Facile One-Pot Synthesis of Nickel Nanoparticles by Hydrothermal Method
Abstract
1. Introduction
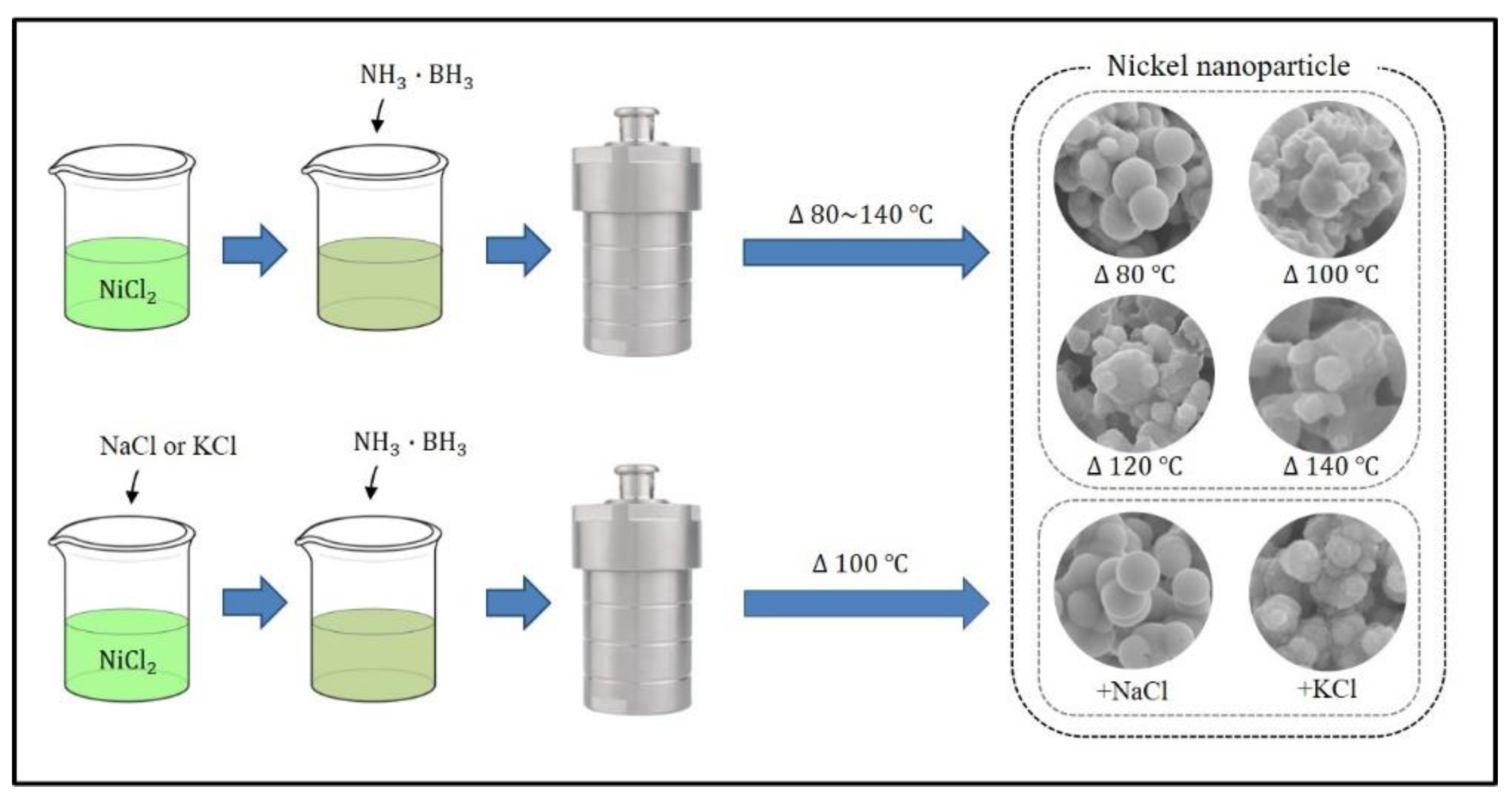
2. Experimental
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devi, H.R.; Bisen, O.Y.; Chen, Z.; Nanda, K.K. Spatially dispersed one-dimensional carbon architecture on oxide framework for oxygen electrochemistry. Chem. Eng. J. 2021, 433, 133649. [Google Scholar] [CrossRef]
- Cheng, W.; Lu, X.F.; Luan, D.; Lou, X.W. NiMn-Based Bimetal–Organic Framework Nanosheets Supported on Multi-Channel Carbon Fibers for Efficient Oxygen Electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 18234–18239. [Google Scholar] [CrossRef] [PubMed]
- Charles, V.; Zhang, X.; Yuan, M.; Zhang, K.; Cui, K.; Zhang, J.; Zhao, T.; Li, Y.; Liu, Z.; Li, B.; et al. CoNi nano-alloy anchored on biomass-derived N-doped carbon frameworks for enhanced oxygen reduction and evolution reactions. Electrochim. Acta 2021, 402, 139555. [Google Scholar] [CrossRef]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Trudel, S.; Berlinguette, C.P. Water Oxidation Catalysis: Electrocatalytic Response to Metal Stoichiometry in Amorphous Metal Oxide Films Containing Iron, Cobalt, and Nickel. J. Am. Chem. Soc. 2013, 135, 11580–11586. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tournet, J.; Dastafkan, K.; Liu, Y.; Ng, Y.H.; Karuturi, S.K.; Zhao, C.; Yin, Z. Noble-Metal-Free Multicomponent Nanointegration for Sustainable Energy Conversion. Chem. Rev. 2021, 121, 10271–10366. [Google Scholar] [CrossRef]
- Paul, S.C.; Dey, S.C.; Molla, A.I.; Islam, S.; Debnath, S.; Miah, M.Y.; Ashaduzzaman; Sarker, M. Nanomaterials as electrocatalyst for hydrogen and oxygen evolution reaction: Exploitation of challenges and current progressions. Polyhedron 2020, 193, 114871. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Z.-H.; Yuan, T.-Q.; Ren, X.; Rong, Z. Raney Ni as a Versatile Catalyst for Biomass Conversion. ACS Catal. 2021, 11, 10508–10536. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, Y. Nanoporous Metals for Heterogeneous Catalysis: Following the Success of Raney Nickel. Chem.–A Eur. J. 2020, 26, 8845–8856. [Google Scholar] [CrossRef]
- Kishi, H.; Yamaoka, N. Multilayer ceramic capacitors with nickel electrodes. In Materials Science Monographs; Nowotny, J., Ed.; Elsevier: Amsterdam, Netherlands, 1995; Volume 81, pp. 613–627. [Google Scholar]
- Im, T.; Pyo, J.; Lee, J.-S.; Lee, C.S. Fabrication of homogeneous nanosized nickel powders using a planetary ball mill: Applications to multilayer ceramic capacitors (MLCCs). Powder Technol. 2020, 382, 118–125. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, J.-H.; Hong, S.-H.; Lee, Y.; Choi, J.-Y. Coating BaTiO3 Nanolayers on Spherical Ni Powders for Multilayer Ceramic Capacitors. Adv. Mater. 2003, 15, 1655–1658. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakamori, T. Steam reforming of methane over nickel catalysts at low reaction temperature. Appl. Catal. A Gen. 2004, 258, 107–114. [Google Scholar] [CrossRef]
- Nieva, M.A.; Villaverde, M.M.; Monzón, A.; Garetto, T.F.; Marchi, A.J. Steam-methane reforming at low temperature on nickel-based catalysts. Chem. Eng. J. 2014, 235, 158–166. [Google Scholar] [CrossRef]
- Vogt, C.; Kranenborg, J.; Monai, M.; Weckhuysen, B.M. Structure Sensitivity in Steam and Dry Methane Reforming over Nickel: Activity and Carbon Formation. ACS Catal. 2019, 10, 1428–1438. [Google Scholar] [CrossRef]
- Wu, H.; La Parola, V.; Pantaleo, G.; Puleo, F.; Venezia, A.M.; Liotta, L.F. Ni-Based Catalysts for Low Temperature Methane Steam Reforming: Recent Results on Ni-Au and Comparison with Other Bi-Metallic Systems. Catalysts 2013, 3, 563–583. [Google Scholar] [CrossRef]
- Lyu, Y.; Jocz, J.N.; Xu, R.; Stavitski, E.; Sievers, C. Nickel Speciation and Methane Dry Reforming Performance of Ni/CexZr1–xO2 Prepared by Different Synthesis Methods. ACS Catal. 2020, 10, 11235–11252. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhao, S.; Tang, Z. Metal–Organic Framework-Based Nanomaterials for Electrocatalytic Oxygen Evolution. Small Methods 2022, 6, e2200773. [Google Scholar] [CrossRef]
- Zhao, S.; Tan, C.; He, C.-T.; An, P.; Xie, F.; Jiang, S.; Zhu, Y.; Wu, K.-H.; Zhang, B.; Li, H.; et al. Structural transformation of highly active metal–organic framework electrocatalysts during the oxygen evolution reaction. Nat. Energy 2020, 5, 881–890. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, L.; Tang, C.; Tan, C.; Cheng, N.; Lai, W.; Wang, Y.; Cheng, Z.; Dong, J.; Kong, Y.; et al. Atomically Dispersed Dual-Site Cathode with a Record High Sulfur Mass Loading for High-Performance Room-Temperature Sodium-Sulfur Batteries. Adv. Mater. 2022, 2206828. [Google Scholar] [CrossRef]
- Zhou, Z.; Kong, Y.; Tan, H.; Huang, Q.; Wang, C.; Pei, Z.; Wang, H.; Liu, Y.; Wang, Y.; Li, S.; et al. Cation-Vacancy-Enriched Nickel Phosphide for Efficient Electrosynthesis of Hydrogen Peroxides. Adv. Mater. 2022, 34, 2106541. [Google Scholar] [CrossRef] [PubMed]
- Tientong, J.; Garcia, S.; Thurber, C.R.; Golden, T.D. Synthesis of Nickel and Nickel Hydroxide Nanopowders by Simplified Chemical Reduction. J. Nanotechnol. 2014, 2014, 193162. [Google Scholar] [CrossRef]
- Ahghari, M.R.; Soltaninejad, V.; Maleki, A. Synthesis of nickel nanoparticles by a green and convenient method as a magnetic mirror with antibacterial activities. Sci. Rep. 2020, 10, 12627. [Google Scholar] [CrossRef] [PubMed]
- Denis, D.K.; Zaman, F.U.; Hou, L.; Chen, G.; Yuan, C. Spray-drying construction of nickel/cobalt/molybdenum based nano carbides embedded in porous carbon microspheres for lithium-ion batteries as anodes. Electrochim. Acta 2022, 424, 140678. [Google Scholar] [CrossRef]
- Li, J.-Y.; Cao, Z.-M.; Hua, Y.; Wei, G.; Yu, X.-Z.; Shang, W.-B.; Lian, H.-Z. Solvothermal Synthesis of Novel Magnetic Nickel Based Iron Oxide Nanocomposites for Selective Capture of Global- and Mono-Phosphopeptides. Anal. Chem. 2019, 92, 1058–1067. [Google Scholar] [CrossRef]
- Eluri, R.; Paul, B. Synthesis of nickel nanoparticles by hydrazine reduction: Mechanistic study and continuous flow synthesis. J. Nanoparticle Res. 2012, 14, 800. [Google Scholar] [CrossRef]
- Park, J.W.; Chae, E.H.; Kim, S.H.; Lee, J.H.; Kim, J.W.; Yoon, S.M.; Choi, J.-Y. Preparation of fine Ni powders from nickel hydrazine complex. Mater. Chem. Phys. 2006, 97, 371–378. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia Borane: An Extensively Studied, Though Not Yet Implemented, Hydrogen Carrier. Energies 2020, 13, 3071. [Google Scholar] [CrossRef]
- Baik, S.Y.; Cho, Y.J.; Lim, Y.R.; Im, H.S.; Jang, D.M.; Myung, Y.; Park, J.; Kang, H.S. Charge-Selective Surface-Enhanced Raman Scattering Using Silver and Gold Nanoparticles Deposited on Silicon–Carbon Core–Shell Nanowires. ACS Nano 2012, 6, 2459–2470. [Google Scholar] [CrossRef]
- Sanyal, U.; Jagirdar, B.R. Metal and Alloy Nanoparticles by Amine-Borane Reduction of Metal Salts by Solid-Phase Synthesis: Atom Economy and Green Process. Inorg. Chem. 2012, 51, 13023–13033. [Google Scholar] [CrossRef]
- Lin, Y.; Mao, W.L. High-pressure storage of hydrogen fuel: Ammonia borane and its related compounds. Chin. Sci. Bull. 2014, 59, 5235–5240. [Google Scholar] [CrossRef]
- Frueh, S.; Kellett, R.; Mallery, C.; Molter, T.; Willis, W.S.; King’Ondu, C.; Suib, S.L. Pyrolytic Decomposition of Ammonia Borane to Boron Nitride. Inorg. Chem. 2010, 50, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Fingerle, M.; Tengeler, S.; Calvet, W.; Mayer, T.; Jaegermann, W. Water Interaction with Sputter-Deposited Nickel Oxide on n-Si Photoanode: Cryo Photoelectron Spectroscopy on Adsorbed Water in the Frozen Electrolyte Approach. J. Electrochem. Soc. 2018, 165, H3148–H3153. [Google Scholar] [CrossRef]
- Kim, K.; Davis, R. Electron spectroscopy of the nickel-oxygen system. J. Electron Spectrosc. Relat. Phenom. 1973, 1, 251–258. [Google Scholar] [CrossRef]
- Barr, T.L. Recent advances in x-ray photoelectron spectroscopy studies of oxides. J. Vac. Sci. Technol. A Vacuum Surfaces, Films 1991, 9, 1793–1805. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Wang, M.; Mei, J.; You, X. Effect of potassium chloride addition on soot formation during ethylene pyrolysis in a flow reactor. Combust. Flame 2021, 223, 118–126. [Google Scholar] [CrossRef]
- Yang, G.; Ma, G.; He, M.; Ji, X.; Li, W.; Youn, H.; Lee, H.; Chen, J. Comparison of Effects of Sodium Chloride and Potassium Chloride on Spray Drying and Redispersion of Cellulose Nanofibrils Suspension. Nanomaterials 2021, 11, 439. [Google Scholar] [CrossRef]
- Rumble, J.R. CRC Handbook of Chemistry and Physics, 102nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Na, C.W.; Myung, Y. Facile One-Pot Synthesis of Nickel Nanoparticles by Hydrothermal Method. Materials 2023, 16, 76. https://doi.org/10.3390/ma16010076
Kim G, Na CW, Myung Y. Facile One-Pot Synthesis of Nickel Nanoparticles by Hydrothermal Method. Materials. 2023; 16(1):76. https://doi.org/10.3390/ma16010076
Chicago/Turabian StyleKim, Gawon, Chan Woong Na, and Yoon Myung. 2023. "Facile One-Pot Synthesis of Nickel Nanoparticles by Hydrothermal Method" Materials 16, no. 1: 76. https://doi.org/10.3390/ma16010076
APA StyleKim, G., Na, C. W., & Myung, Y. (2023). Facile One-Pot Synthesis of Nickel Nanoparticles by Hydrothermal Method. Materials, 16(1), 76. https://doi.org/10.3390/ma16010076





