Photoinhibitive Properties of α-MoO3 on Its Composites with TiO2, ZnO, BiOI, AgBr, and Cu2O
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of α-MoO3 and Composites
2.2. Characterization Methods
2.3. The Assessment of the Photocatalytic Activity
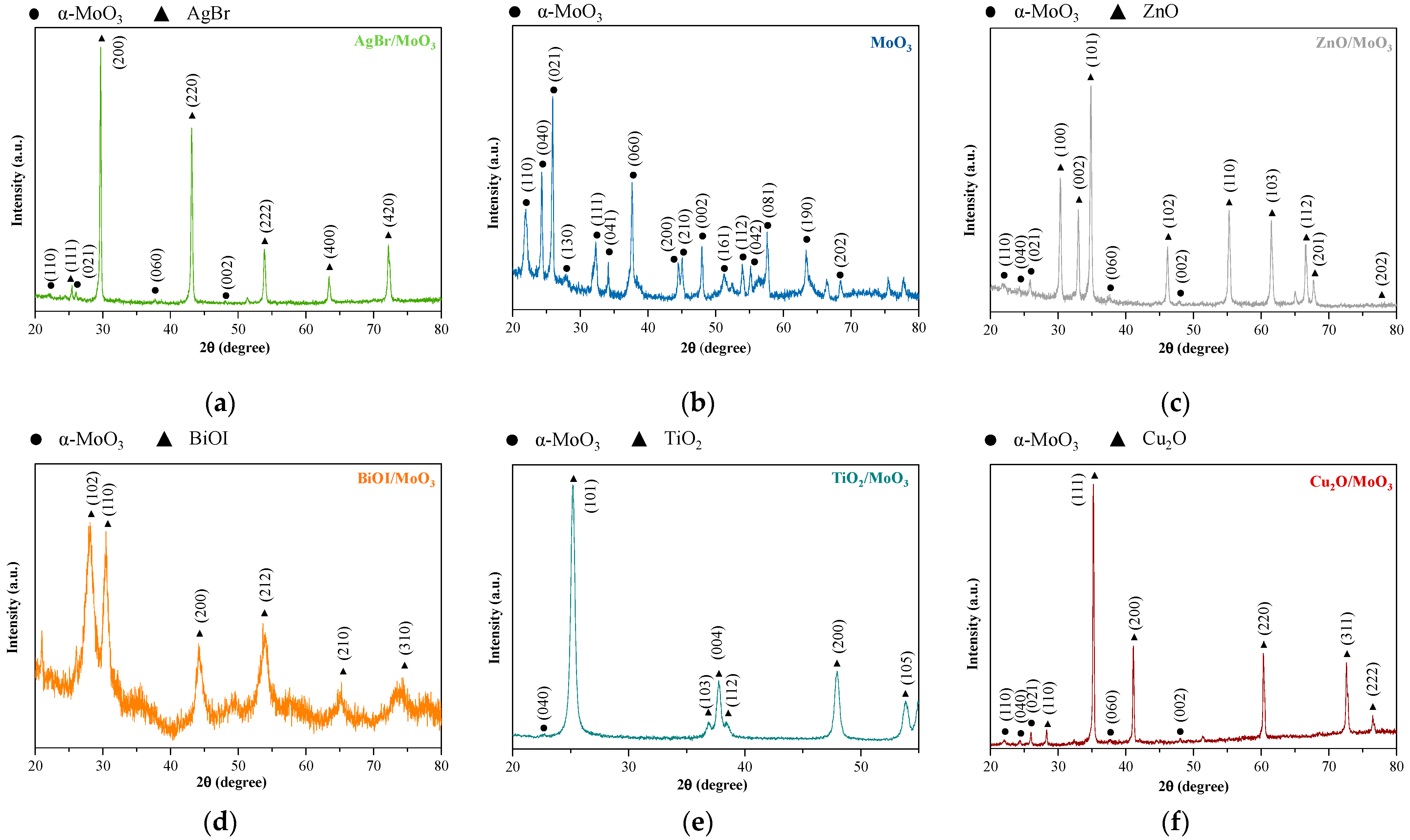
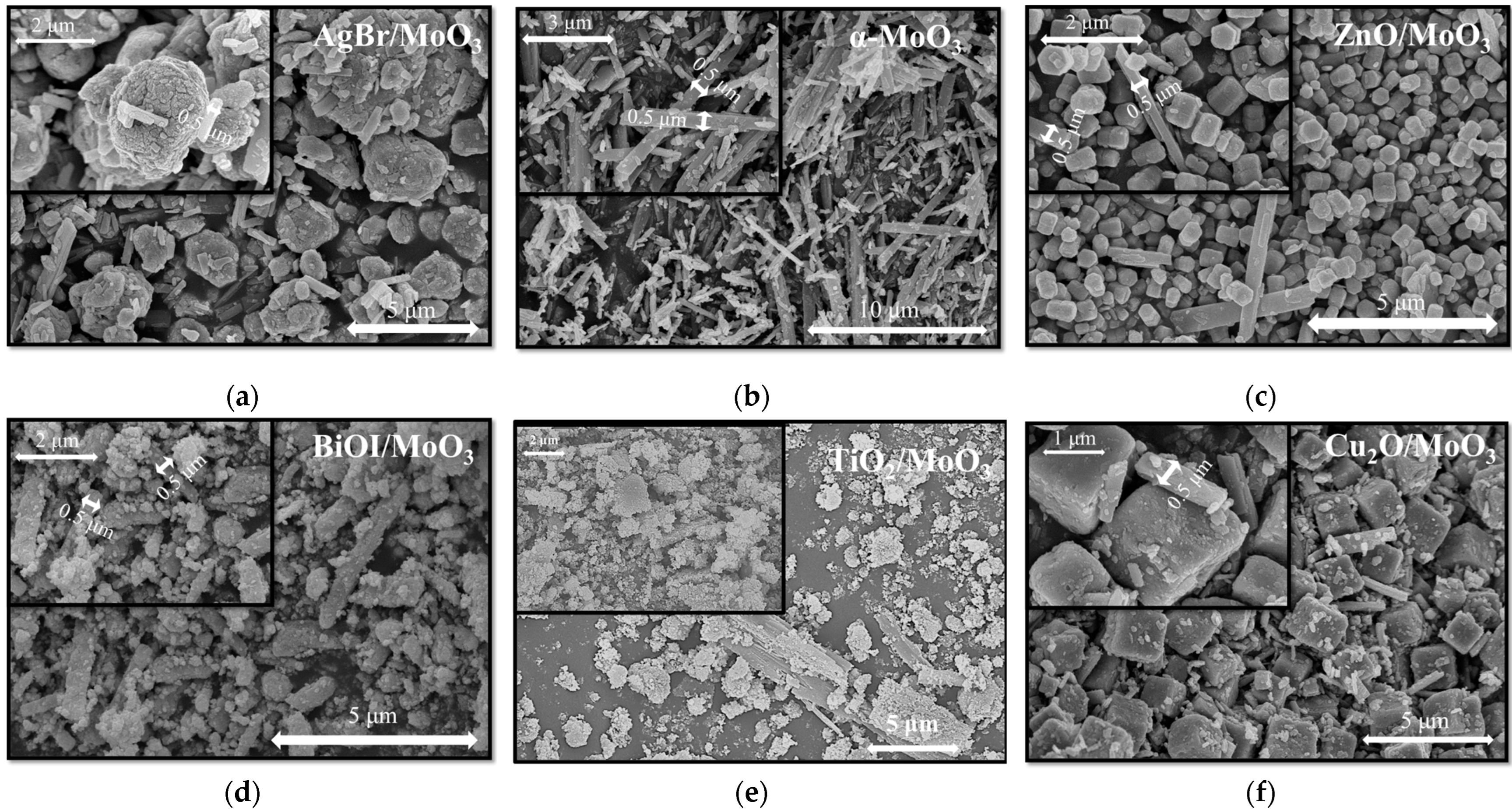
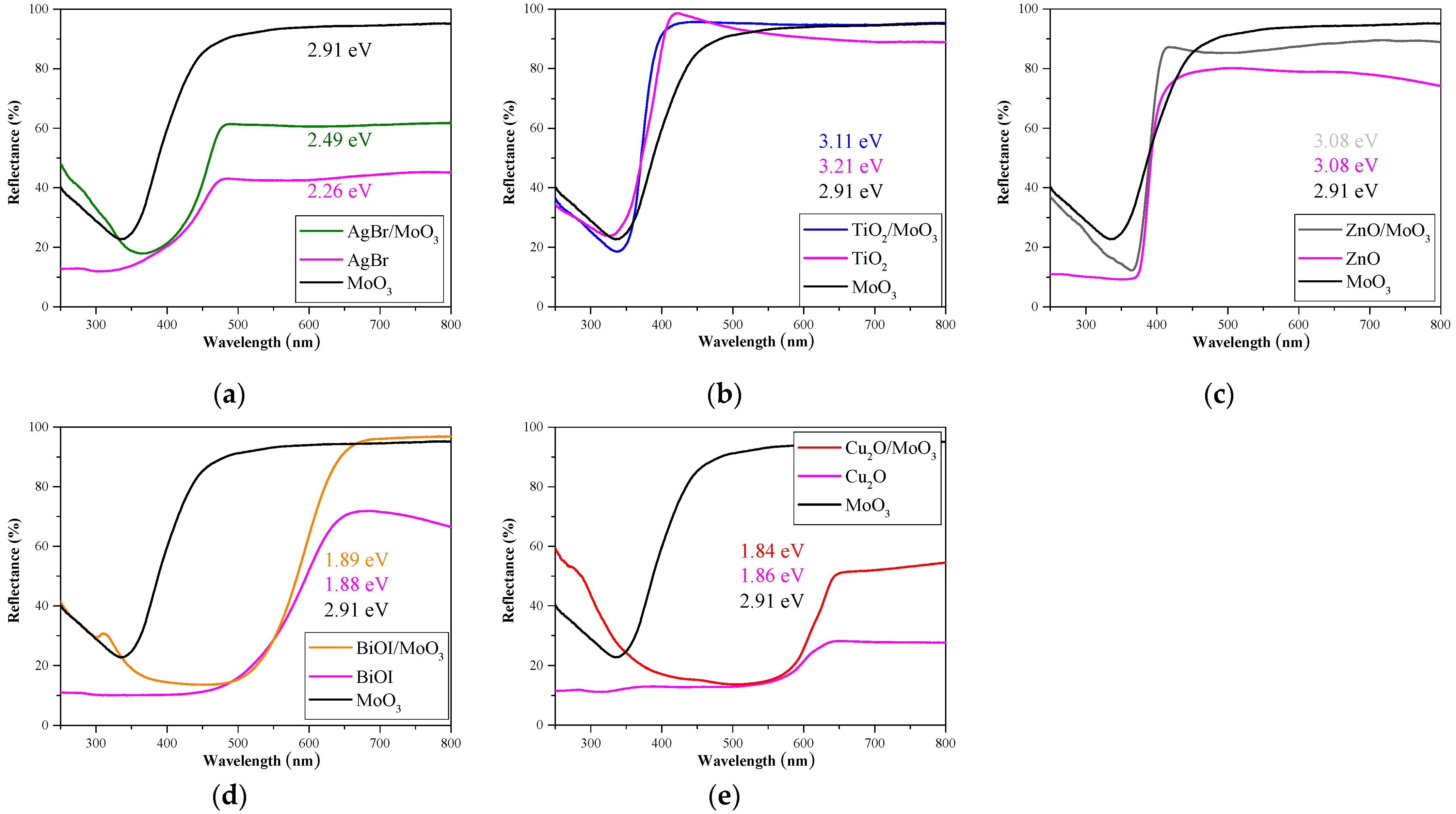
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kedves, E.-Z.; Pap, Z.; Hernadi, K.; Baia, L. Significance of the surface and bulk features of hierarchical TiO2 in their photocatalytic properties. Ceram. Int. 2021, 47, 7088–7100. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, X.; Qu, F.; Umar, A.; Wu, X. Hierarchical WO3 nanostructures assembled by nanosheets and their applications in wastewater purification. J. Alloys Compd. 2016, 689, 570–574. [Google Scholar] [CrossRef]
- Bárdos, E.; Király, A.K.; Pap, Z.; Baia, L.; Garg, S.; Hernádi, K. The effect of the synthesis temperature and duration on the morphology and photocatalytic activity of BiOX (X = Cl, Br, I) materials. Appl. Surf. Sci. 2019, 479, 745–756. [Google Scholar] [CrossRef]
- Tóth, Z.-R.; Pap, Z.; Kiss, J.; Baia, L.; Gyulavári, T.; Czekes, Z.; Todea, M.; Magyari, K.; Kovács, G.; Hernadi, K. Shape tailoring of AgBr microstructures: Effect of the cations of different bromide sources and applied surfactants. RSC Adv. 2021, 11, 9709–9720. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Molnár, C.; Štangar, U.L.; Cristea, V.-M.; Pap, Z.; Hernadi, K.; Baia, L. Optimization Method of the Solvothermal Parameters Using Box–Behnken Experimental Design—The Case Study of ZnO Structural and Catalytic Tailoring. Nanomaterials 2021, 11, 1334. [Google Scholar] [CrossRef]
- Fodor, S.; Baia, L.; Baán, K.; Kovács, G.; Pap, Z.; Hernadi, K. The Effect of the Reducing Sugars in the Synthesis of Visible-Light-Active Copper(I) Oxide Photocatalyst. Molecules 2021, 26, 1149. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Guernelli, M.; Montalti, M. Extending photocatalysis to the visible and NIR: The molecular strategy. Nanoscale 2021, 13, 9147–9159. [Google Scholar] [CrossRef]
- Muhmood, T.; Uddin, A. Fabrication of spherical-graphitic carbon nitride via hydrothermal method for enhanced photo-degradation ability towards antibiotic. Chem. Phys. Lett. 2020, 753, 137604. [Google Scholar] [CrossRef]
- Muhmood, T.; Cai, Z.; Lin, S.; Xiao, J.; Hu, X. Dimensions controllable synthesis of silver Nano-morphologies via moderate one step methodology. Adv. Powder Technol. 2021, 32, 3388–3394. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Abdullah, A.Z.; Mohamed, A.R. Investigation on visible-light photocatalytic degradation of 2,4-dichlorophenoxyacetic acid in the presence of MoO3/ZnO nanorod composites. J. Mol. Catal. A Chem. 2013, 370, 123–131. [Google Scholar] [CrossRef]
- Kedves, E.-Z.; Bárdos, E.; Gyulavári, T.; Pap, Z.; Hernadi, K.; Baia, L. Dependence of cationic dyes’ adsorption upon α-MoO3 structural properties. Appl. Surf. Sci. 2022, 573, 151584. [Google Scholar] [CrossRef]
- Diniz, J.; Nunes, C.D.; Monteiro, O.C. Novel approach to synthesise MoO3-TiO2 nanocomposites for the photo-assisted oxidation of benzyl alcohol to benzaldehyde. Inorg. Chem. Commun. 2020, 119, 108099. [Google Scholar] [CrossRef]
- Selvakumar, G.; Palanivel, C. A study on synthesis, characterization and catalytic applications of MoO3-ZnO nanocompositematerial. Mater. Sci. Energy Technol. 2022, 5, 36–44. [Google Scholar] [CrossRef]
- Feng, B.; Wu, Z.; Liu, J.; Zhu, K.; Li, Z.; Jin, X.; Hou, Y.; Xi, Q.; Cong, M.; Liu, P.; et al. Combination of ultrafast dye-sensitized-assisted electron transfer process and novel Z-scheme system: AgBr nanoparticles interspersed MoO3 nanobelts for enhancing photocatalytic performance of RhB. Appl. Catal. B Environ. 2017, 206, 242–251. [Google Scholar] [CrossRef]
- Huan, C.; Wang, P.; He, B.; Cai, Y.; Ke, Q. Oxygen deficient α-MoO3 with enhanced adsorption and state-quenching of H2O for gas sensing: A DFT study. J. Mater. Chem. C 2022, 10, 1839–1849. [Google Scholar] [CrossRef]
- Minubayeva, Z.; Seward, T.M. Molybdic acid ionisation under hydrothermal conditions to 300 °C. Geochim. Cosmochim. Acta 2010, 74, 4365–4374. [Google Scholar] [CrossRef]
- Barka, N.; Assabbane, A.; Nounah, A.; Dussaud, J.; Ait Ichou, Y. Photocatalytic degradation of methyl orange with immobilized TiO2 nanoparticles: Effect of pH and some inorganic anions photocatalytic degradation of methyl orange with immobilized tio 2 nanoparticles: Effect of ph and some inorganic anions. Artic. Phys. Chem. News 2008, 41, 85–88. [Google Scholar]
- Kedves, E.-Z.; Székely, I.; Baia, L.; Baia, M.; Csavdári, A.; Pap, Z. The Comparison of the Photocatalytic Performance Shown by TiO2 and TiO2/WO3 Composites—A Parametric and Kinetic Study. J. Nanosci. Nanotechnol. 2018, 19, 356–365. [Google Scholar] [CrossRef]
- Bárdos, E.; Márta, V.; Baia, L.; Todea, M.; Kovács, G.; Baán, K.; Garg, S.; Pap, Z.; Hernadi, K. Hydrothermal crystallization of bismuth oxybromide (BiOBr) in the presence of different shape controlling agents. Appl. Surf. Sci. 2020, 518, 146184. [Google Scholar] [CrossRef]
- Roy Choudhury, A.K. 4-Instrumental Colourant Formulation; Roy Choudhury, A.K., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 117–173. ISBN 978-1-78242-367-6. [Google Scholar]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F. Under vacuum synthesis of type-I heterojunction between red phosphorus and graphene like carbon nitride with enhanced catalytic, electrochemical and charge separation ability for photodegradation of an acute toxicity category-III compound. Appl. Catal. B 2018, 238, 568–575. [Google Scholar] [CrossRef]
- Yu, L.; Xi, J.; Li, M.-D.; Chan, H.T.; Su, T.; Phillips, D.L.; Chan, W.K. The degradation mechanism of methyl orange under photo-catalysis of TiO2. Phys. Chem. Chem. Phys. 2012, 14, 3589–3595. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.T.; Le, S.T.T.; Channei, D.; Khanitchaidecha, W.; Nakaruk, A. Photodegradation mechanisms of phenol in the photocatalytic process. Res. Chem. Intermed. 2016, 42, 5961–5974. [Google Scholar] [CrossRef]
- Dan, Z.; Yang, Y.; Qin, F.; Wang, H.; Chang, H. Facile Fabrication of Cu2O Nanobelts in Ethanol on Nanoporous Cu and Their Photodegradation of Methyl Orange. Materials 2018, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Prado-Chay, D.A.; Cortés-Jácome, M.A.; Angeles-Chávez, C.; Oviedo-Roa, R.; Martínez-Magadán, J.M.; Zuriaga-Monroy, C.; Hernández-Hernández, I.J.; Mayoral, P.R.; Gómora-Herrera, D.R.; Toledo-Antonio, J.A. Synthesis and Photocatalytic Activity of Cu2O Microspheres upon Methyl Orange Degradation. Top. Catal. 2020, 63, 586–600. [Google Scholar] [CrossRef]
- Kedves, E.-Z.; Fodor, C.; Fazekas, Á.; Székely, I.; Szamosvölgyi, Á.; Sápi, A.; Kónya, Z.; Cristian Pop, L.; Baia, L.; Pap, Z. α-MoO3 with inhibitive properties in Fenton reactions and insights on its general impact on OH radical based advanced oxidation processes. Appl. Surf. Sci. 2023, 624, 156914. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kedves, E.-Z.; Bárdos, E.; Ravasz, A.; Tóth, Z.-R.; Mihálydeákpál, S.; Kovács, Z.; Pap, Z.; Baia, L. Photoinhibitive Properties of α-MoO3 on Its Composites with TiO2, ZnO, BiOI, AgBr, and Cu2O. Materials 2023, 16, 3621. https://doi.org/10.3390/ma16103621
Kedves E-Z, Bárdos E, Ravasz A, Tóth Z-R, Mihálydeákpál S, Kovács Z, Pap Z, Baia L. Photoinhibitive Properties of α-MoO3 on Its Composites with TiO2, ZnO, BiOI, AgBr, and Cu2O. Materials. 2023; 16(10):3621. https://doi.org/10.3390/ma16103621
Chicago/Turabian StyleKedves, Endre-Zsolt, Enikő Bárdos, Alpár Ravasz, Zsejke-Réka Tóth, Szilvia Mihálydeákpál, Zoltán Kovács, Zsolt Pap, and Lucian Baia. 2023. "Photoinhibitive Properties of α-MoO3 on Its Composites with TiO2, ZnO, BiOI, AgBr, and Cu2O" Materials 16, no. 10: 3621. https://doi.org/10.3390/ma16103621
APA StyleKedves, E.-Z., Bárdos, E., Ravasz, A., Tóth, Z.-R., Mihálydeákpál, S., Kovács, Z., Pap, Z., & Baia, L. (2023). Photoinhibitive Properties of α-MoO3 on Its Composites with TiO2, ZnO, BiOI, AgBr, and Cu2O. Materials, 16(10), 3621. https://doi.org/10.3390/ma16103621







