density (kg/m3) | Apparent Density (kg/m3) | Mass Loss (%) | Crushing Index (%) |
|---|---|---|---|---|---|
| 4.9 | 0 | 1620 | 2560 | 4 | 22 |
| Mud Content (%) | Sludge Content (%) | Needle and Flake Content (%) | Crushing Index (%) | Stacking Density (kg/m3) | Apparent Density (kg/m3) |
|---|---|---|---|---|---|
| 0.1 | 0 | 3 | 7 | 1610 | 2630 |
| Water Reduction Rate (%) | Water Secretion Rate (%) | Gas Content (%) | 28 d Shrinkage Ratio (%) | Solids Content (%) |
|---|---|---|---|---|
| 27 | 24 | 3.9 | 103 | 12.04 |
| Specimen | Dosage of Each Component (kg/m3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Water | OPC | ITP | FA | LS | ITS | ITR | Water Reducer | |
| IFL-0 | 176.4 | 420 | 0 | 0 | 0 | 740 | 1 110 | 5.2 |
| IFL-1 | 176.4 | 378 | 21 | 10.5 | 10.5 | 740 | 1 110 | 5.2 |
| IFL-2 | 176.4 | 336 | 42 | 21 | 21 | 740 | 1 110 | 5.2 |
| IFL-3 | 176.4 | 294 | 63 | 31.5 | 31.5 | 740 | 1 110 | 5.2 |
| IFL-4 | 176.4 | 252 | 84 | 42 | 42 | 740 | 1 110 | 5.2 |
| IFL-5 | 184.8 | 336 | 42 | 21 | 21 | 740 | 1 110 | 5.2 |
| IFL-6 | 193.2 | 336 | 42 | 21 | 21 | 740 | 1 110 | 5.2 |
| IFL-7 | 201.6 | 336 | 42 | 21 | 21 | 740 | 1 110 | 5.2 |
| Specimen | CRRs (%) | w/b | Standard Curing 28 d (g) | Acid Erosion 60 d (g) | ML (%) |
|---|---|---|---|---|---|
| IFL-0 | 0 | 0.42 | 2365 | 2306 | 2.49 |
| IFL-1 | 10 | 0.42 | 2520 | 2501 | 0.75 |
| IFL-2 | 20 | 0.42 | 2498 | 2501 | −0.12 |
| IFL-3 | 30 | 0.42 | 2466 | 2458 | 0.32 |
| IFL-4 | 40 | 0.42 | 2516 | 2502 | 0.56 |
| Specimen | CRRs (%) | w/b | Standard Curing 28 d (g) | Acid Erosion 60 d (g) | ML (%) |
|---|---|---|---|---|---|
| IFL-2 | 20 | 0.42 | 2498 | 2501 | −0.12 |
| IFL-5 | 20 | 0.44 | 2478 | 2473 | 0.20 |
| IFL-6 | 20 | 0.46 | 2501 | 2495 | 0.24 |
| IFL-7 | 20 | 0.48 | 2523 | 2513 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, Y.; Liu, W.; Gu, X.; Wang, Q.; Zhang, S.; Gao, J. Effect of Iron Tailing Powder-Based Ternary Admixture on Acid Corrosion Resistance of Concrete. Materials 2023, 16, 3688. https://doi.org/10.3390/ma16103688
Zhang B, Zhang Y, Liu W, Gu X, Wang Q, Zhang S, Gao J. Effect of Iron Tailing Powder-Based Ternary Admixture on Acid Corrosion Resistance of Concrete. Materials. 2023; 16(10):3688. https://doi.org/10.3390/ma16103688
Chicago/Turabian StyleZhang, Bing, Yannian Zhang, Wenliang Liu, Xiaowei Gu, Qingjie Wang, Shaowu Zhang, and Jian Gao. 2023. "Effect of Iron Tailing Powder-Based Ternary Admixture on Acid Corrosion Resistance of Concrete" Materials 16, no. 10: 3688. https://doi.org/10.3390/ma16103688
APA StyleZhang, B., Zhang, Y., Liu, W., Gu, X., Wang, Q., Zhang, S., & Gao, J. (2023). Effect of Iron Tailing Powder-Based Ternary Admixture on Acid Corrosion Resistance of Concrete. Materials, 16(10), 3688. https://doi.org/10.3390/ma16103688






