An Anthracene-Based Bis-Stilbene Derivative as Luminescent Materials for Organic Light Emitting Diodes
Abstract
1. Introduction
2. Methodology and Experimental
2.1. Synthesis
2.1.1. Synthesis of 10-Bromoanthracene-9-carbaldehyde
2.1.2. Synthesis of 10-(9H-Carbazol-9-yl)anthracene-9-carbaldehyde
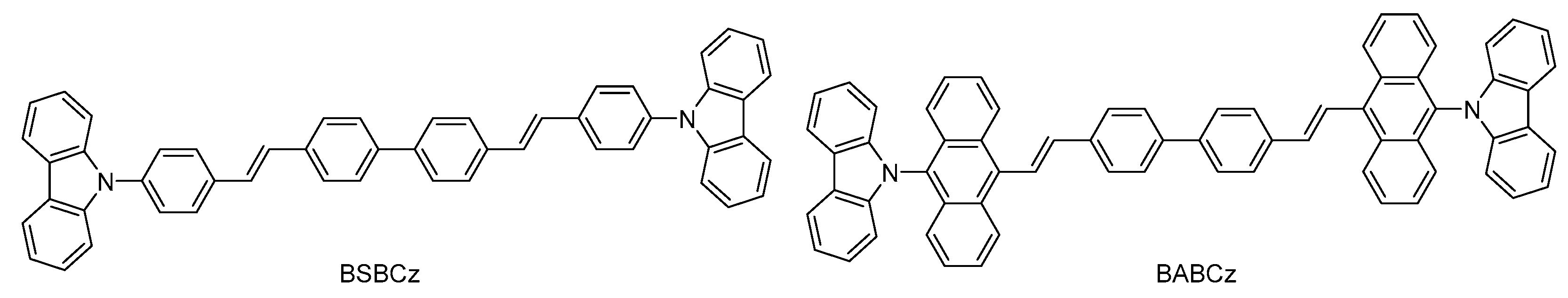
2.1.3. Synthesis of 4,4′-bis[(N-carbazole)anthracylic-vinyl]biphenyl (BABCz)
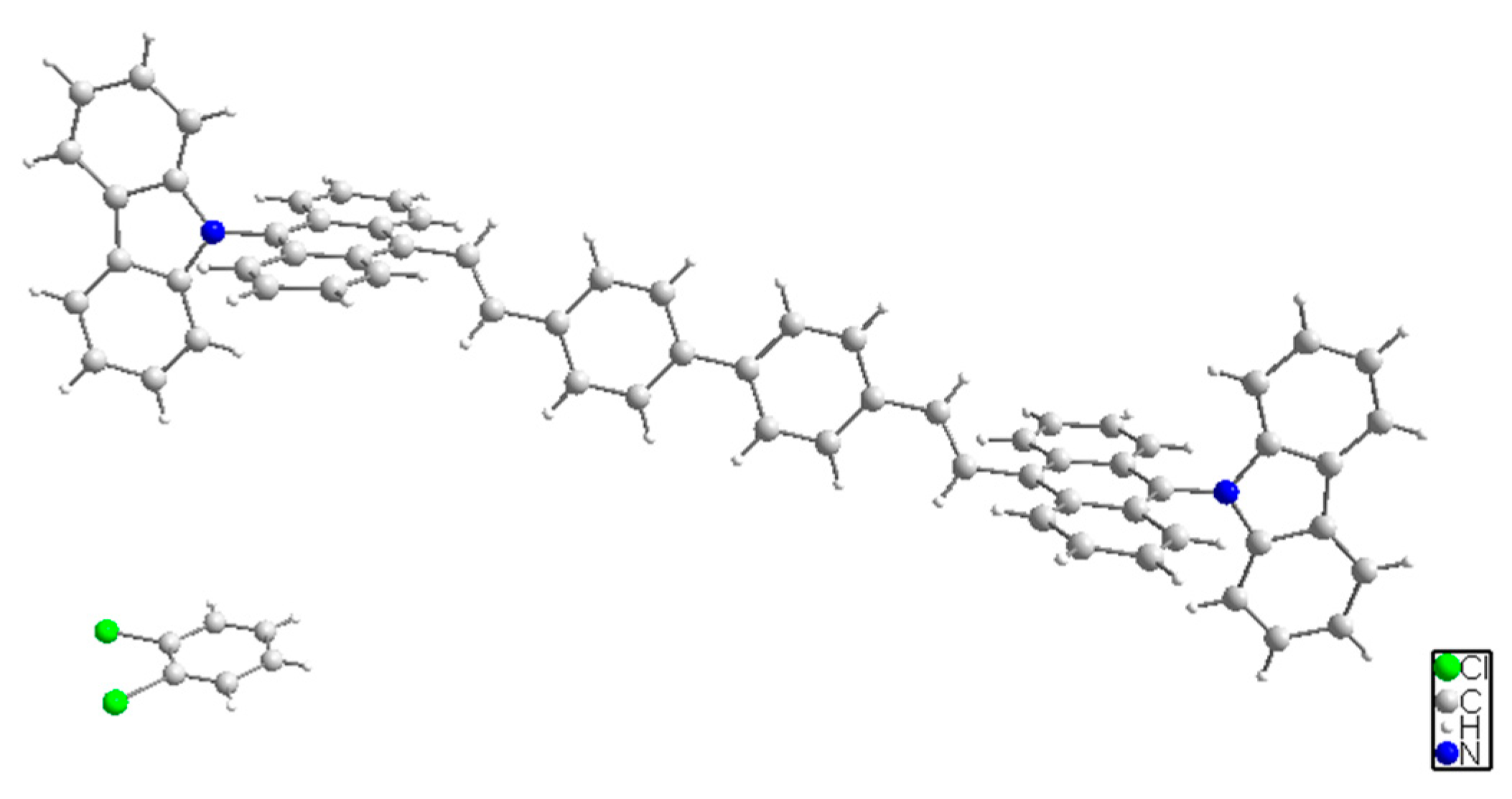
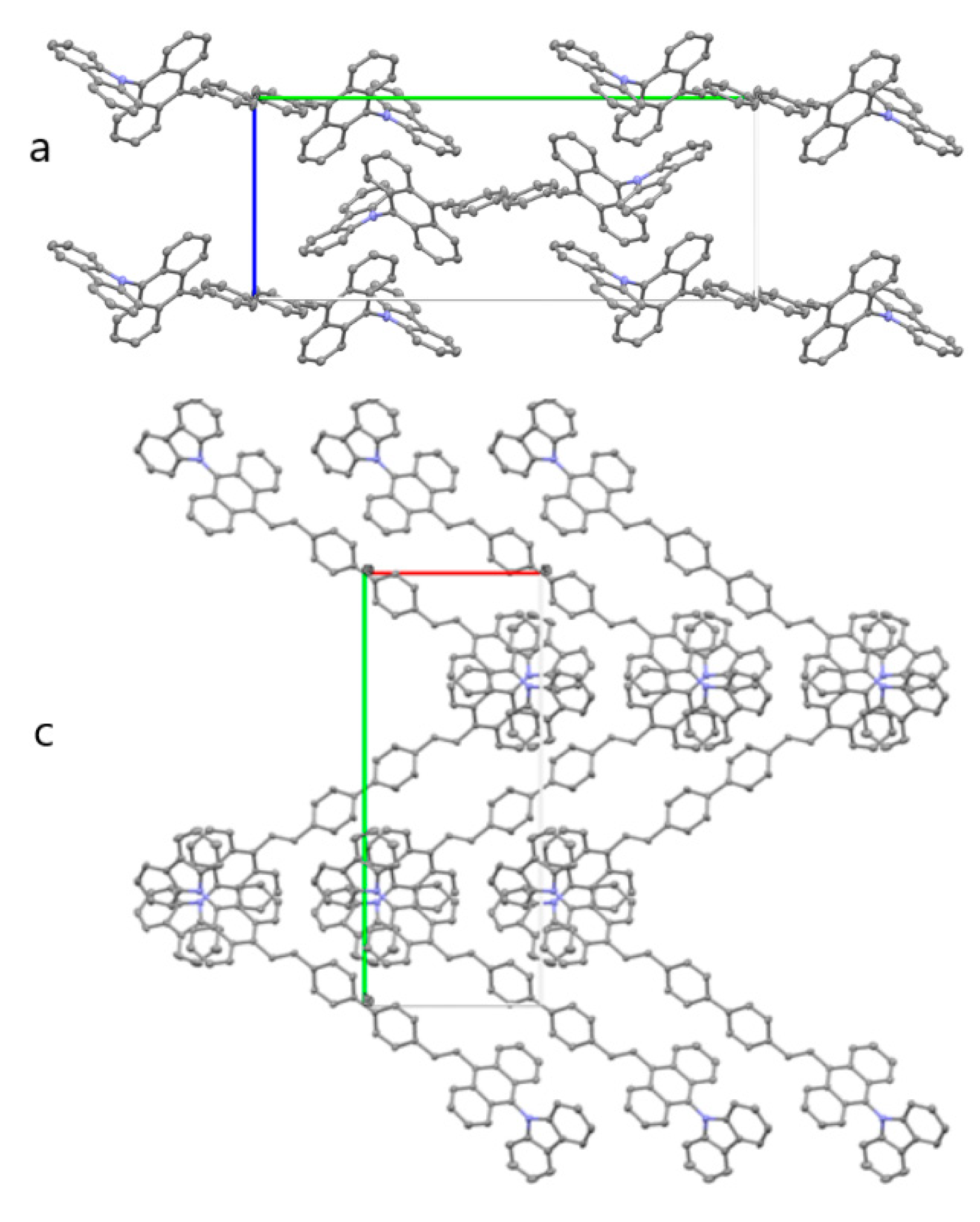
2.2. Single Crystal Structure of BABCz
2.3. Preparation of BABCz Films
2.4. OLED Device Fabrication
3. Results and Discussion
3.1. Synthesis and Structural Analysis
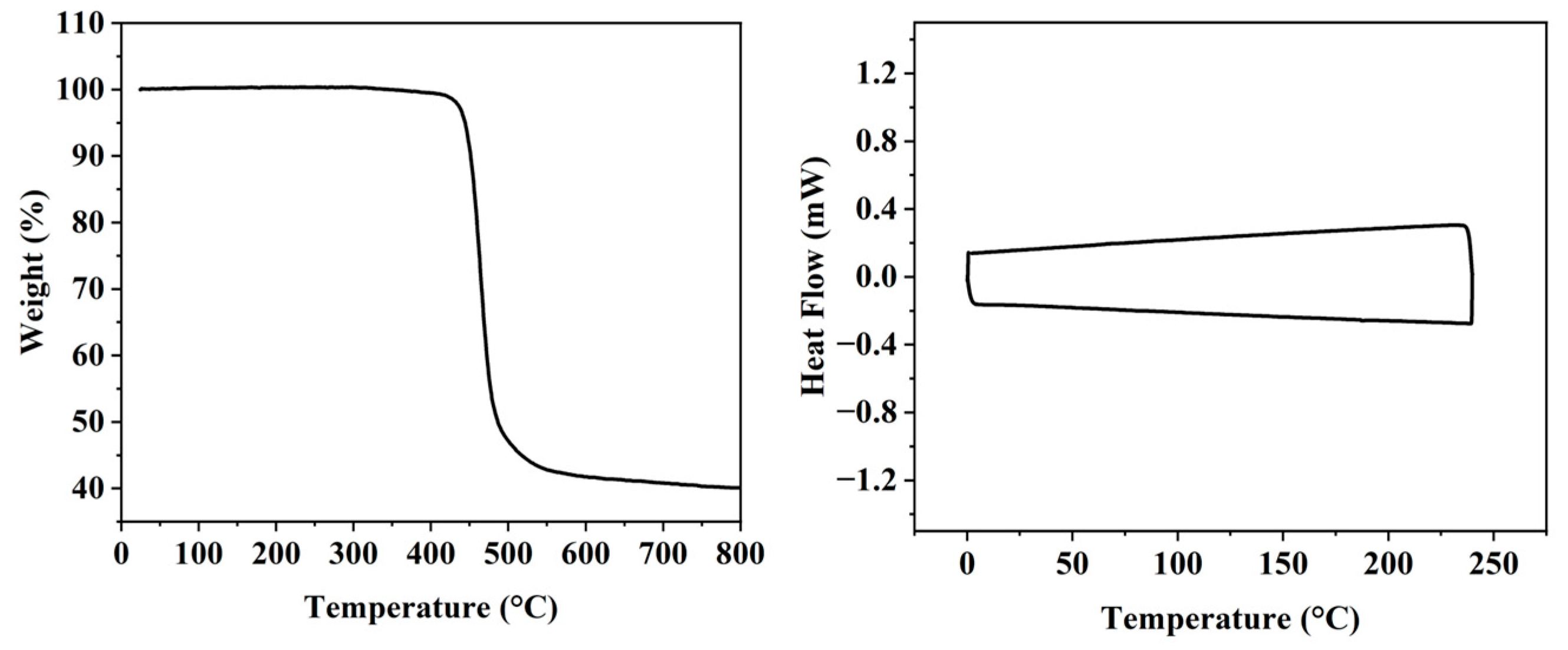
3.2. Performance Testing
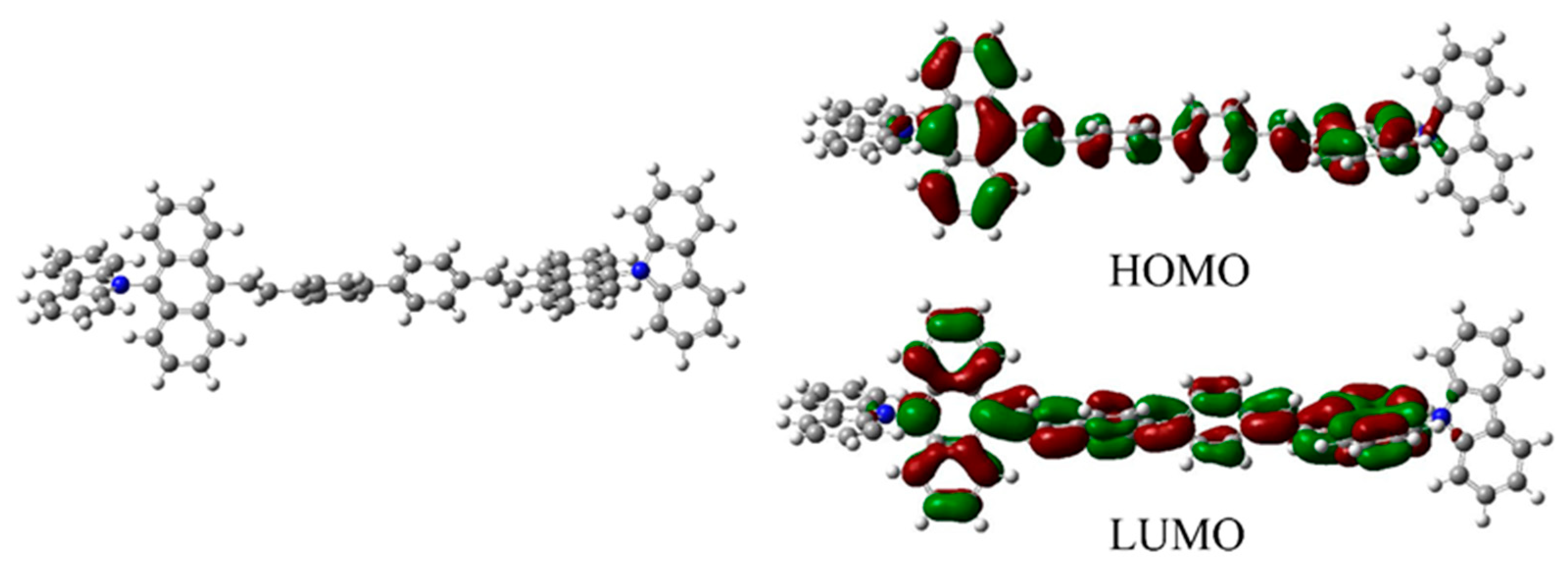
3.3. Theoretical Calculations and Electrochemical Properties
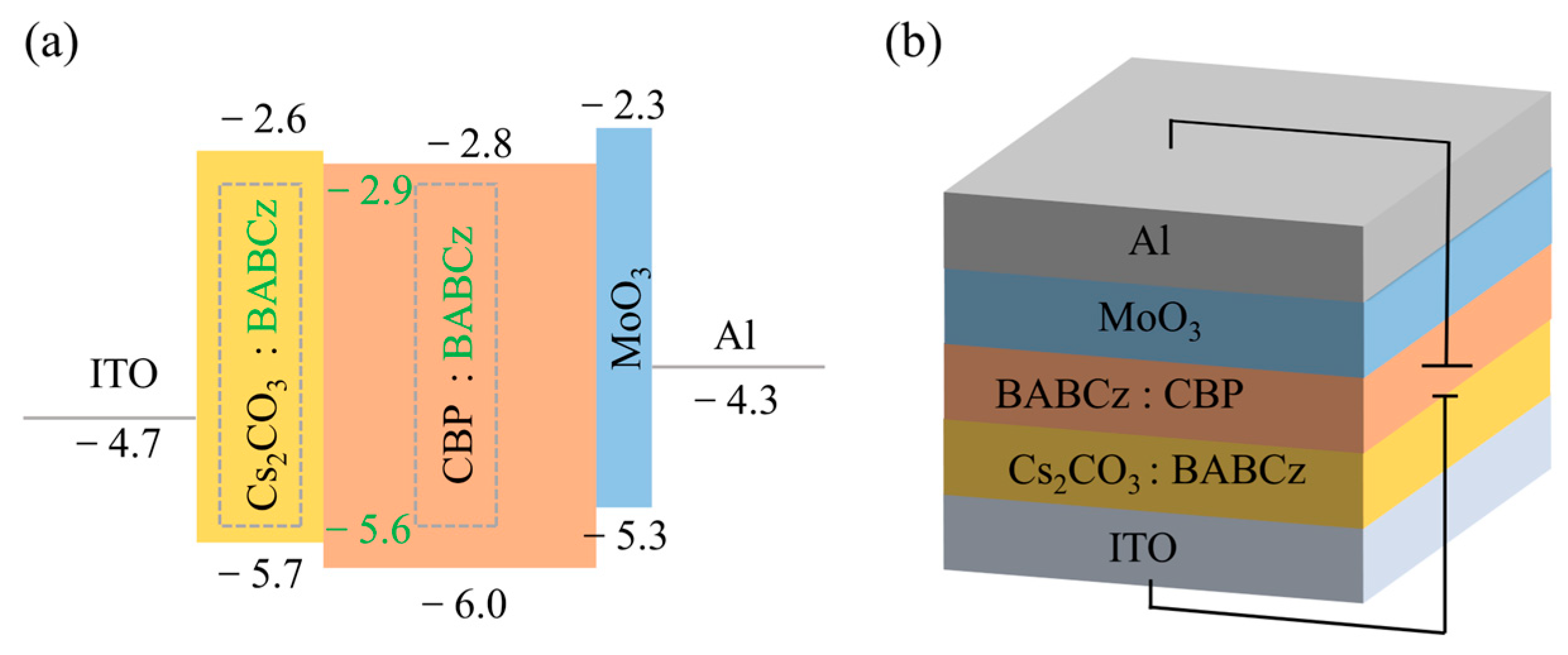
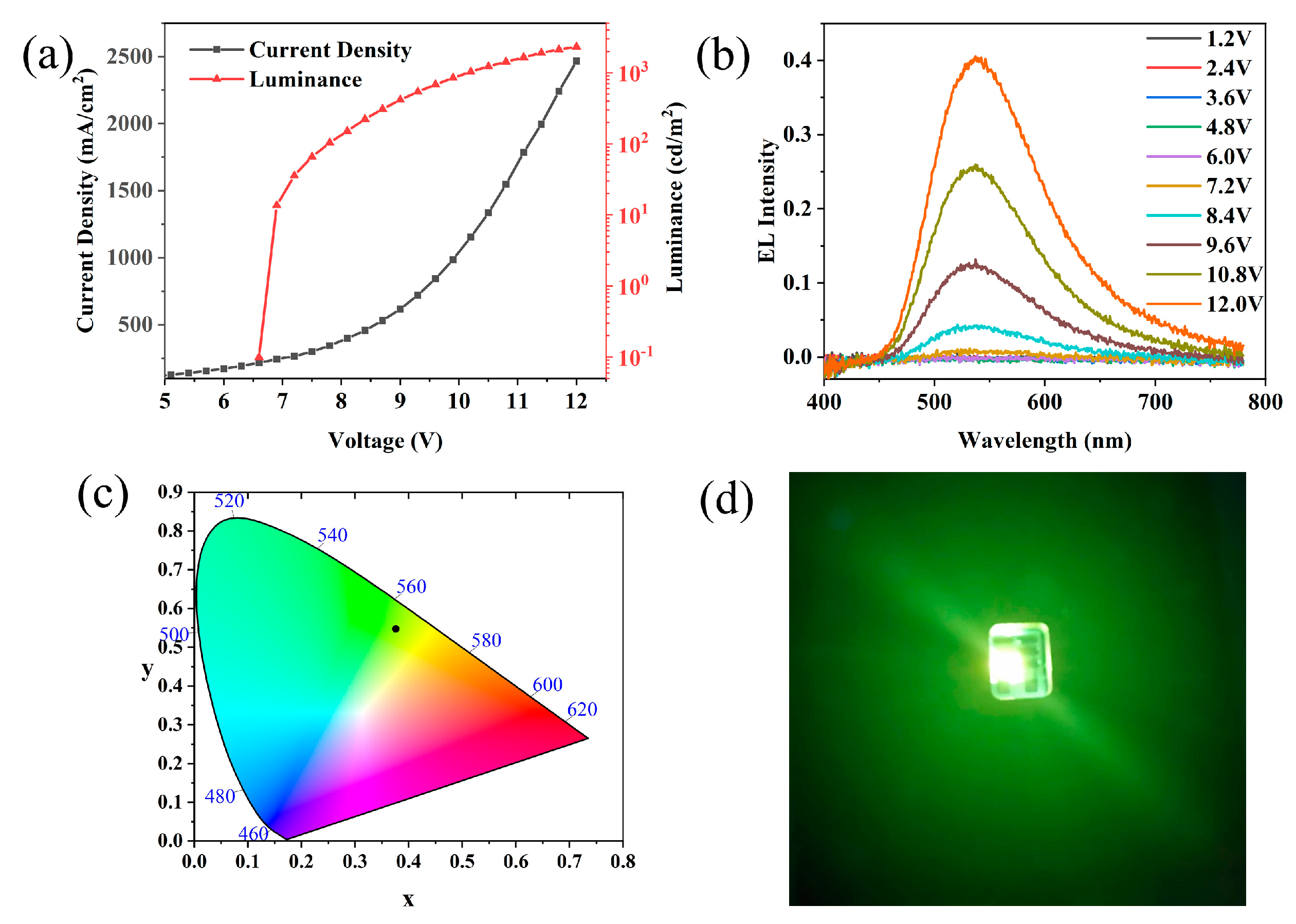
3.4. Device Fabrication and Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amado-Briseño, M.Á.; Hernández-Ortíz, O.J.; Rodríguez, M.A.V.; Ayala, K.A.; del Pozo Melero, G.; Herrero, B.R.; Zanabria, A.G.H.; Roa, A.E.; Vázquez-García, R.A. Mechanosynthesis of 2,2′-((1E,1′E)-(2,5-bis(octyloxy)-1,4-phenylene)bis(ethene-2,1-diyl))bis(6-bromoquinoline): Optical, electroluminescence, electrical, electrochemical, and morphological studies. J. Mater. Sci. Mater. Electron. 2021, 33, 126–138. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H.; Guo, J.; Wang, J.; Qiu, N.; Xiao, S.; Chi, J.; Yang, D.; Ma, D.; Zhao, Z.; et al. Robust Luminescent Molecules with High-Level Reverse Intersystem Crossing for Efficient Near Ultraviolet Organic Light-Emitting Diodes. Angew. Chem. Int. Ed. Engl. 2022, 61, e202116810. [Google Scholar] [PubMed]
- Filipek, P.; Karoń, K.; Hellwig, H.; Szłapa-Kula, A.; Filapek, M. The Role of Intermolecular Interaction on Aggregation-Induced Emission Phenomenon and OLED Performance. Materials 2022, 15, 8525. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.; Dong, H.; Meng, L.; Jiang, L.; Jiang, L.; Wang, Y.; Yu, J.; Sun, Y.; Hu, W.; et al. High mobility emissive organic semiconductor. Nat. Commun. 2015, 6, 10032. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Fan, J.X.; Yang, S.; Liu, D.; Ng, C.F.; Dong, H.; Ren, A.M.; Miao, Q. Halogenated Tetraazapentacenes with Electron Mobility as High as 27.8 cm(2) V(-1) s(-1) in Solution-Processed n-Channel Organic Thin-Film Transistors. Adv. Mater. 2018, 30, e1803467. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Li, M.; He, G.; Guo, X. Interface Engineering in Organic Field-Effect Transistors: Principles, Applications, and Perspectives. Chem. Rev. 2020, 120, 2879–2949. [Google Scholar] [CrossRef]
- Fukuda, K.; Yu, K.; Someya, T. The Future of Flexible Organic Solar Cells. Adv. Energy Mater. 2020, 10, 2000765. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and Semitransparent Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1701791. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Ou, Q.; Yu, P.; Wang, G.; Duan, Y.; Geng, H.; Peng, Q.; Shuai, Z.; Liao, Y. Molecular Design Strategy for Simultaneously Strong Luminescence and High Mobility: Multichannel CH-pi Interaction. J. Phys. Chem. Lett. 2021, 12, 938–946. [Google Scholar] [CrossRef]
- Kozlov, V.G.; Bulović, V.; Burrows, P.E.; Forrest, S.R. Laser action in organic semiconductor waveguide and double-heterostructure devices. Nature 1997, 389, 362–364. [Google Scholar] [CrossRef]
- Berggren, M.; Dodabalapur, A.; Slusher, R.E. Stimulated emission and lasing in dye-doped organic thin films with Forster transfer. Appl. Phys. Lett. 1997, 71, 2230–2232. [Google Scholar] [CrossRef]
- Ishow, E.; Brosseau, A.; Clavier, G.; Nakatani, K.; Tauc, P.; Fiorini-Debuisschert, C.; Neveu, S.; Sandre, O.; Léaustic, A. Multicolor Emission of Small Molecule-Based Amorphous Thin Films and Nanoparticles with a Single Excitation Wavelength. Chem. Mater. 2008, 20, 6597–6599. [Google Scholar] [CrossRef]
- Rabbani-Haghighi, H.; Forget, S.; Chénais, S.; Siove, A.; Castex, M.-C.; Ishow, E. Laser operation in nondoped thin films made of a small-molecule organic red-emitter. Appl. Phys. Lett. 2009, 95, 033305. [Google Scholar] [CrossRef]
- Jordan, G.; Flämmich, M.; Rüther, M.; Kobayashi, T.; Blau, W.J.; Suzuki, Y.; Kaino, T. Light amplification at 501 nm and large nanosecond optical gain in organic dye-doped polymeric waveguides. Appl. Phys. Lett. 2006, 88, 161114. [Google Scholar] [CrossRef]
- Ribierre, J.C.; Tsiminis, G.; Richardson, S.; Turnbull, G.A.; Samuel, I.D.W.; Barcena, H.S.; Burn, P.L. Amplified spontaneous emission and lasing properties of bisfluorene-cored dendrimers. Appl. Phys. Lett. 2007, 91, 081108. [Google Scholar] [CrossRef]
- Ma, D. Status and Prospects of Aggregation-Induced Emission Materials in Organic Optoelectronic Devices. Top. Curr. Chem. 2021, 379, 16. [Google Scholar] [CrossRef]
- Sadchikova, E.V.; Safronov, N.E.; Beliaev, N.A.; Nenajdenko, V.G.; Belskaya, N.P. Isoxazolyl-Derived 1,4-Dihydroazolo[5,1-c][1,2,4]Triazines: Synthesis and Photochemical Properties. Molecules 2023, 28, 3192. [Google Scholar] [CrossRef]
- Nakanotani, H.; Furukawa, T.; Hosokai, T.; Hatakeyama, T.; Adachi, C. Light Amplification in Molecules Exhibiting Thermally Activated Delayed Fluorescence. Adv. Opt. Mater. 2017, 5, 1700051. [Google Scholar] [CrossRef]
- Kim, D.-H.; D’aléo, A.; Chen, X.-K.; Sandanayaka, A.D.S.; Yao, D.; Zhao, L.; Komino, T.; Zaborova, E.; Canard, G.; Tsuchiya, Y.; et al. High-efficiency electroluminescence and amplified spontaneous emission from a thermally activated delayed fluorescent near-infrared emitter. Nat. Photonics 2018, 12, 98–104. [Google Scholar] [CrossRef]
- Sandanayaka, A.S.D.; Matsushima, T.; Bencheikh, F.; Terakawa, S.; Potscavage, W.J.P., Jr.; Qin, C.; Fujihara, T.; Goushi, K.; Ribierre, J.-C.; Adachi, C. Indication of current-injection lasing from an organic semiconductor. Appl. Phys. Express 2019, 12, 061010. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, F.; Wang, C.; Yu, J.; Wei, B. Enhanced performance of SubPc planar heterojunction solar cells with a novel luminescent sensitizer of 4,4′-bis[(N-carbaz ole)styryl]biphenyl. Synth. Met. 2017, 229, 52–56. [Google Scholar] [CrossRef]
- Oyama, Y.; Mamada, M.; Shukla, A.; Moore, E.G.; Lo, S.-C.; Namdas, E.B.; Adachi, C. Design Strategy for Robust Organic Semiconductor Laser Dyes. ACS Mater. Lett. 2020, 2, 161–167. [Google Scholar] [CrossRef]
- Mamada, M.; Fukunaga, T.; Bencheikh, F.; Sandanayaka, A.S.D.; Adachi, C. Low Amplified Spontaneous Emission Threshold from Organic Dyes Based on Bis-stilbene. Adv. Funct. Mater. 2018, 28, 1802130. [Google Scholar] [CrossRef]
- Mai, V.T.N.; Shukla, A.; Senevirathne, A.M.C.; Allison, I.; Lim, H.; Lepage, R.J.; McGregor, S.K.M.; Wood, M.; Matsushima, T.; Moore, E.G.; et al. Lasing Operation under Long-Pulse Excitation in Solution-Processed Organic Gain Medium: Toward CW Lasing in Organic Semiconductors. Adv. Opt. Mater. 2020, 8, 2001234. [Google Scholar] [CrossRef]
- Aleshin, A.N.; Lee, J.Y.; Chu, S.W.; Kim, J.S.; Park, Y.W. Mobility studies of field-effect transistor structures basedon anthracene single crystals. Appl. Phys. Lett. 2004, 84, 5383–5385. [Google Scholar] [CrossRef]
- Katoh, R.; Suzuki, K.; Furube, A.; Kotani, M.; Tokumaru, K. Fluorescence Quantum Yield of Aromatic Hydrocarbon Crystals. J. Phys. Chem. C 2009, 113, 2961–2965. [Google Scholar] [CrossRef]
- Huang, J.; Su, J.-H.; Tian, H. The development of anthracene derivatives for organic light-emitting diodes. J. Mater. Chem. 2012, 22, 10977–10989. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, L.; Murtaza, I.; Liang, X.; Meng, H.; Huang, W. A thermally stable anthracene derivative for application in organic thin film transistors. Org. Electron. 2017, 43, 105–111. [Google Scholar] [CrossRef]
- Avanesjan, O.S.; Benderskii, V.A.; Brikenstein, V.K.; Broude, V.L.; Korshunov, L.I.; Lavrushko, A.G.; Tartakovskii, I.I. Anthracene Crystals Under Intensive Optical Pumping. Mol. Cryst. Liq. Cryst. 1974, 29, 165–174. [Google Scholar] [CrossRef]
- Shi, J.; Tang, C.W. Anthracene derivatives for stable blue-emitting organic electroluminescence devices. Appl. Phys. Lett. 2002, 80, 3201–3203. [Google Scholar] [CrossRef]
- Liu, W.; Ying, S.; Guo, R.; Qiao, X.; Leng, P.; Zhang, Q.; Wang, Y.; Ma, D.; Wang, L. Nondoped blue fluorescent organic light-emitting diodes based on benzonitrile-anthracene derivative with 10.06% external quantum efficiency and low efficiency roll-off. J. Mater. Chem. C 2019, 7, 1014–1021. [Google Scholar] [CrossRef]
- Kwak, S.W.; Lee, K.M.; Lee, J.-E.; Yoo, J.; Yi, Y.; Kwon, H.; Lee, H.; Park, M.H.; Chung, Y. Synthesis and Electroluminescence Properties of 3-(Trifluoromethyl)phenyl-Substituted 9,10-Diarylanthracene Derivatives for Blue Organic Light-Emitting Diodes. Appl. Sci. 2017, 7, 1109. [Google Scholar] [CrossRef]
- Johannes, A.Z.; Pingak, R.K.; Bukit, M. Tauc Plot Software: Calculating energy gap values of organic materials based on Ultraviolet-Visible absorbance spectrum. IOP Conf. Series Mater. Sci. Eng. 2020, 823, 012030. [Google Scholar] [CrossRef]

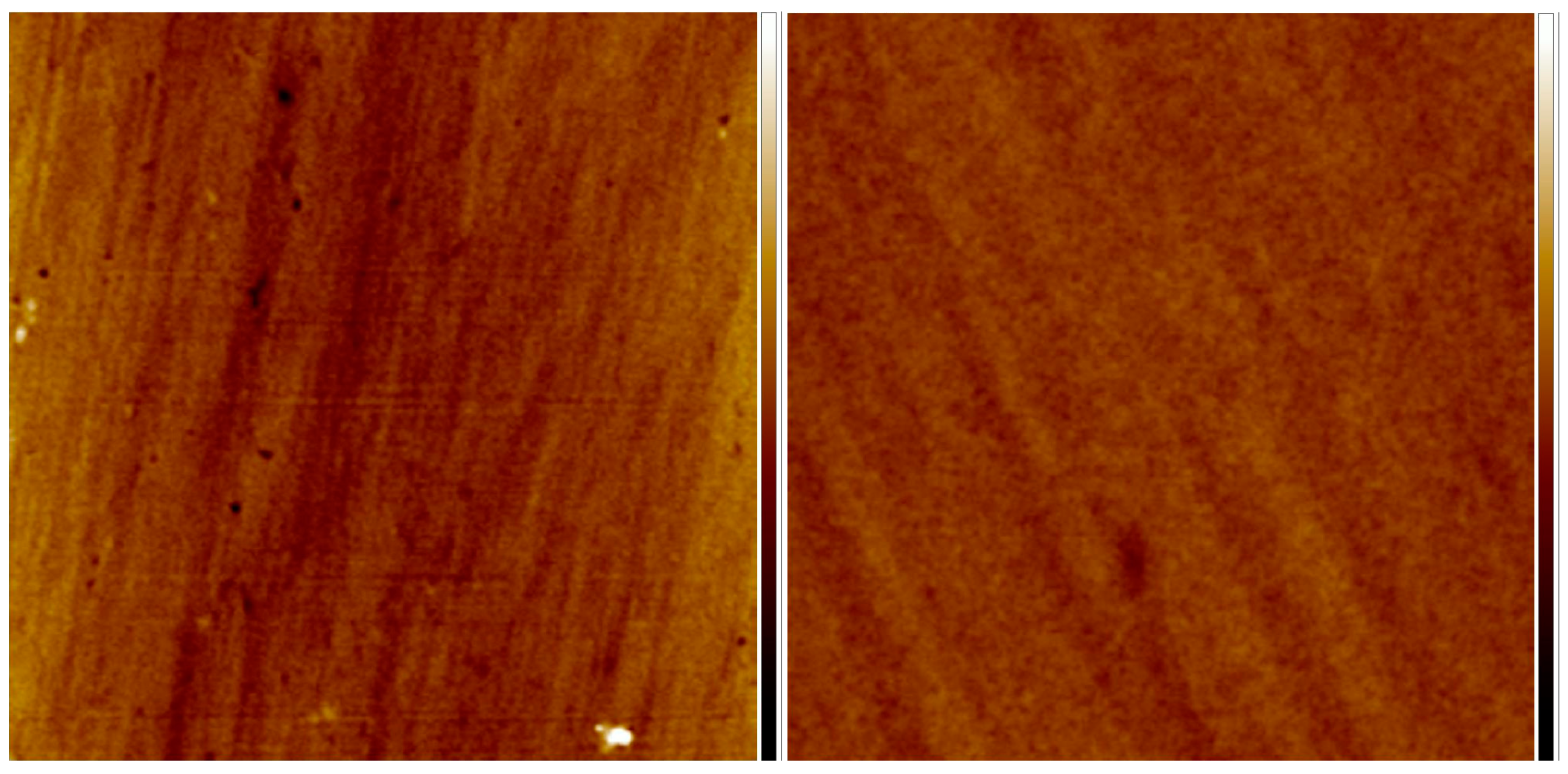
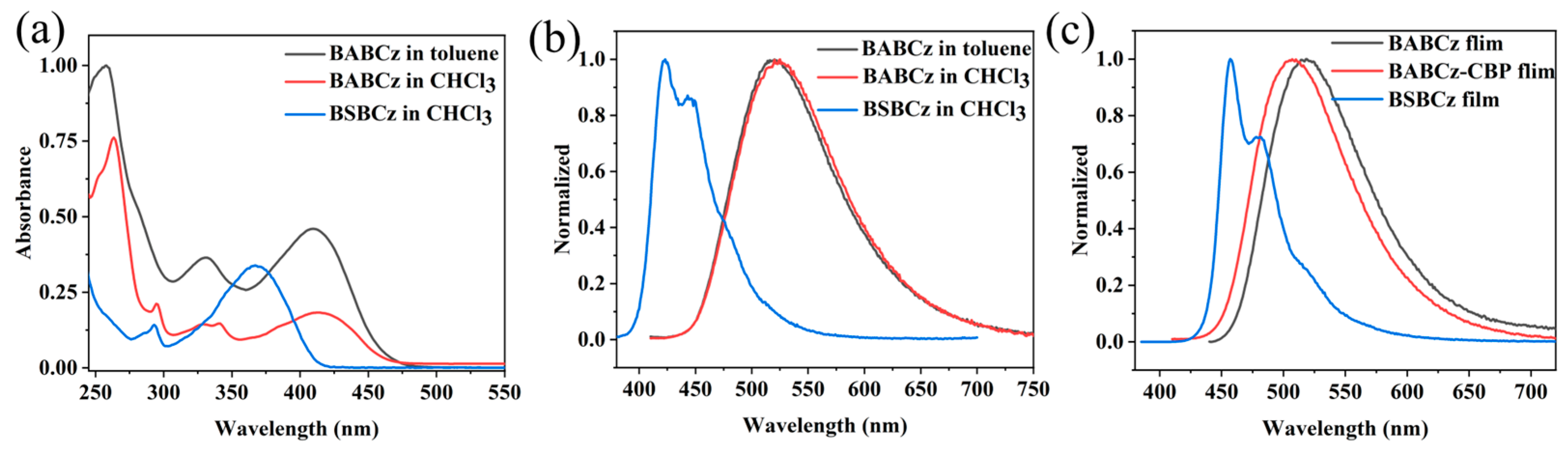

| Materials | λPL (nm) | ΦPL (a) | τ (ns) | kr/108(s−1) (c) |
|---|---|---|---|---|
| BABCz(CHCl3 solution) | 523 | 8.93% | 1.42 | 0.63 |
| BSBCz(CHCl3 solution) | 423, 443 | 78.6% | 1.06 | 7.42 |
| BABCz(vacuum-deposited pure film) (b) | 518 | - | - | - |
| BSBCz(vacuum-deposited pure film) | 457, 481 | 70.2% | 1.09 | 6.44 |
| BABCz(vacuum-deposited doped film) | 507 | 23.33% | 1.76 | 1.33 |
| Method | HOMO (eV) | LUMO (eV) | Band Gap (eV) | |
|---|---|---|---|---|
| BABCz | CV | −5.65 | −2.93 | 2.72 |
| Calculation | −5.21 | −2.20 | 3.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wu, H.; Bian, G.; Song, L. An Anthracene-Based Bis-Stilbene Derivative as Luminescent Materials for Organic Light Emitting Diodes. Materials 2023, 16, 3685. https://doi.org/10.3390/ma16103685
Wang H, Wu H, Bian G, Song L. An Anthracene-Based Bis-Stilbene Derivative as Luminescent Materials for Organic Light Emitting Diodes. Materials. 2023; 16(10):3685. https://doi.org/10.3390/ma16103685
Chicago/Turabian StyleWang, Hui, Houlin Wu, Guangling Bian, and Ling Song. 2023. "An Anthracene-Based Bis-Stilbene Derivative as Luminescent Materials for Organic Light Emitting Diodes" Materials 16, no. 10: 3685. https://doi.org/10.3390/ma16103685
APA StyleWang, H., Wu, H., Bian, G., & Song, L. (2023). An Anthracene-Based Bis-Stilbene Derivative as Luminescent Materials for Organic Light Emitting Diodes. Materials, 16(10), 3685. https://doi.org/10.3390/ma16103685








