Developments on Constitutive Material Model for Architectural Soda-Lime Silicate (SLS) Glass and Evaluation of Key Modelling Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Study Methods
2.2. Experimental Study
3. Results
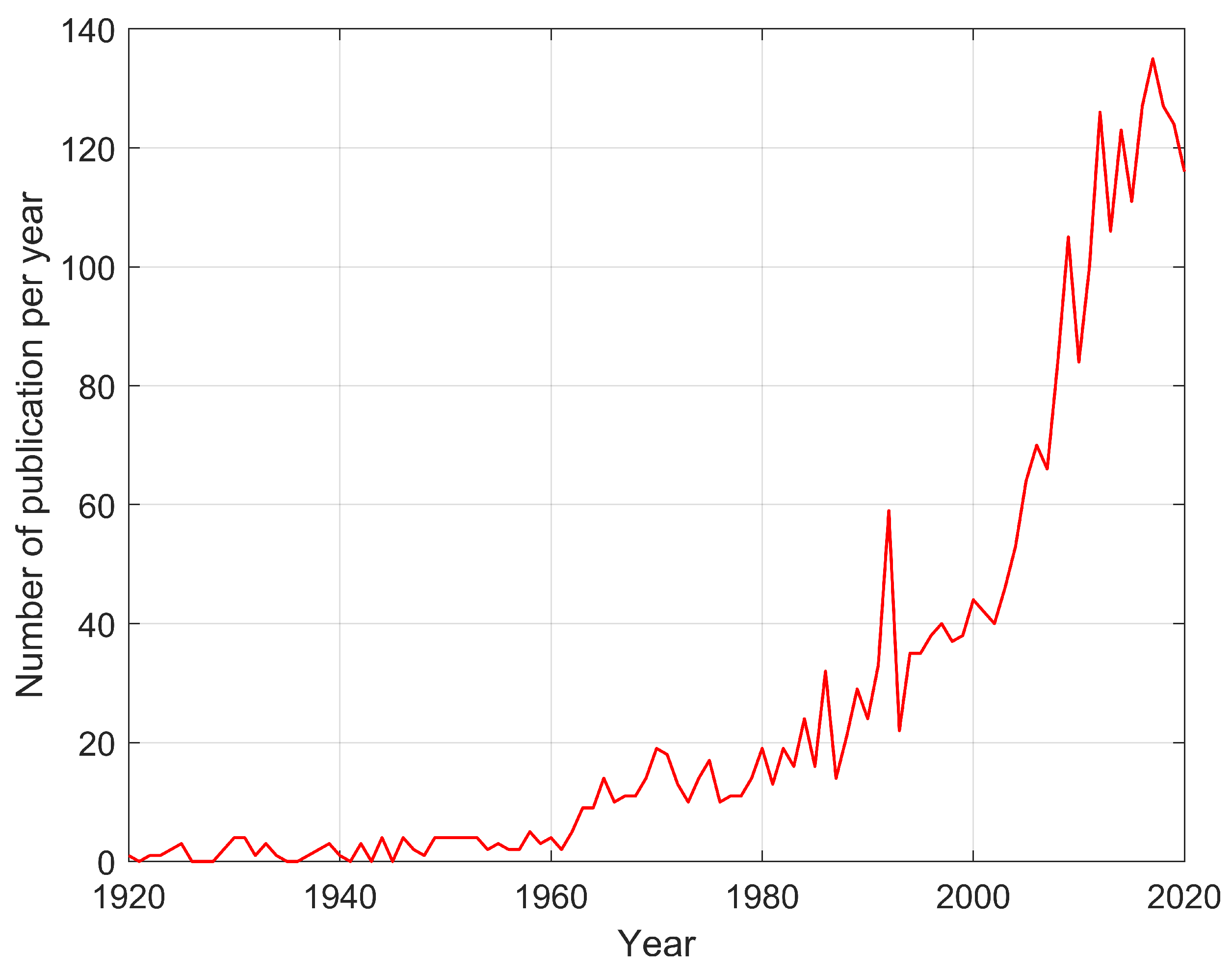
3.1. Literature Study
- (1)
- Glass state ();
- (2)
- Supercooled liquid state ();
- (3)
- Liquid state ().
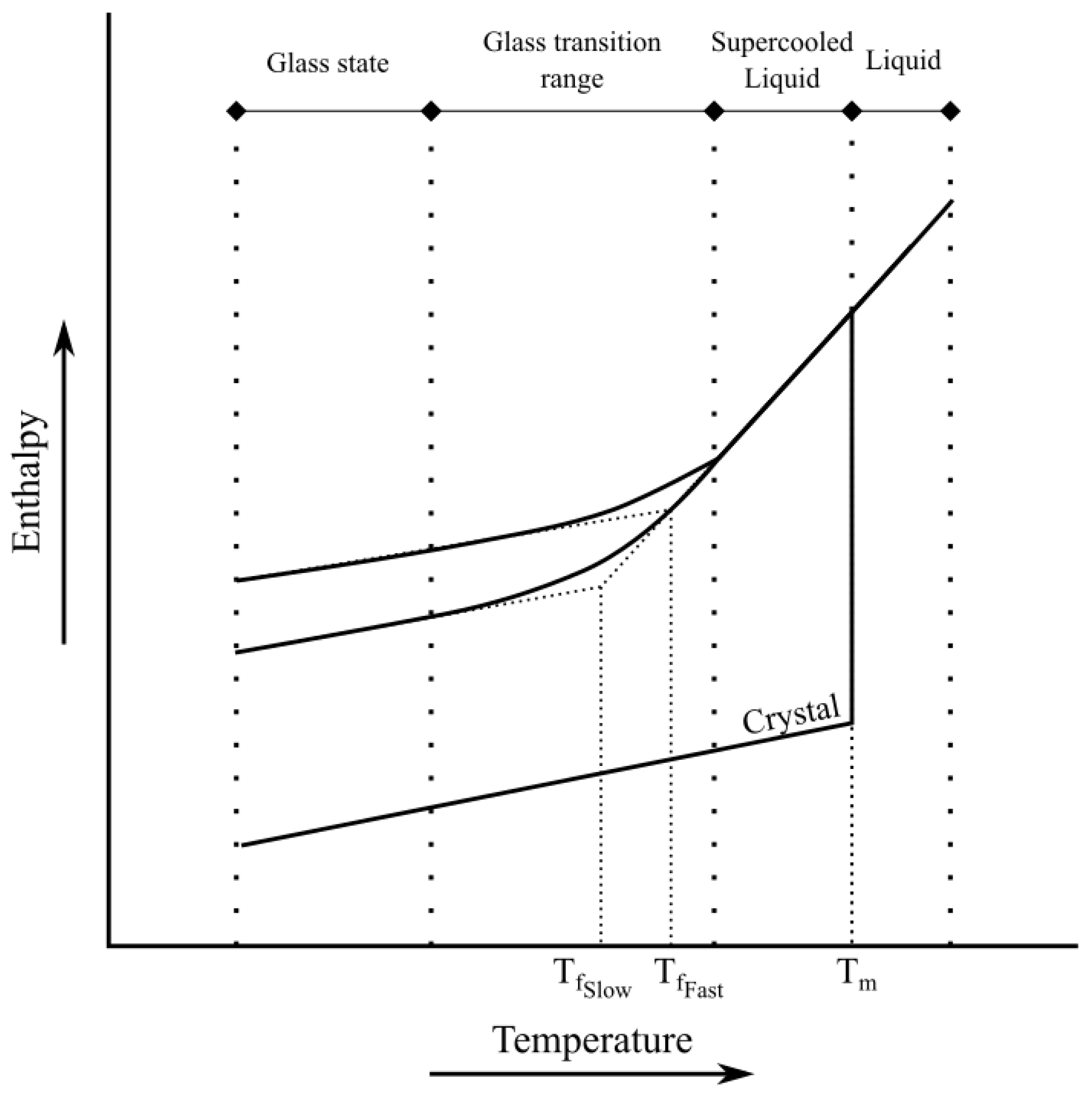
Nielsen [52] Work
- Temperature-dependent viscoelasticity and thermorheologically simple behavior.
- Structural relaxation.
- Liquid state ()— is not a part of the equation anymore;
- Supercooled liquid state ()—in this case by cooling, lags behind the real temperature;
- Glass state ()— is not part of equation anymore.
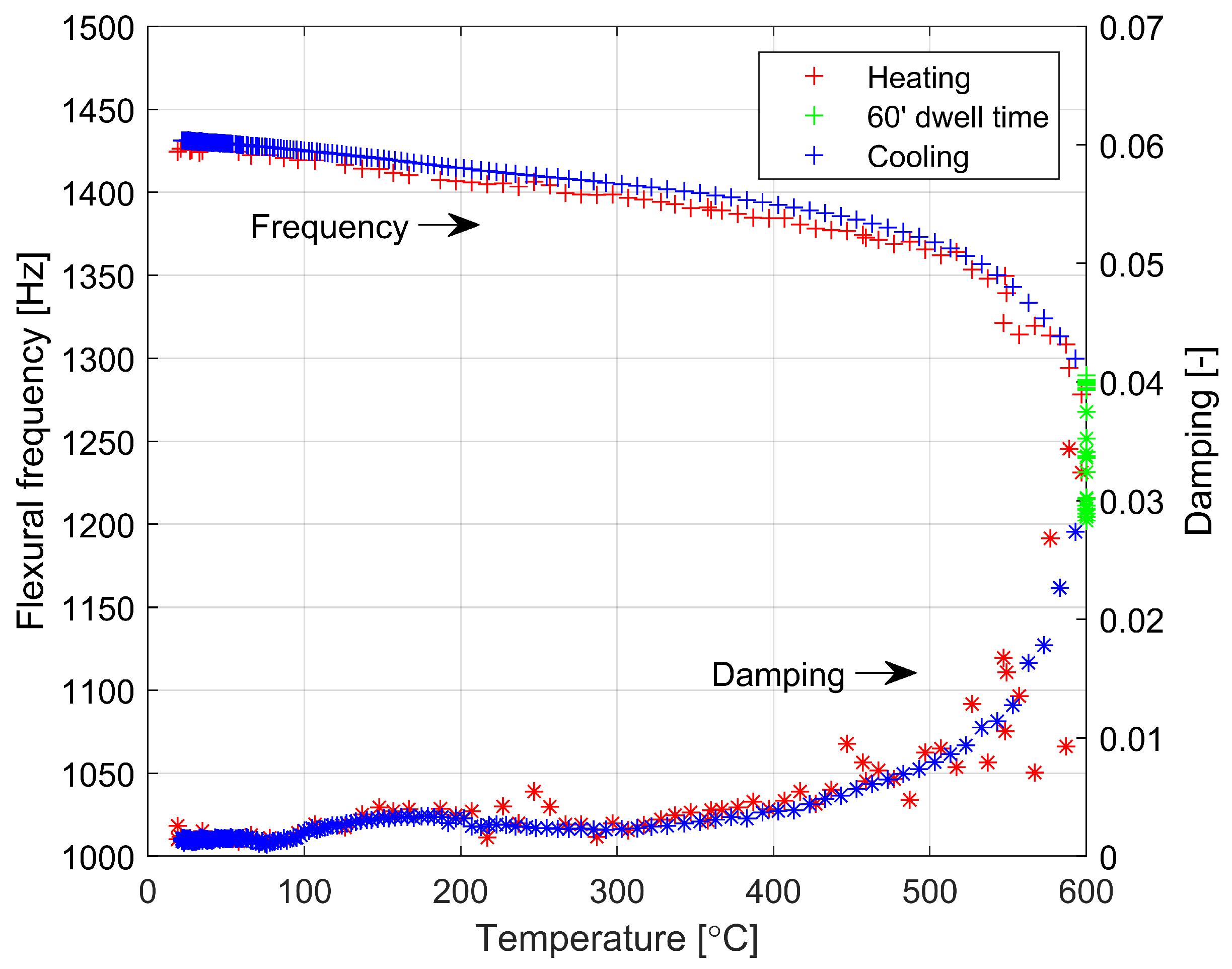
3.2. Experimental Study of Key Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shelby, J. Introduction to Glass Science and Technology; EngineeringPro collection; Royal Society of Chemistry: London, UK, 2005. [Google Scholar]
- Zanotto, E.D.; Mauro, J.C. The glassy state of matter: Its definition and ultimate fate. J. Non-Cryst. Solids 2017, 471, 490–495. [Google Scholar] [CrossRef]
- Harzing, A.W. Publish or Perish. 2007. Available online: https://harzing.com/resources/publish-or-perish (accessed on 20 October 2022).
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. Proc. Int. Aaai Conf. Web Soc. Media 2009, 3, 361–362. [Google Scholar] [CrossRef]
- EN 572-1:2012+A1:2016; Glass in Building—Basic Soda Lime Silicate Glass Products—Part 1: Definitions and General Physical and Mechanical Properties. European Committee for Standardization: Brussels, Belgium, 2016.
- Pilkington, L.A.B. Review lecture: The float glass process. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1969, 314, 1–25. [Google Scholar]
- Chen, X.; Chen, X.; Chan, A.; Cheng, Y.; Wang, H. A FDEM Parametric Investigation on the Impact Fracture of Monolithic Glass. Buildings 2022, 12, 271. [Google Scholar] [CrossRef]
- Respondek, Z.; Kozłowski, M.; Wiśniowski, M. Deflections and Stresses in Rectangular, Circular and Elliptical Insulating Glass Units. Materials 2022, 15, 2427. [Google Scholar] [CrossRef]
- Kozłowski, M.; Bedon, C.; Honfi, D. Numerical Analysis and 1D/2D Sensitivity Study for Monolithic and Laminated Structural Glass Elements under Thermal Exposure. Materials 2018, 11, 1447. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, J. Applications for curved glass in buildings. J. Facade Des. Eng. 2014, 2, 67–83. [Google Scholar] [CrossRef]
- Malewski, A.; Kozłowski, M.; Sumelka, W.; Połedniok, M. Large scale architectural glass slumping process-challenges and limitations. Arch. Civ. Eng. 2020, 66, 485–505. [Google Scholar]
- Vollers, K.J. Developing Doublecurved Architectural Glass. In Proceedings of the DS 30: 7th International Design Conference (DESIGN 2002), Dubrovnik, Croatia, 14–17 May 2002. [Google Scholar]
- Fildhuth, T.; Knippers, J.; Bindji-Odzili, F.; Baldassini, N.; Pennetier, S. Recovery behaviour of laminated cold bent glass–Numerical analysis and testing. Chall. Glass 2014, 4, 113. [Google Scholar]
- Galuppi, L.; Royer-Carfagni, G. Optimal cold bending of laminated glass. Int. J. Solids Struct. 2015, 67, 231–243. [Google Scholar] [CrossRef]
- Galuppi, L.; Royer-Carfagni, G. Cold-lamination-bending of glass: Sinusoidal is better than circular. Compos. Part B Eng. 2015, 79, 285–300. [Google Scholar] [CrossRef]
- Vákár, L.I.; Gaal, M. Cold bendable, Laminated Glass–New possibilities in design. Struct. Eng. Int. 2004, 14, 95–97. [Google Scholar] [CrossRef]
- Vollers, K. Usage of Cold Bent Glass Panes as an Approximation for Doublecurved. In Proceedings of the CTBUH 2004 Seoul Conference, Seoul, Republic of Korea, 10–13 October 2004. [Google Scholar]
- Bijster, J.; Noteboom, C.; Eekhout, M. Glass Entrance Van Gogh Museum Amsterdam. Glass Struct. Eng. 2016, 1, 205–231. [Google Scholar] [CrossRef][Green Version]
- Liu, W.; Ruan, H.; Zhang, L. Revealing structural relaxation of optical glass through the temperature dependence of Young’s modulus. J. Am. Ceram. Soc. 2014, 97, 3475–3482. [Google Scholar] [CrossRef]
- Turnbull, D. Under what conditions can a glass be formed? Contemp. Phys. 1969, 10, 473–488. [Google Scholar] [CrossRef]
- Ediger, M.D.; Angell, C.A.; Nagel, S.R. Supercooled liquids and glasses. J. Phys. Chem. 1996, 100, 13200–13212. [Google Scholar] [CrossRef]
- Ediger, M.; Harrowell, P. Perspective: Supercooled liquids and glasses. J. Chem. Phys. 2012, 137, 080901. [Google Scholar] [CrossRef]
- Mauro, J.C.; Yue, Y.; Ellison, A.J.; Gupta, P.K.; Allan, D.C. Viscosity of glass-forming liquids. Proc. Natl. Acad. Sci. USA 2009, 106, 19780–19784. [Google Scholar] [CrossRef]
- Angell, C.A. Formation of glasses from liquids and biopolymers. Science 1995, 267, 1924–1935. [Google Scholar] [CrossRef]
- Debenedetti, P.G.; Stillinger, F.H. Supercooled liquids and the glass transition. Nature 2001, 410, 259–267. [Google Scholar] [CrossRef]
- Dyre, J.C. Colloquium: The glass transition and elastic models of glass-forming liquids. Rev. Mod. Phys. 2006, 78, 953. [Google Scholar] [CrossRef]
- Angell, C.A.; Ngai, K.L.; McKenna, G.B.; McMillan, P.F.; Martin, S.W. Relaxation in glassforming liquids and amorphous solids. J. Appl. Phys. 2000, 88, 3113–3157. [Google Scholar] [CrossRef]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1925, 8, 339–355. [Google Scholar] [CrossRef]
- Narayanaswamy, O.; Gardon, R. Calculation of residual stresses in glass. J. Am. Ceram. Soc. 1969, 52, 554–558. [Google Scholar] [CrossRef]
- Bartenev, G. The phenomenon of the hardening of glass. J. Tech. Phys. 1948, 18, 383–388. [Google Scholar]
- Indenbom, V. Theory of Tempering of Glass. Z. Tekh. Fiz. 1954, 24, 925–928. [Google Scholar]
- Aggarwala, B.; Saibel, E. Tempering stresses in an infinite glass plate. Phys. Chem. Glasses 1961, 2, 137–140. [Google Scholar]
- Lee, E.; Rogers, T.; Woo, T. Residual stresses in a glass plate cooled symmetrically from both surfaces. J. Am. Ceram. Soc. 1965, 48, 480–487. [Google Scholar] [CrossRef]
- Lee, E.; Radok, J.; Woodward, W. Stress analysis for linear viscoelastic materials. Trans. Soc. Rheol. 1959, 3, 41–59. [Google Scholar] [CrossRef]
- Morland, L.W.; Lee, E. Stress analysis for linear viscoelastic materials with temperature variation. Trans. Soc. Rheol. 1960, 4, 233–263. [Google Scholar] [CrossRef]
- Lee, E.; Rogers, T.G. Solution of viscoelastic stress analysis problems using measured creep or relaxation functions. J. Appl. Mech. Mar. 1963, 30, 127–133. [Google Scholar] [CrossRef]
- Kurkjian, C.R. Relaxation of torsional stress in the transformation range of a soda-lime-silica glass. Phys. Chem. Glasses 1963, 4, 128–136. [Google Scholar]
- Schwarzl, F.; Staverman, A. Time-temperature dependence of linear viscoelastic behavior. J. Appl. Phys. 1952, 23, 838–843. [Google Scholar] [CrossRef]
- Gardon, R.; Narayanaswamy, O. Stress and volume relaxation in annealing flat glass. J. Am. Ceram. Soc. 1970, 53, 380–385. [Google Scholar] [CrossRef]
- Narayanaswamy, O. A model of structural relaxation in glass. J. Am. Ceram. Soc. 1971, 54, 491–498. [Google Scholar] [CrossRef]
- Tool, A.Q. Relation between inelastic deformability and thermal expansion of glass in its annealing range. J. Am. Ceram. Soc. 1946, 29, 240–253. [Google Scholar] [CrossRef]
- Goldstein, M. Viscous liquids and the glass transition: A potential energy barrier picture. J. Chem. Phys. 1969, 51, 3728–3739. [Google Scholar] [CrossRef]
- Ritland, H. Limitations of the fictive temperature concept. J. Am. Ceram. Soc. 1956, 39, 403–406. [Google Scholar] [CrossRef]
- Narayanaswamy, O. Stress and structural relaxation in tempering glass. J. Am. Ceram. Soc. 1978, 61, 146–152. [Google Scholar] [CrossRef]
- Markovsky, A.; Soules, T.F.; Boyd, D. An efficient and stable algorithm for calculating fictive temperatures. J. Am. Ceram. Soc. 1984, 67, c56–c57. [Google Scholar] [CrossRef]
- Nielsen, J.H.; Thiele, K.; Schneider, J.; Meyland, M.J. Compressive zone depth of thermally tempered glass. Constr. Build. Mater. 2021, 310, 125238. [Google Scholar] [CrossRef]
- Gardon, R. Thermal tempering of glass. In Glass Science and Technology; Elsevier: Amsterdam, The Netherlands, 1980; Volume 5, pp. 145–216. [Google Scholar]
- Laufs, W.; Sedlacek, G. Stress distribution in thermally tempered glass panes near the edges, corners and holes. Part 1. Temperature distributions during the tempering process of glass panes. Glass Sci. Technol. (Frankf.) 1999, 72, 7–14. [Google Scholar]
- Carré, H.; Daudeville, L. Numerical simulation of soda-lime silicate glass tempering. J. Phys. IV 1996, 6, C1-175. [Google Scholar] [CrossRef]
- Pourmoghaddam, N.; Schneider, J. Finite-element analysis of the residual stresses in tempered glass plates with holes or cut-outs. Glass Struct. Eng. 2018, 3, 17–37. [Google Scholar] [CrossRef]
- Daudeville, L.; Carre, H. Thermal tempering simulation of glass plates: Inner and edge residual stresses. J. Therm. Stresses 1998, 21, 667–689. [Google Scholar] [CrossRef]
- Nielsen, J.H. Tempered Glass: Bolted Connections and Related Problems; Technical University of Denmark (DTU): Lyngby, Denmark, 2009. [Google Scholar]
- Nielsen, J.H.; Olesen, J.F.; Poulsen, P.N.; Stang, H. Finite Element Implementation of a Glass Tempering Model in Three Dimensions. Comput. Struct. 2010, 88, 963–972. [Google Scholar] [CrossRef]
- Dassault Systèmes Simulia Corp. Abaqus Software; Dassault Systèmes Simulia Corp.: Johnston, RI, USA, 2022. [Google Scholar]
- Centelles, X.; Pelayo, F.; Lamela-Rey, M.J.; Fernández, A.I.; Salgado-Pizarro, R.; Castro, J.R.; Cabeza, L.F. Viscoelastic characterization of seven laminated glass interlayer materials from static tests. Constr. Build. Mater. 2021, 279, 122503. [Google Scholar] [CrossRef]
- Laidler, K.J.; Meiser, J.H. Physical Chemistry; Houghton Mifflin: Boston, MA, USA, 1999. [Google Scholar]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Zachariasen, W.H. The atomic arrangement in glass. J. Am. Chem. Soc. 1932, 54, 3841–3851. [Google Scholar] [CrossRef]
- Carré, H.; Daudeville, L. Load-bearing capacity of tempered structural glass. J. Eng. Mech. 1999, 125, 914–921. [Google Scholar] [CrossRef]
- Haldimann, M.; Luible, A.; Overend, M. Structural Use of Glass; IABSE: Zürich, Switzerland, 2008; Volume 10. [Google Scholar]
- Lu, W.; Duan, Q.; Chen, H.; Li, H.; Liu, Y.; Wang, Q.; Sun, J. Thermal response and resistance optimization of various types of point-supported glass facades. Constr. Build. Mater. 2019, 224, 610–621. [Google Scholar] [CrossRef]
- Ohlberg, S.; Woo, T. Thermal stress calculations based on a linear viscoelastic deviatoric response and a fictive temperature component for the volumetric response. J. Non-Cryst. Solids 1974, 14, 280–286. [Google Scholar] [CrossRef]
- Soules, T.F.; Busbey, R.F.; Rekhson, S.M.; Markovsky, A.; BURKE, M.A. Finite-element calculation of stresses in glass parts undergoing viscous relaxation. J. Am. Ceram. Soc. 1987, 70, 90–95. [Google Scholar] [CrossRef]
- Pilette, C.F.; Taylor, D.A. Thermal stresses in double-glazed windows. Can. J. Civ. Eng. 1988, 15, 807–814. [Google Scholar] [CrossRef]
- Plumb, R.C. Antique windowpanes and the flow of supercooled liquids. J. Chem. Educ. 1989, 66, 994. [Google Scholar] [CrossRef]
- Pelletier, J.; Perez, J.; Duffrene, L. Mechanical response of an oxide glass to mechanical loading—Shear and volume relaxation effects: Physical analysis. Acta Mater. 2000, 48, 1397–1408. [Google Scholar] [CrossRef]





| Parameter | Parameter Description | Unit | Impact on the Model | |
|---|---|---|---|---|
| 1 | Reference temperature | K | High | |
| 2 | Liquid glass thermal expansion coeff. | 10 K | High | |
| 3 | , | Shear relaxation and bulk relaxation moduli | Pa | High |
| 4 | , | Relaxation times | s | High |
| 5 | Thermal conductivity | W m K | Medium | |
| 6 | Specific heat capacity | J kg K | Low | |
| 7 | Solid glass thermal expansion coeff. | 10 K | Low | |
| 8 | Density | kg/m | N/A | |
| 9 | H | Activation energy (total) | J/mol | N/A |
| 10 | Ideal Gas constant | J/mol | Physical constant | |
| 11 | h | Forced convection constant | W m K | Environment variable |
| 12 | Initial Temperature | K | Environment variable | |
| 13 | Ambient Temperature | K | Environment variable |
| Paper | Date | E | |||
|---|---|---|---|---|---|
| Lee et al. [33] | 1965 | 811 | - | - | - |
| Narayanaswamy and Gardon [29] | 1969 | 811 | - | 7.58 | - |
| Narayanaswamy and Gardon [29] | 1970 | 811 | - | - | - |
| Ohlberg and Woo [62] | 1974 | - | 9.5 (24.5) | - | - |
| Soules et al. [63] | 1987 | 746 | 9.2 (18.4) | - | - |
| Pilette and Taylor [64] | 1988 | - | 8.0 | 7.0 | - |
| Plumb [65] | 1989 | 823 | - | - | - |
| Carré and Daudeville [49] | 1996 | 869 | 9.1 (27.1) | 7.2 | - |
| Daudeville and Carre [51] | 1998 | 869 | 9.1 (25.1) | 7.0 | - |
| Carré and Daudeville [59] | 1999 | 864 | 9.0 (25.0) | 7.0 | - |
| Laufs and Sedlacek [48] | 1999 | 853 | - | - | 1000 (at 853 K) |
| Pelletier et al. [66] | 2000 | 813 | 9.8 | 6.2 | - |
| Shelby [1] | 2005 | 823–853 | 9.5 | 7.0–7.5 | - |
| Nielsen [52] | 2009 | 869 | 9.1 (25.1) | 7.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malewski, A.; Kozłowski, M.; Podwórny, J.; Środa, M.; Sumelka, W. Developments on Constitutive Material Model for Architectural Soda-Lime Silicate (SLS) Glass and Evaluation of Key Modelling Parameters. Materials 2023, 16, 397. https://doi.org/10.3390/ma16010397
Malewski A, Kozłowski M, Podwórny J, Środa M, Sumelka W. Developments on Constitutive Material Model for Architectural Soda-Lime Silicate (SLS) Glass and Evaluation of Key Modelling Parameters. Materials. 2023; 16(1):397. https://doi.org/10.3390/ma16010397
Chicago/Turabian StyleMalewski, Andrzej, Marcin Kozłowski, Jacek Podwórny, Marcin Środa, and Wojciech Sumelka. 2023. "Developments on Constitutive Material Model for Architectural Soda-Lime Silicate (SLS) Glass and Evaluation of Key Modelling Parameters" Materials 16, no. 1: 397. https://doi.org/10.3390/ma16010397
APA StyleMalewski, A., Kozłowski, M., Podwórny, J., Środa, M., & Sumelka, W. (2023). Developments on Constitutive Material Model for Architectural Soda-Lime Silicate (SLS) Glass and Evaluation of Key Modelling Parameters. Materials, 16(1), 397. https://doi.org/10.3390/ma16010397










