Adsorptive Stripping Voltammetry for Determination of Vanadium: A Review
Abstract
1. Introduction
| Working Electrode | Complexing Agent | Catalytic System | LOD (mol L−1) | Accumulation Time (s) | Linear Range (mol L−1) | Investigated Interferents | Ref. |
|---|---|---|---|---|---|---|---|
| GCE/PbF | Cupferron | – | 2.8 × 10−12 | 15 | 1 × 10−11–2 × 10−10 | Ca(II), Cd(II), Cu(II), Mg(II), Ni(II), Bi(III) and Fe(III), Triton X–100 | [48] |
| HMDE | Cupferron | VO2(+)–cupferron–BrO3− | 4.9 × 10−12 | 15 | – | Ag(I), TI(I), Cd(II), Cu(II), Co(II), Mn(II), Ni(II), Pb(II), Zn(II), Ti(IV), Bi(III), Cr(III), Hg(II), Te(VI), As(III), Ga(III), Fe(III), Sb(III), Ge(IV), Sn(IV), Pt(IV), Zr(IV), UO2(2+) and MoO2(2+) | [45] |
| HMDE | Quercetin−5−sulfonic acid | VO2(+)–QSA–BrO3− | 4.5 × 10−12 | 30 | no data–7 × 10−9 | Na(I), K(I), Ag(I), Be(II), Ca(II), Co(II), Cu(II), Ni(II), Pb(II), Zn(II), Cr(III), Al(III), Sb(III), Zr(IV), Ti(IV), As(V) and MoO2(2+) | [62] |
| HMDE | Chloranilic acid | VO2(+)–chloranilic acid–BrO3− | 9.0 × 10−12 | 100 | 2 × 10−10–5 × 10−8 | Cu(II), Cd(II), Pb(II), Sn(II), Bi(III), Fe(III), Sb(III), Se(IV), Sn(IV), Te(IV), UO2(2+) and MoO2(2+), Triton X–100 | [52] |
| Hg(Ag)FE | Chloranilic acid | 1 × 10−11 | 90 | 2.5 × 10−10–1 × 10−7 | Cd(II), Cu(II), Mn(II), Pb(II), Sn(II), Zn(II), Bi(III), Fe(III), Se(IV), UO2(2+) and WO2(2+), Triton X–100 and HA | [53] | |
| HMDE | 2,3–dihydroxynapthlhalene | VO2(+)–DHN–BrO3− | 1.5 × 10−11 4 × 10−12 | 60 600 | 5 × 10−11–4 × 10−9 | Cd(II), Co(II), Cu(II), Fe(II), Mn(II), Ni(II), Pb(II), Sn(II), Zn(II), Al(III), As(III), Bi(III), Cr(III), Fe(III), Se(IV), As(V), Cr(VI), MoO2(2+) and Se(VI), SDS, DTAC and Triton X–100 | [63] |
| MWEs | Gallic acid | VO2(+)–GA–BrO3− | 1.7 × 10−11 | 120 | 1 × 10−10–2 × 10−8 | Ni(II), Sn(II), Fe(III), Al(III), Se(IV), As(V), Cr(VI) and MoO2(2+), Triton X–100 | [60] |
| MFE | Cupferron | VO2(+)–cupferron–BrO3− | 1.6 × 10−10 | 90 | 2 × 10−9–6.9 × 10−10 | Cd(II), Cu(II), Fe(II), Mn(II), Ni(II), Pb(II), Sr(II), Zn(II), Fe(III), Ti(IV), WO2(2+) and MoO2(2+) | [46] |
| HMDE | 2,3–dihydroxybenzaldehyde | VO2(+)–2,3–DHBA–BrO3− | 2 × 10−10 | 30 | 5 × 10−10–5 × 10−8 | Cd(II), Co(II), Cu(II), Fe(II), Mn(II), Ni(II), Pb(II) and Zn(II) | [59] |
| HMDE | Cupferron | – | 2.0 × 10−10 | 50 | 2 × 10−9–2 × 10−6 | Ag(I), K(I), Li(I), Na(I), Ca(II), Cd(II), Co(II), Cu(II), Mg(II), Mn(II), Ni(II), Ba(II), Zn(II), Al(III), Cr(III), La(III), Fe(III) and Sb(III), Triton X–100 | [50] |
| BiFµE | Cupferron | – | 2.5 × 10−10 | 60 | 8 × 10−10–1 × 10−7 | Tl(I), Cd(II), Co(II), Cu(II), Hg(II), Mn(II), Sn(II), Zn(II), Ni(II), Pb(II), Au(III), Bi(III), Cr(III), Fe(III), Ga(III), Sb(III), In(III), Pt(IV), Ti(IV), WO2(2+) and MoO2(2+), Triton X–100 and HA | [47] |
| MFE | Pyrogallol | – | 3 × 10−10 | 120 | nd–1.5 × 10−6 | Cd(II), Co(II), Cu(II), Mg(II), Ni(II), Pb(II), Sn(II), Zn(II), Al(III), Cr(III), Fe(III) and As(V), DTAC, SDS and Triton X–100 | [61] |
| HMDE | DMG + catechol | – | 3 × 10−10 | 900 | nd–1 × 10−7 | – | [65] |
| PbFE | Cupferron | – | 3.2 × 10−10 | 30 | 1 × 10−9–7 × 10−8 | Cu(II), Mn(II), Ni(II), Au(III), Bi(III), Ga(III), In(III), Sb(III), Cr(III), Fe(III), Ge(IV), Pt(IV), Ti(IV) and MoO2(2+). Triton X–100, SDS, CTAB and Rhamnolipids | [49] |
| HMDE | Catechol | VO2(+)–catechol–BrO3− | 6 × 10−10 7 × 10−11 | 15 120 | – | Cd(II), Co(II), Cu(II), Mn(II), Ni(II), Zn(II), Al(III), As(III), Fe(III), In(III), Sb(III), Se(IV), Ti(IV), Cr(VI), MoO2(2+) and UO2(2+), Sodium salt, DBS, Hyamine 1622 and Triton X–100 | [55] |
| ABPE | Alizarin violet | – | 6 × 10−10 | 90 | 8 × 10−10–1 × 10−7 | Ag(I), Ba(II), Be(II), Ca(II), Cd(II), Co(II), Cu(II), Mg(II), Mn(II), Ni(II), Pb(II), Zn(II), Al(III), Bi(III), Cr(III), Fe(III), Ga(III), In(III), Sb(III), Sc(III), Se(IV), Th(IV), Ti(IV), Sn(IV), Zr(IV), WO2(2+) and MoO2(2+), Triton X–100 | [57] |
| MME | cupferron–oxalic acid–1,3–diphenylguanidine | – | 9 × 10−10 | 30 | 3.79 × 10−9–2.84 × 10−7 | Tl(I), Ag(I), Ba(II), Cd(II), Co(II), Cu(II), Mn(II), Ni(II), Pb(II), Sr(II), Zn(II), Al(III), As(III), Bi(III), Cr(III), Fe(III), Se(IV), Ti(IV), Te(IV), Se(IV), As(V), Nb(V), Cr(VI), MoO2(2+), Se(VI), UO2(2+) and WO2(2+) | [64] |
| HMDE | DMG + catechol | – | 2.52 × 10−9 | 900 | >3 × 10−7 | – | [64] |
| HMDE | Chloranilic acid | – | 2.7 × 10−9 | 15 | 4.59 × 10−8–2.9 × 10−7 | – | [54] |
| BiFE | Chloranilic acid | VO2(+)–chloranilic acid–BrO3− | 3.9 × 10−9 | 600 | 9.8 × 10−8–5 × 10−7 | Ag(I), Au(I), Be(II), Cd(II), Cu(II), Ni(II), Pb(II), Pt(II), Hg(II), Ti(II), Zn(II), Cr(III), Fe(III), Al(III), Sb(III), MoO2(2+) and UO2(2+) | [51] |
| HMDE | Pyrocatechol violet | – | 1 × 10−8 | 180 | 1 × 10−8–6 × 10−7 | Ag(I), Tl(I), Ca(II), Co(II), Cu(II), Hg(II), Mn(II), Ni(II), Sn(II), Zn(II), Pd(II), Pb(II), Al(III), Fe(III), Rh(III), Ir(IV), Ti(IV), Os(IV), Ce(IV) and Pt(IV), Triton X–100 | [58] |
| HMDE | Chromoxane cyanine R | – | 1 × 10−7 | 180 | 3 × 10−7–2.4 × 10−5 | Ag(I), Cd(II), Ca(II), Ba(II), Mg(II), Zn(II), Co(II), Ni(II), Fe(II), Mn(II), Sr(II), Hg(II), Cr(III), Ga(III), In(III), La(III), Fe(III), Eu(III), Hf(III), Al(III) and WO2(2+), SDS and Triton X–100 | [56] |
2. Adsorptive Stripping Voltammetry of Vanadium
2.1. Procedures Based on Complexation Reactions
2.2. Catalytic Systems
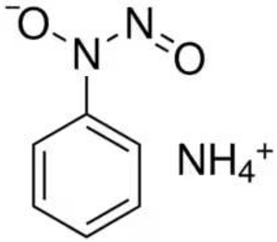
2.2.1. Catalytic System VO2(+)–Cupferron–BrO3−
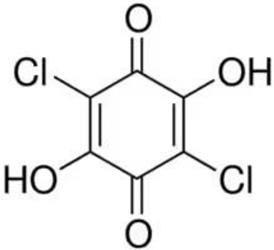
2.2.2. Catalytic System VO2(+)–Chloranilic Acid–BrO3−
2.2.3. Catalytic System VO2(+)–Catechol–BrO3−
- Heat a mixture of catechol and bromate ion solutions in a microwave oven, which resulted in the formation of orthobenzoquinone, i.e., the oxidized form of catechol.
- Reduction of orthobenzoquinone to catechol and the formation of a complex with vanadium(V), which is the stage of adsorption.
- In the range of potentials −0.8 V–0.1 V, the vanadium(V)–catechol complex is reduced to vanadium(IV)–catechol.
- The vanadium(IV)–catechol complex is chemically oxidized to the vanadium(V) form by bromate ions, where the vanadium(V)–catechol complex is ready for repeated reduction, thanks to which the catalytic increase in the reduction current is visible [55].
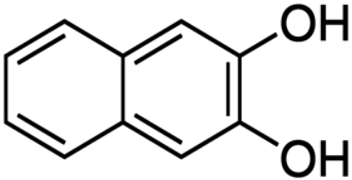
2.2.4. Catalytic system VO2(+)–2,3–DHBA–BrO3−
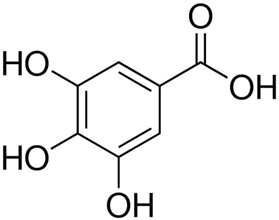
2.2.5. Catalytic System VO2(+)–GA–BrO3−
2.2.6. Catalytic System VO2(+)–QSA–BrO3−
- Oxidation of vanadium(II) and vanadium(III) ions to VO2(+), followed by the formation of the VO2(+)–QSA complex.
- On the surface of the HMDE working electrode, the VO2(+)–QSA complex is adsorbed and then reduced to the V(III)–QSA complex.
- During stripping, the V(III)–QSA complex is reduced to V(II). V(II) is chemically oxidized to VO2(+), forming the VO2(+)–QSA complex, and the reduction is repeated, increasing the signal [62].
2.3. Working Electrodes
2.4. Influence of Interferents on Vanadium Determination
2.4.1. Influence of Foreign Ions
2.4.2. Influence of the Organic Matrix of Samples
3. Simultaneous Determination of Vanadium Ions with Other Ions
4. Application
| Complexing Agent | Sample | Found VO2(+) by AdSV Method | Found VO2(+) by AAS Method | Ref. | ||
|---|---|---|---|---|---|---|
| Pb(II) (ng g−1) | VO2(+) (ng g−1) | Pb(II) (ng g−1) | VO2(+) (ng g−1) | |||
| Cupferron | Potato | 231.37 ± 0.64 | 440.38 ± 1.21 | 233.02 ± 1.63 | 439.34 ± 1.37 | [50] |
| Rice | 2.40 ± 0.13 | 115.98 ± 1.15 | 2.96 ± 0.28 | 116.57 ± 1.01 | ||
| Flour | 10.81 ± 0.36 | 98.06 ± 0.56 | 10.17 ± 0.49 | 96.97 ± 0.79 | ||
| Chromoxane cyanine R | Sample | Found VO2(+) by AdSV Method | Found VO2(+) by ICP–OES Method | |||
| MoO2(2+)Mo(VI) (ng mL−1) | VO2(+) (ng mL−1) | MoO2(2+) (ng mL−1) | VO2(+) (ng mL−1) | |||
| Tomato | 2.54 ± 0.08 | 1.06 ± 0.04 | 2.50 ± 0.06 | 1.05 ± 0.05 | [56] | |
| Carrot | 1.92 ± 0.04 | 0.76 ± 0.05 | 1.95 ± 0.04 | 0.76 ± 0.05 | ||
| Tea | 3.41 ± 0.13 | 1.53 ± 0.06 | 3.40 ± 0.09 | 1.55 ± 0.06 | ||
| Cucumber | 1.86 ± 0.06 | 0.96 ± 0.05 | 1.90 ± 0.06 | 0.98 ± 0.04 | ||
| Complexing Agent | Sample | Added VO2(+) (nmol L−1) | Found VO2(+) (nmol L−1) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Cupferron | Ciemiega river water | 5.0 10.0 | 5.2 9.9 | [47] | |||
| Tap water | 5.0 10.0 | 4.9 9.6 | |||||
| Rainwater | 5.0 10.0 | 5.3 10.4 | |||||
| Cupferron | Vistula River | - 5.0 10.0 | 4.7 10.2 14.5 | [49] | |||
| Chloranilic acid | Vistula river | - 2.5 5.0 10.0 | 6.47 ± 0.31 9.06 ± 0.37 11.1 ± 0.4 – | [53] | |||
| Rudawa river | - 2.5 5.0 10.0 | 5.27 ± 0.11 7.61 ± 0.16 10.5 ± 0.2 – | |||||
| Wilga river | - 2.5 5.0 10.0 | 3.17 ± 0.16 5.44 ± 0.22 8.09 ± 0.23 – | |||||
| Snow | - 2.5 5.0 10.0 | 14.3 ± 0.4 – 18.7 ± 0.5 25.0 ± 0.5 | |||||
| Alizarin violet | Tap water | - | 9.87 | [57] | |||
| River water | - | 22.5 | |||||
| Mineral water | - | 9.10 | |||||
| Cupferron | Ilam city water | Added VO2(+) (ng mL−1) | Found VO2(+) (ng mL−1) | [50] | |||
| - 10.0 30.0 | 0.48 ± 0.08 11.01 ± 0.55 30.99 ± 0.24 | ||||||
| Zayandehrood river water | - 10.0 30.0 | 0.91 ± 0.18 10.54 ± 0.30 30.75 ± 0.24 | |||||
| Mineral water | - 10.0 30.0 | 0.15 ± 0.09 10.41 ± 0.21 30.85 ± 0.20 | |||||
| Ilam University laboratory water | - 10.0 30.0 | – 10.08 ± 0.12 30.38 ± 0.19 | |||||
| Chromoxane cyanine R | River water | - 2.0 5.0 | 5.67 ± 0.17 7.57 ± 0.20 10.51 ± 0.32 | [56] | |||
| Tap water | - 2.0 5.0 | – 1.96 ± 0.10 4.88 ± 0.15 | |||||
| Well water | - 2.0 5.0 | – 1.94 ± 0.10 4.93 ± 0.12 | |||||
| Complexing Agent | Sample | Certified Reference | Found VO2(+) | Ref. | |||
| Cupferron | SPS–WW1 Wastewater | 100.0 ± 0.5 (ng mL−1) | 96.2 ± 1.7 (ng mL−1) | [48] | |||
| Cupferron | CRM (estuarine water) | 0.51 ± 0.61 (nmol L−1) | 0.496 ± 0.51 (nmol L−1) | [49] | |||
| Pyrogallol | Urban particulate matter 1648 | 140 ± 3 (µg L−1) | 137.3 ± 7.7 (µg L−1) | [61] | |||
| Peach leaves 1547 | 0.37 ± 0.03 (µg L−1) | 0.34 ± 0.04 (µg L−1) | |||||
| Apple leaves 1515 | 0.26 ± 0.03 (µg L−1) | 0.25 ± 0.05 (µg L−1) | |||||
| Bovine liver 1577b | 0.123 (µg L−1) | 0.117 ± 0.020 (µg L−1) | |||||
| Complexing Agent | Sample | Found | Ref. | ||||
| Ur(VI) (µg L−1) | Sb(III) (µg L−1) | MoO2(2+) (µg L−1) | VO2(+) (µg L−1) | ||||
| Chloranilic acid | NASS sea water standard | 2.86 ± 0.43 | – | 8.28 ± 0.25 | – | [54] | |
| Sewage of uranium slag heap | 14.03 ± 1.98 | – | 2.650 ± 0.40 | 6.968 ± 2.20 | |||
| DMG+Catechol | Cu(II) (nmol L−1) | Ni(II) (nmol L−1) | VO2(+) (nmol L−1) | [65] | |||
| seawater reference material CASS–4 | 9.7 ± 0.9 | 9.7 ± 0.9 | 9.7 ± 0.9 | ||||
| DMG+Catechol | Co(II) (nmol L−1) | Cu(II) (nmol L−1) | Fe(III) (nmol L−1) | Ni(II) (nmol L−1) | VO2(+) (nmol L−1) | [66] | |
| seawater reference material CASS–4 | 0.56 ± 0.08 | 8.5 ± 0.8 | 14 ± 3 | 5.9 ± 0.5 | 19 ± 2 | ||
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barceloux, D.G. Vanadium. J. Toxicol. Clin. Toxicol. 1999, 37, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Gomez, M.; Sanchez, D.J.; Llobet, J.M.; Keen, C.L. Toxicology of vanadium compounds in diabetic rats: The action of chelating agents on vanadium accumulation. Mol. Cell. Biochem. 1995, 153, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Saha, R.; Saha, B. Toxicity of inorganic vanadium compounds. Res. Chem. Intermed. 2015, 41, 4873–4897. [Google Scholar] [CrossRef]
- Pyrzynska, K. Recent developments in spectrophotometric methods for determination of Vanadium. Microchim. Acta 2005, 149, 159–164. [Google Scholar] [CrossRef]
- Milcheva, N.P.; Genç, F.; Racheva, P.V.; Delchev, V.B.; Andruch, V.; Gavazov, K.B. An environmentally friendly cloud point extraction–spectrophotometric determination of trace vanadium using a novel reagent. J. Mol. Liq. 2021, 334, 116086. [Google Scholar] [CrossRef]
- Pinto, J.J.; García-Vargas, M.; Moreno, C. A bulk liquid membrane–flow injection (BLM–FI) coupled system for the preconcentration and determination of vanadium in saline waters. Talanta 2013, 103, 161–165. [Google Scholar] [CrossRef]
- Linshan, B.; Wei, Z.; Xinhua, L.; Laiping, L. Kinetic spectrophotometric determination of vanadium in steels based on the catalytic oxidation of thionine by potassium bromate. Rare Met. 2007, 26, 85–88. [Google Scholar] [CrossRef]
- Paleologos, E.K.; Giokas, D.L.; Tzouwara–Karayanni, S.M.; Karayannis, M.I. Spectrofluorometric determination of vanadium based on the formation of a ternary complex between vanadium, peroxides, and 2–α–pyridylthioquinaldinamide. application to the determination of hydrogen peroxide and peroxy acids. Anal. Chem. 2002, 74, 100–106. [Google Scholar] [CrossRef]
- Heinemann, G.; Vogt, W. Quantification of vanadium in serum by electrothermal atomic absorption spectrometry. Clin. Chem. 1996, 42, 1275–1282. [Google Scholar] [CrossRef]
- Asadollahi, T.; Dadfarnia, S.; Shabani, A.M.H. Separation/preconcentration and determination of vanadium with dispersive liquid–liquid microextraction based on solidification of floating organic drop (DLLME-SFO) and electrothermal atomic absorption spectrometry. Talanta 2010, 82, 208–212. [Google Scholar] [CrossRef]
- Naeemullah; Tuzen, M. A new portable switchable hydrophilicity microextraction method for determination of vanadium in microsampling micropipette tip syringe system couple with ETAAS. Talanta 2019, 194, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Berton, P.; Martinis, E.M.; Wuilloud, R.G. Development of an on-line temperature-assisted ionic liquid dispersive microextraction system for sensitive determination of vanadium in environmental and biological samples. J. Hazard. Mater. 2010, 176, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Filik, H.; Aksu, D. Determination of vanadium in food samples by cloud point extraction and graphite furnace atomic absorption spectroscopy. Food Anal. Methods 2012, 5, 359–365. [Google Scholar] [CrossRef]
- Ali, J.; Tuzen, M.; Kazi, T.G. Green and innovative technique develop for the determination of vanadium in different types of water and food samples by eutectic solvent extraction method. Food Chem. 2020, 306, 125638. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, R.; Adamczyk, A.; Otto, M. Determination of vanadium in soils and sediments by the slurry sampling graphite furnace atomic absorption spectrometry using permanent modifiers. Talanta 2013, 113, 19–25. [Google Scholar] [CrossRef]
- Naeemullah; Kazi, T.G.; Tuzen, M. Magnetic stirrer induced dispersive ionic-liquid microextraction for the determination of vanadium in water and food samples prior to graphite furnace atomic absorption spectrometry. Food Chem. 2015, 172, 161–165. [Google Scholar] [CrossRef]
- Souza, V.S.; Teixeira, L.S.G.; Korn, M.G.A.; Cerqueira, U.M.F.M.; Bezerra, M.A. Determination of total contents and volatile and non-volatile fractions of nickel and vanadium in gasohol by graphite furnace atomic absorption spectrometry after extraction induced by emulsion-breaking. Fuel 2019, 242, 479–486. [Google Scholar] [CrossRef]
- López-García, I.; Marín-Hernández, J.J.; Hernández-Córdoba, M. Graphite furnace atomic absorption spectrometric determination of vanadium after cloud point extraction in the presence of graphene oxide. Spectrochim. Acta Part B At. Spectrosc. 2018, 143, 42–47. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Jiang, S.-J. Electrothermal vaporization inductively coupled plasma mass spectrometry for determination of vanadium and chromium in soils. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 63–70. [Google Scholar] [CrossRef]
- Dahlquist, R.L.; Knoll, J.W. Inductively coupled plasma–atomic emission spectrometry: Analysis of biological materials and soils for major, trace, and ultra–trace elements. Appl. Spectrosc. 1978, 32, 1–29. [Google Scholar] [CrossRef]
- Wuilloud, R.G.; Wuilloud, J.C.; Olsina, R.A.; Martinez, L.D. Speciation and preconcentration of vanadium(v) and vanadium(iv) in water samples by flow injection-inductively coupled plasma optical emission spectrometry and ultrasonic nebulization. Analyst 2001, 126, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Nixon, D.E.; Neubauer, K.R.; Eckdahl, S.J.; Butz, J.A.; Burritt, M.F. Evaluation of a tunable bandpass reaction cell for an inductively coupled plasma mass spectrometer for the determination of chromium and vanadium in serum and urine. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 951–966. [Google Scholar] [CrossRef]
- Trovo, P.L.V.; Fregolente, L.G.; Amaral, C.D.B.; Gonzalez, M.H. Determination of vanadium in water samples from Brazilian mineral spring (Ibirá Spa) using ICP-MS. Environ. Nanotechnol. Monit. Manag. 2017, 8, 48–52. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Queiroz, A.S.; Fernandes, M.S.; dos Santos, H.C. Application of factorial designs and Doehlert matrix in optimization of experimental variables associated with the preconcentration and determination of vanadium and copper in seawater by inductively coupled plasma optical emission spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 1939–1950. [Google Scholar] [CrossRef]
- de Azevedo Mello, P.; Fagundes Pereira, J.S.; de Moraes, D.P.; Dressler, V.L.; de Moraes Flores, É.M.; Knapp, G. Nickel, vanadium and sulfur determination by inductively coupled plasma optical emission spectrometry in crude oil distillation residues after microwave–induced combustion. J. Anal. At. Spectrom. 2009, 24, 911. [Google Scholar] [CrossRef]
- Mortada, W.I.; El-Defrawy, M.M.; Erfan, E.; El-Asmy, H.A. Cloud point extraction coupled with back-extraction for speciation of inorganic vanadium in water and determination of total vanadium in food samples by ICP-OES. J. Food Composit. Anal. 2022, 108, 104445. [Google Scholar] [CrossRef]
- dos Anjos, S.L.; Alves, J.C.; Rocha Soares, S.A.; Araujo, R.G.O.; de Oliveira, O.M.C.; Queiroz, A.F.S.; Ferreira, S.L.C. Multivariate optimization of a procedure employing microwave-assisted digestion for the determination of nickel and vanadium in crude oil by ICP OES. Talanta 2018, 178, 842–846. [Google Scholar] [CrossRef]
- Pyrzyska, K. Determination of vanadium species in environmental samples. Talanta 2004, 64, 823–829. [Google Scholar] [CrossRef]
- He, W.; Wang, K.; Yang, J. Spectrophotometric methods for determination of vanadium: A review. Toxicol. Environ. Chem. 2018, 100, 20–31. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Lee, N.-M.; Lin, S.-Y.; Liu, C.-Y. Determination of vanadium, molybdenum and tungsten in complex matrix samples by chelation ion chromatography and on-line detection with inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2002, 466, 161–174. [Google Scholar] [CrossRef]
- Sugiyama, M.; Tamada, T.; Hori, T. Liquid chromatography—Catalytic analysis detection for highly sensitive and automated fractional determination of vanadium(IV) and -(V). Anal. Chim. Acta 2001, 431, 141–148. [Google Scholar] [CrossRef]
- Jen, J.-F.; Wu, M.-H.; Yang, T.C. Simultaneous determination of vanadium(IV) and vanadium(V) as EDTA complexes by capillary zone electrophoresis. Anal. Chim. Acta 1997, 339, 251–257. [Google Scholar] [CrossRef]
- Chen, Z.; Naidu, R. On-column complexation and simultaneous separation of vanadium(IV) and vanadium(V) by capillary electrophoresis with direct UV detection. Anal. Bioanal. Chem. 2002, 374, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Kitazumi, I.; Nakashima, Y.; Himeno, S. Simultaneous electrophoretic determination of vanadium(V) and vanadium(IV) based on the complex formation with a Mo(VI)–P(V) reagent. J. Chromatogr. A 2001, 939, 123–129. [Google Scholar] [CrossRef]
- Nakano, S. Flow-injection chemiluminescent determination of vanadium(IV) and total vanadium by means of catalysis on the periodate oxidation of purpurogallin. Talanta 2002, 58, 1263–1270. [Google Scholar] [CrossRef]
- Souza, V.S.; Teixeira, L.S.G.; Bezerra, M.A. Application of multivariate designs in the development of a method for vanadium determination in natural waters by HR-CS GF AAS after cloud-point extraction. Microchem. J. 2016, 129, 318–324. [Google Scholar] [CrossRef]
- Azevedo Lemos, V.; Bastos Santos, L.; Santos Assis, R. Deep eutectic solvent in ultrasound-assisted liquid-phase microextraction for determination of vanadium in food and environmental waters. Microchem. J. 2022, 180, 107543. [Google Scholar] [CrossRef]
- Nunes, L.S.; Korn, M.G.A.; Lemos, V.A. A novel direct-immersion single-drop microextraction combined with digital colorimetry applied to the determination of vanadium in water. Talanta 2021, 224, 121893. [Google Scholar] [CrossRef]
- Ensafi, A.; Naderi, B. Catalytic determination of ultra trace amounts of vanadium with detection by linear sweep voltammetry. Fresenius J. Anal. Chem. 1997, 358, 480–483. [Google Scholar] [CrossRef]
- Girousi, S.T.; Gherghi, I.; Voulgaropoulos, A.N.; Stratis, J.A. Voltammetric Determination of vanadium, by using 1,10–phenanthroline as a complexing agent. Int. J. Environ. Anal. Chem. 1999, 75, 83–91. [Google Scholar] [CrossRef]
- Browning, E.; Horlick, G.; Tan, S.H.; Vaughan, M.A.; Shao, Y. Determination of chromium(VI) and vanadium(V) using an on–line anodic stripping voltammetry flow cell with detection by inductively coupled plasma mass spectrometry. Anal. Chem. 1994, 66, 1540–1547. [Google Scholar]
- Farias, P.A.M.; Takase, I. Cathodic stripping voltammetry of vanadium based on adsorptive accumulation of its solochrome violet RS complex at the static mercury drop electrode. Electroanalysis 1992, 4, 823–828. [Google Scholar] [CrossRef]
- Qureshi, M.S.; bin Mohd Yusoff, A.R.; Shah, A.; Nafady, A.; Sirajuddin. A new sensitive electrochemical method for the determination of vanadium(IV) and vanadium(V) in Benfield sample. Talanta 2015, 132, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Stadlober, M.; Kalcher, K.; Raber, G. Anodic stripping voltammetric determination of vanadium(V) using a carbon paste electrode modified in situ with cetyltrimethylammonium bromide. Electroanalysis 1997, 9, 225–230. [Google Scholar] [CrossRef]
- Wang, J.; Tian, B.; Lu, J. Adsorptive–catalytic stripping measurements of ultratrace vanadium in the presence of cupferron and bromate. Talanta 1992, 39, 1273–1276. [Google Scholar] [CrossRef]
- Greenway, G.M.; Wolfbauer, G. On–line determination of vanadium by adsorptive stripping voltammetry. Anal. Chim. Acta 1995, 312, 15–25. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Adamczyk, M.; Wlazlowska, E. The use of a solid bismuth microelectrode for vanadium quantification by adsorptive stripping voltammetry in environmental water samples. Molecules 2022, 27, 2168. [Google Scholar] [CrossRef]
- Tyszczuk–Rotko, K.; Gorylewski, D.; Kozak, J. Supporting electrolyte manipulation for simple improvement of the sensitivity of trace Vanadium(V) determination at a lead–coated glassy carbon electrode. Sensors 2022, 22, 8209. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Adamczyk, M. Application of electrochemical sensor based on lead film electrode in trace Vanadium(V) determination by adsorptive stripping voltammetry. IEEE Sens. J. 2019, 19, 5916–5922. [Google Scholar] [CrossRef]
- Abbasi, S.; Khani, H.; Sahraei, R. A highly sensitive adsorptive stripping voltammetric method for simultaneous determination of lead and vanadium in foodstuffs. Food Anal. Methods 2012, 5, 272–278. [Google Scholar] [CrossRef]
- Wang, J.; Lu, D.; Thongngamdee, S.; Lin, Y.; Sadik, O.A. Catalytic adsorptive stripping voltammetric measurements of trace vanadium at bismuth film electrodes. Talanta 2006, 69, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Bobrowski, A.; Nowak, K.; Zarebski, J. Novel voltammetric methods for vanadium determination by exploitation of catalytic adsorptive vanadium–chloranilic acid–bromate system. Anal. Chim. Acta 2005, 543, 150–155. [Google Scholar] [CrossRef]
- Piech, R.; Baś, B.; Paczosa–Bator, B.; Kubiak, W.W. Adsorptive stripping voltammetric determination of vanadium(V) witch chloranilic acid using cyclic renewable mercury film silver based electrode. J. Electroanal. Chem. 2009, 633, 333–338. [Google Scholar] [CrossRef]
- Sander, S. Simultaneous adsorptive stripping voltammetric determination of molybdenum(VI), uranium(VI), vanadium(V), and antimony(III). Anal. Chim. Acta 1999, 394, 81–89. [Google Scholar] [CrossRef]
- Vega, M.; van den Berg, C.M. Determination of vanadium in sea water by catalytic adsorptive cathodic stripping voltammetry. Anal. Chim. Acta 1994, 293, 19–28. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Khayamian, T.; Khaloo, S.S. Simultaneous determination of trace amounts of vanadium and molybdenum in water and foodstuff samples using adsorptive cathodic stripping voltammetry. Int. J. Food Sci. Technol. 2008, 43, 416–422. [Google Scholar] [CrossRef]
- Deng, P.H.; Fei, J.J.; Feng, Y.L. Determination of trace vanadium(V) by adsorptive anodic stripping voltammetry on an acetylene black paste electrode in the presence of alizarin violet. J. Electroanal. Chem. 2010, 648, 85–91. [Google Scholar] [CrossRef]
- Vukomanovic, D.V.; Vanloon, G.W. Voltammetric determination of vanadium with adsorptive preconcentration of the pyrocatechol violet complex. Talanta 1994, 14, 387–394. [Google Scholar] [CrossRef]
- Povar, I.; Cazac, T.; Chiriac, L.; Sandulescu, R. Catalytic–adsorptive determination of vanadium in solutions of 2,3–dihydroxybenzaldehide and bromate–ions. J. Electroanal. Chem. 2011, 661, 275–279. [Google Scholar] [CrossRef]
- Dansby–Sparks, R.; Chambers, J.Q.; Xue, Z.L. Trace vanadium analysis by catalytic adsorptive stripping voltammetry using mercury–coated micro–wire and polystyrene–coated bismuth film electrodes. Anal. Chim. Acta 2009, 643, 19–25. [Google Scholar] [CrossRef]
- Adeloju, S.B.O.; Pablo, F. Adsorptive cathodic stripping voltammetric determination of ultra–trace concentrations of vanadium on a glassy carbon mercury film electrode. Anal. Chim. Acta 1994, 288, 157–166. [Google Scholar] [CrossRef]
- Rojas–Romo, C.; Arancibia, V.; Costa, D.M.; Tapia, R.A. Highly sensitive determination of Vanadium(V) by catalytic adsorptive stripping voltammetry. Substituent effect on sensitivity III. Sens. Actuators B Chem. 2015, 224, 772–779. [Google Scholar] [CrossRef]
- Li, H.; Smart, R.B. Catalytic stripping voltammetry of vanadium in the presence of dihydroxynaphthalene and bromate. Anal. Chim. Acta 1996, 333, 131–138. [Google Scholar] [CrossRef]
- Schneider, A.B.; Nascimento, P.C.; Bohrer, D.; de Carvalho, L.M.; Guarda, A.; Krause, C.; Wiethan, B.A.; Koschinsky, A. determination of zirconium and vanadium in natural waters by adsorptive stripping voltammetry in the presence of cupferron, oxalic acid and 1,3–diphenylguanidine. Electroanalysis 2015, 27, 1864–1870. [Google Scholar] [CrossRef]
- Cobelo–García, A.; Santos–Echeandía, J.; Prego, R.; Nieto, O. Direct simultaneous determination of Cu, Ni and Y in seawater using adsorptive cathodic stripping voltammetry with mixed ligands. Electroanalysis 2005, 17, 906–911. [Google Scholar] [CrossRef]
- Santos–Echeandía, J. Direct simultaneous determination of Co, Cu, Fe, Ni and v in pore waters by means of adsorptive cathodic stripping voltammetry with mixed ligands. Talanta 2011, 85, 506–512. [Google Scholar] [CrossRef]
- Alshawi, J.M.S.; Mohammed, Q.M.; Alesary, H.F.; Ismail, H.K.; Barton, S. Voltammetric determination of Hg2+, Zn2+, and Pb2+ ions using a PEDOT/NTA-modified electrode. ACS Omega 2022, 7, 20405–20419. [Google Scholar] [CrossRef]
- Mohammed, Q.M.; Ismail, H.K.; Alesary, H.F.; Barton, S. Use of a Schiff base-modified conducting polymer electrode for electrochemical assay of Cd(II) and Pb(II) ions by square wave voltammetry. Chem. Pap. 2022, 76, 715–729. [Google Scholar] [CrossRef]
- Korolczuk, M.; Grabarczyk, M.; Rutyna, I. An adsorptive stripping voltammetry procedure for ultra-trace determination of U(VI) using double accumulation step on two lead-film working electrodes. Talanta 2014, 130, 342–346. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Baś, B.; Korolczuk, M. Application of a renewable silver based mercury film electrode to the determination of Cr(VI) in soil samples. Microchim. Acta 2009, 164, 465–470. [Google Scholar] [CrossRef]
- Grabarczyk, M. Catalytic adsorptive stripping voltammetric determination of Cr(VI) in EDTA extracts from solid samples. Electrochim. Acta 2006, 51, 2333–2337. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/PL/en/product/aldrich/675636 (accessed on 5 May 2023).
- Available online: https://www.sigmaaldrich.com/PL/en/product/aldrich/c8136 (accessed on 5 May 2023).
- Available online: https://www.sigmaaldrich.com/PL/en/product/supelco/phl82372 (accessed on 5 May 2023).
- Available online: https://www.sigmaaldrich.com/PL/en/substance/gallicacid17012149917 (accessed on 5 May 2023).
- Available online: https://www.sigmaaldrich.com/PL/en/product/aldrich/37760 (accessed on 5 May 2023).
- Available online: https://www.sigmaaldrich.com/PL/en/product/aldrich/189839 (accessed on 5 May 2023).
- Bobrowski, A.; Zarębski, J. Catalytic systems in adsorptive stripping voltammetry. Electroanalysis 2000, 12, 1177–1186. [Google Scholar] [CrossRef]
- Porada, R.; Jedlińska, K.; Lipińska, J.; Baś, B. Review–voltammetric sensors with laterally placed working electrodes: A review. J. Electrochem. Soc. 2020, 167, 037536. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wlazłowska, E.; Grabarczyk, M. Adsorptive Stripping Voltammetry for Determination of Vanadium: A Review. Materials 2023, 16, 3646. https://doi.org/10.3390/ma16103646
Wlazłowska E, Grabarczyk M. Adsorptive Stripping Voltammetry for Determination of Vanadium: A Review. Materials. 2023; 16(10):3646. https://doi.org/10.3390/ma16103646
Chicago/Turabian StyleWlazłowska, Edyta, and Malgorzata Grabarczyk. 2023. "Adsorptive Stripping Voltammetry for Determination of Vanadium: A Review" Materials 16, no. 10: 3646. https://doi.org/10.3390/ma16103646
APA StyleWlazłowska, E., & Grabarczyk, M. (2023). Adsorptive Stripping Voltammetry for Determination of Vanadium: A Review. Materials, 16(10), 3646. https://doi.org/10.3390/ma16103646