Controlled Silanization of Transparent Conductive Oxides as a Precursor of Molecular Recognition Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Modification Procedure
2.2. Electrochemical Measurements
2.3. Contact Angle Measurements
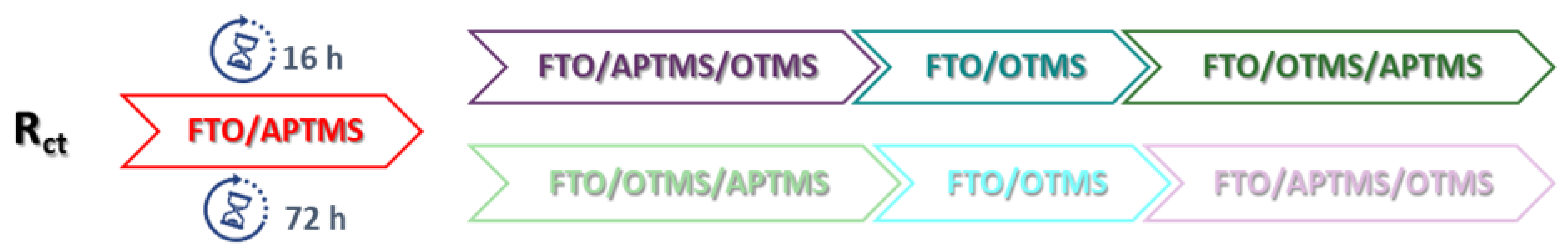
3. Results and Discussion
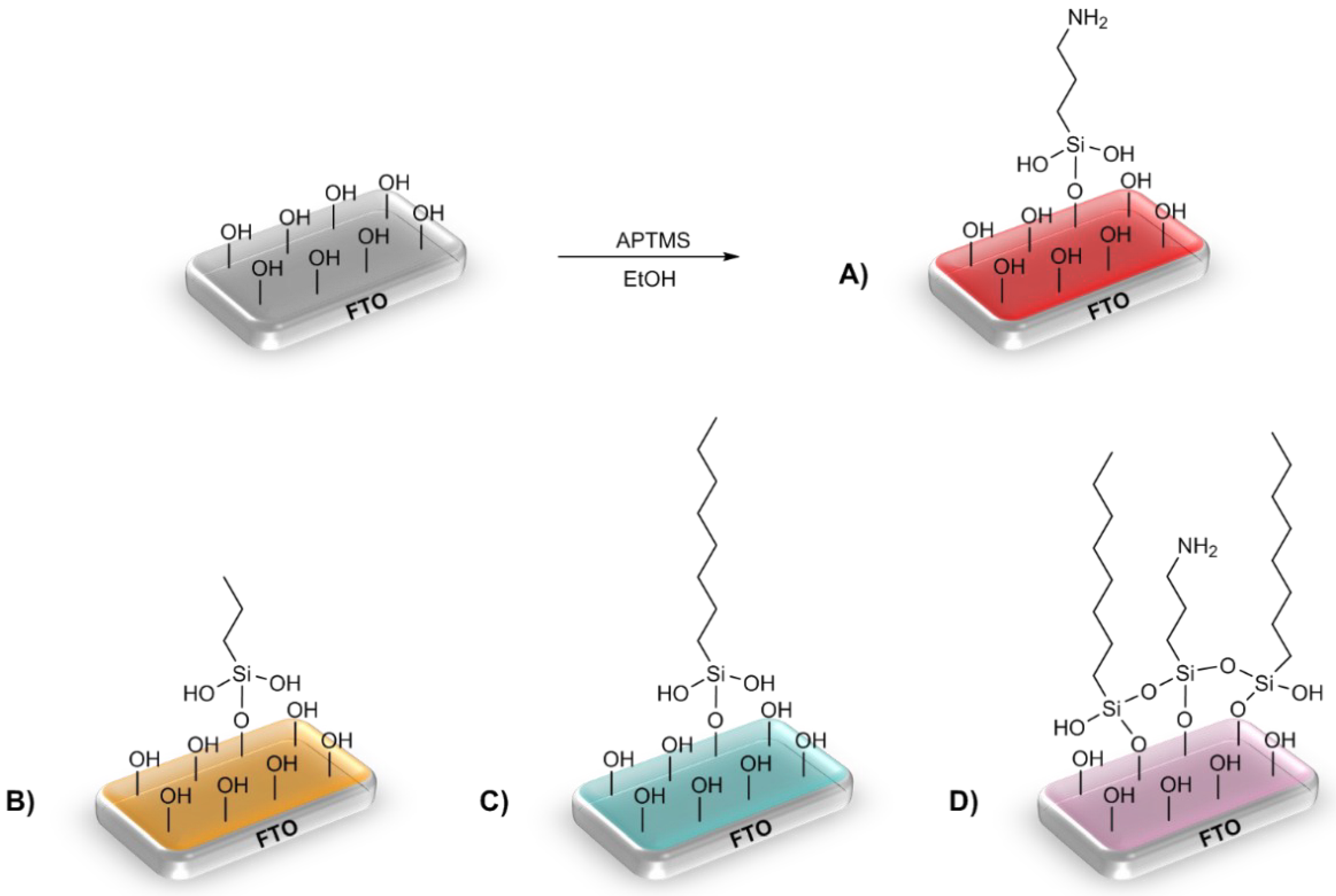
3.1. Alkoxysilane Modification
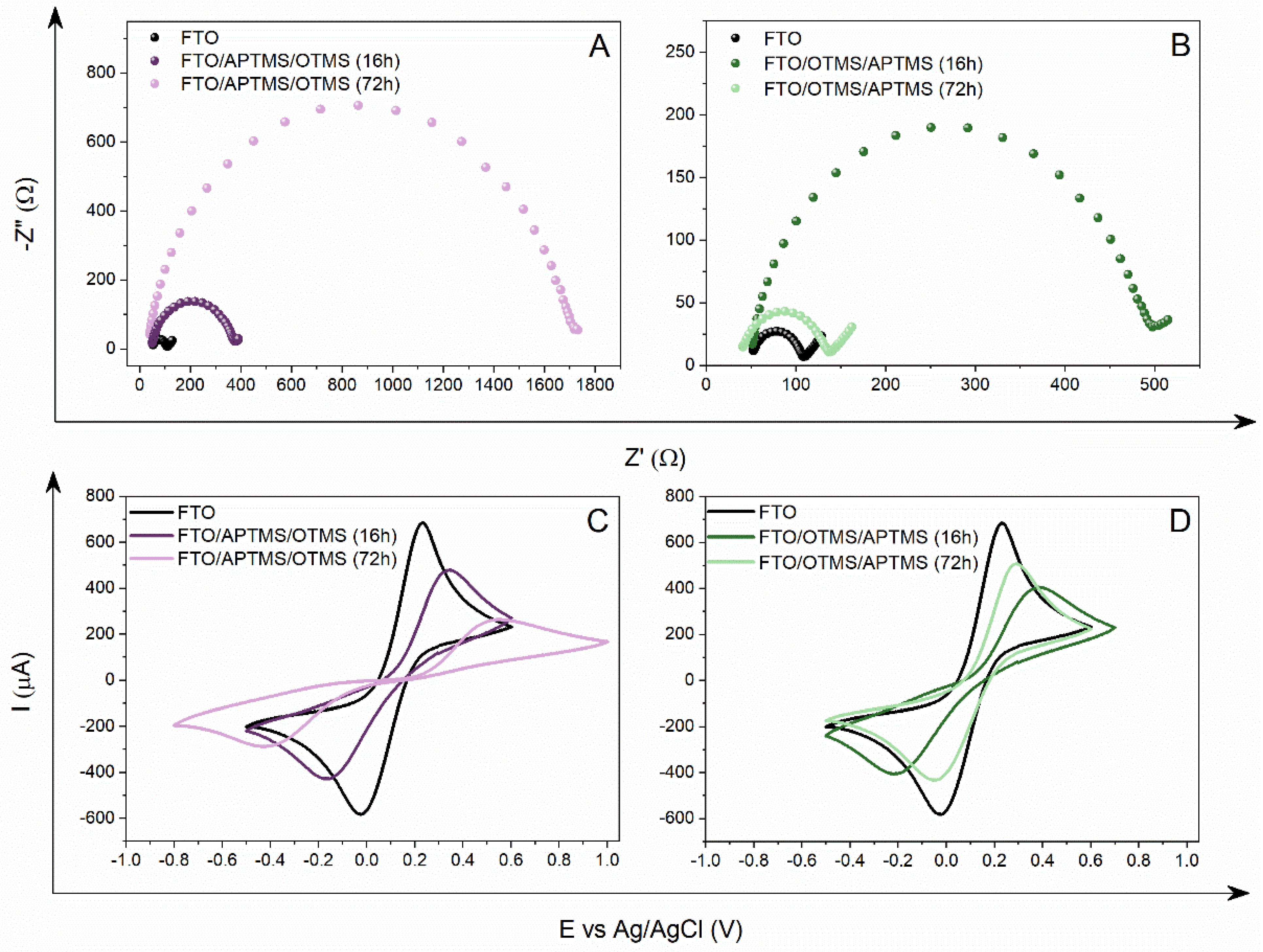
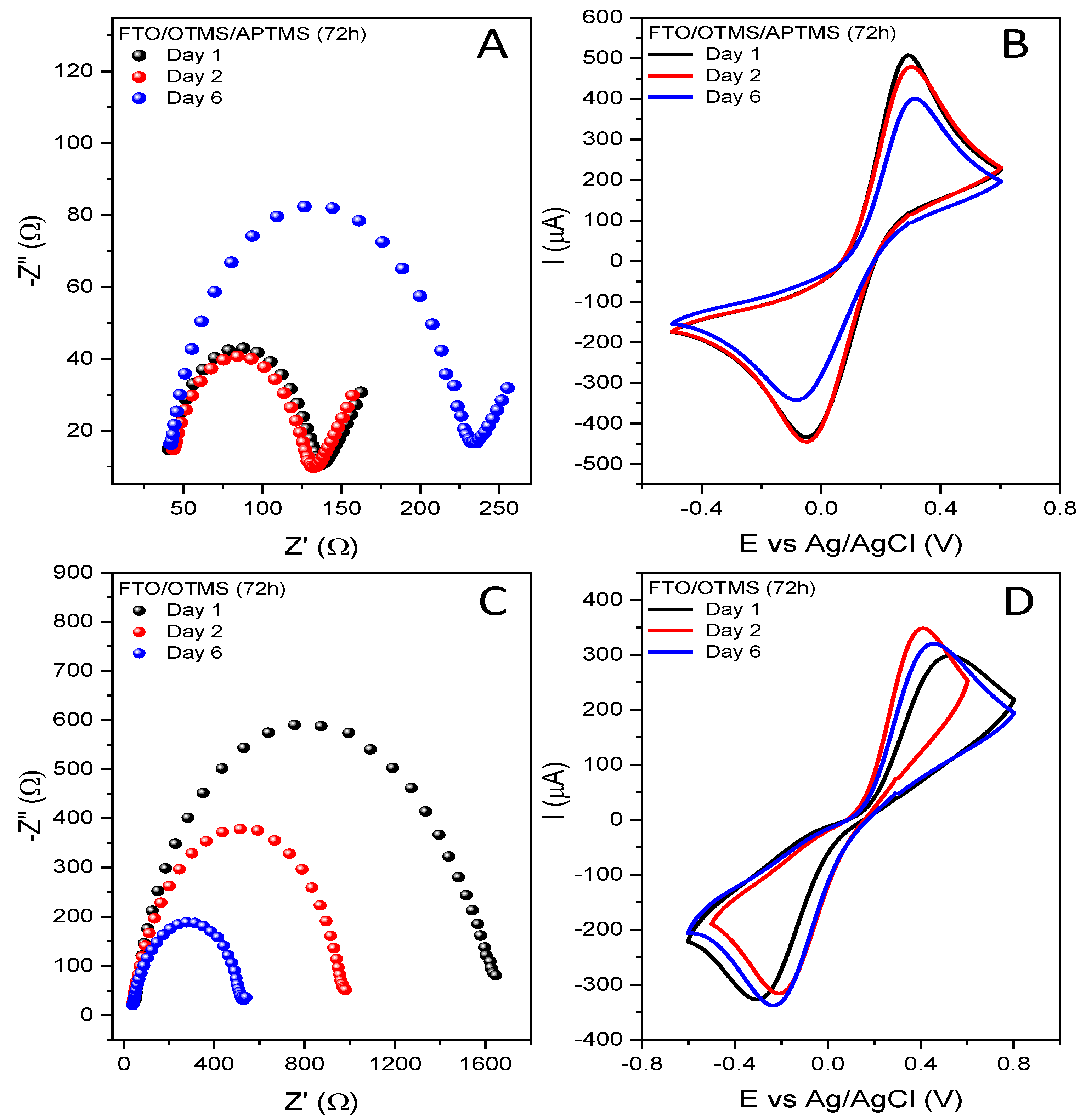
3.2. Double-Alkoksysilane Modification
3.3. Two-Step Mixed Alkoksysilane Modification
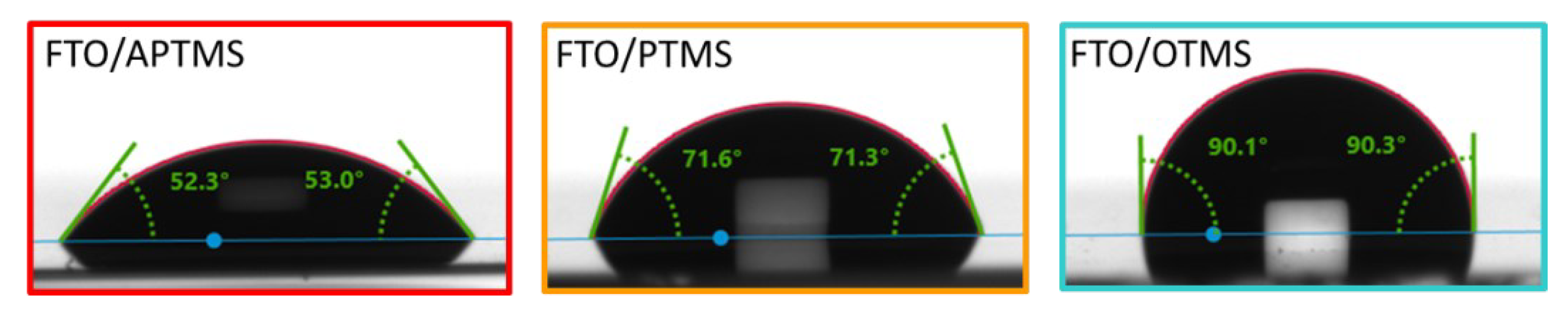
3.4. Wettability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swebocki, T.; Niedziałkowski, P.; Cirocka, A.; Szczepańska, E.; Ossowski, T.; Wcisło, A. In Pursuit of Key Features for Constructing Electrochemical Biosensors—Electrochemical and Acid-Base Characteristic of Self-Assembled Monolayers on Gold. Supramol. Chem. 2020, 32, 256–266. [Google Scholar] [CrossRef]
- Prodromidis, M.I. Impedimetric Immunosensors—A Review. Electrochim. Acta 2010, 55, 4227–4233. [Google Scholar] [CrossRef]
- Ho, J.A.; Hsu, W.-L.; Liao, W.-C.; Chiu, J.-K.; Chen, M.-L.; Chang, H.-C.; Li, C.-C. Ultrasensitive Electrochemical Detection of Biotin Using Electrically Addressable Site-Oriented Antibody Immobilization Approach via Aminophenyl Boronic Acid. Biosens. Bioelectron. 2010, 26, 1021–1027. [Google Scholar] [CrossRef]
- Niedzialkowski, P.; Slepski, P.; Wysocka, J.; Chamier-Cieminska, J.; Burczyk, L.; Sobaszek, M.; Wcislo, A.; Ossowski, T.; Bogdanowicz, R.; Ryl, J. Multisine Impedimetric Probing of Biocatalytic Reactions for Label-Free Detection of DEFB1 Gene: How to Verify That Your Dog Is Not Human? Sens. Actuators B Chem. 2020, 323, 128664. [Google Scholar] [CrossRef]
- Niedziałkowski, P.; Bojko, M.; Ryl, J.; Wcisło, A.; Spodzieja, M.; Magiera-Mularz, K.; Guzik, K.; Dubin, G.; Holak, T.A.; Ossowski, T.; et al. Ultrasensitive Electrochemical Determination of the Cancer Biomarker Protein SPD-L1 Based on a BMS-8-Modified Gold Electrode. Bioelectrochemistry 2021, 139, 107742. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Tang, T.; Jed Harrison, D.; Lee, W.E.; Jemere, A.B. A Regenerating Ultrasensitive Electrochemical Impedance Immunosensor for the Detection of Adenovirus. Biosens. Bioelectron. 2015, 68, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G.F.; Moore, E.J. Electrochemical Immunosensors for Food Analysis: A Review of Recent Developments. Anal. Lett. 2017, 50, 1–32. [Google Scholar] [CrossRef]
- Jayanthi, V.S.P.K.S.A.; Das, A.B.; Saxena, U. Recent Advances in Biosensor Development for the Detection of Cancer Biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef]
- Gabriunaite, I.; Valiūnienė, A.; Poderyte, M.; Ramanavicius, A. Silane-Based Self-Assembled Monolayer Deposited on Fluorine Doped Tin Oxide as Model System for Pharmaceutical and Biomedical Analysis. J. Pharm. Biomed. Anal. 2020, 177, 112832. [Google Scholar] [CrossRef]
- Miyata, T.; Hikosaka, T.; Minami, T. High Sensitivity Chlorine Gas Sensors Using Multicomponent Transparent Conducting Oxide Thin Films. Sens. Actuators B Chem. 2000, 69, 16–21. [Google Scholar] [CrossRef]
- Minami, T. Chapter Five—Transparent Conductive Oxides for Transparent Electrode Applications. In Semiconductors and Semimetals; Svensson, B.G., Pearton, S.J., Jagadish, C., Eds.; Oxide Semiconductors; Elsevier: Amsterdam, The Netherlands, 2013; Volume 88, pp. 159–200. [Google Scholar]
- Shim, Y.-S.; Moon, H.G.; Kim, D.H.; Jang, H.W.; Kang, C.-Y.; Yoon, Y.S.; Yoon, S.-J. Transparent Conducting Oxide Electrodes for Novel Metal Oxide Gas Sensors. Sens. Actuators B Chem. 2011, 160, 357–363. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Hu, S. Nanocomposites of Graphene and Graphene Oxides: Synthesis, Molecular Functionalization and Application in Electrochemical Sensors and Biosensors. A Review. Microchim Acta 2017, 184, 1–44. [Google Scholar] [CrossRef]
- Kim, C.-L.; Jung, C.-W.; Oh, Y.-J.; Kim, D.-E. A Highly Flexible Transparent Conductive Electrode Based on Nanomaterials. NPG Asia Mater. 2017, 9, e438. [Google Scholar] [CrossRef]
- Takamatsu, S.; Takahata, T.; Muraki, M.; Iwase, E.; Matsumoto, K.; Shimoyama, I. Transparent Conductive-Polymer Strain Sensors for Touch Input Sheets of Flexible Displays. J. Micromech. Microeng. 2010, 20, 075017. [Google Scholar] [CrossRef]
- Lee, K.-T.; Liu, D.-M.; Liang, Y.-Y.; Matsushita, N.; Ikoma, T.; Lu, S.-Y. Porous Fluorine-Doped Tin Oxide as a Promising Substrate for Electrochemical Biosensors—Demonstration in Hydrogen Peroxide Sensing. J. Mater. Chem. B 2014, 2, 7779–7784. [Google Scholar] [CrossRef]
- Banyamin, Z.; Kelly, P.; West, G.; Boardman, J. Electrical and Optical Properties of Fluorine Doped Tin Oxide Thin Films Prepared by Magnetron Sputtering. Coatings 2014, 4, 732–746. [Google Scholar] [CrossRef]
- Bierwagen, O. Indium Oxide—A Transparent, Wide-Band Gap Semiconductor for (Opto)Electronic Applications. Semicond. Sci. Technol. 2015, 30, 024001. [Google Scholar] [CrossRef]
- Ouerfelli, J.; Djobo, S.O.; Bernède, J.C.; Cattin, L.; Morsli, M.; Berredjem, Y. Organic Light Emitting Diodes Using Fluorine Doped Tin Oxide Thin Films, Deposited by Chemical Spray Pyrolysis, as Anode. Mater. Chem. Phys. 2008, 112, 198–201. [Google Scholar] [CrossRef]
- Jasiecki, S.; Czupryniak, J.; Ossowski, T.; Schroeder, G. FTO Coated Glass Electrode Functionalization with Transition Metal Cations Receptors via Electrostatic Self-Assembly. Int. J. Electrochem. Sci. 2013, 8, 12543–12556. [Google Scholar]
- Ahuja, T.; Rajesh; Kumar, D.; Tanwar, V.K.; Sharma, V.; Singh, N.; Biradar, A.M. An Amperometric Uric Acid Biosensor Based on Bis[Sulfosuccinimidyl] Suberate Crosslinker/3-Aminopropyltriethoxysilane Surface Modified ITO Glass Electrode. Thin Solid Film. 2010, 519, 1128–1134. [Google Scholar] [CrossRef]
- Kim, C.O.; Hong, S.-Y.; Kim, M.; Park, S.-M.; Park, J.W. Modification of Indium–Tin Oxide (ITO) Glass with Aziridine Provides a Surface of High Amine Density. J. Colloid Interface Sci. 2004, 277, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Pruna, R.; Palacio, F.; Martínez, M.; Blázquez, O.; Hernández, S.; Garrido, B.; López, M. Organosilane-Functionalization of Nanostructured Indium Tin Oxide Films. Interface Focus 2016, 6, 20160056. [Google Scholar] [CrossRef] [PubMed]
- Muthurasu, A.; Ganesh, V. Electrochemical Characterization of Self-Assembled Monolayers (SAMs) of Silanes on Indium Tin Oxide (ITO) Electrodes—Tuning Electron Transfer Behaviour across Electrode–Electrolyte Interface. J. Colloid Interface Sci. 2012, 374, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Göbel, G.; Talke, A.; Lisdat, F. FTO—An Electrode Material for the Stable Electrochemical Determination of Dopamine. Electroanalysis 2018, 30, 225–229. [Google Scholar] [CrossRef]
- Wang, P.; Li, S.; Kan, J. A Hydrogen Peroxide Biosensor Based on Polyaniline/FTO. Sens. Actuators B Chem. 2009, 137, 662–668. [Google Scholar] [CrossRef]
- Naderi Asrami, P.; Mozaffari, S.A.; Saber Tehrani, M.; Aberoomand Azar, P. A Novel Impedimetric Glucose Biosensor Based on Immobilized Glucose Oxidase on a CuO-Chitosan Nanobiocomposite Modified FTO Electrode. Int. J. Biol. Macromol. 2018, 118, 649–660. [Google Scholar] [CrossRef]
- Valiūnienė, A.; Kavaliauskaitė, G.; Virbickas, P.; Ramanavičius, A. Prussian Blue Based Impedimetric Urea Biosensor. J. Electroanal. Chem. 2021, 895, 115473. [Google Scholar] [CrossRef]
- sadat Vajedi, F.; Dehghani, H. A High-Sensitive Electrochemical DNA Biosensor Based on a Novel ZnAl/Layered Double Hydroxide Modified Cobalt Ferrite-Graphene Oxide Nanocomposite Electrophoretically Deposited onto FTO Substrate for Electroanalytical Studies of Etoposide. Talanta 2020, 208, 120444. [Google Scholar] [CrossRef]
- Terracciano, M.; Rea, I.; Politi, J.; De Stefano, L. Optical Characterization of Aminosilane-Modified Silicon Dioxide Surface for Biosensing. J. Eur. Opt. Soc.-Rapid Publ. 2013, 8, 13075. [Google Scholar] [CrossRef]
- Acres, R.G.; Ellis, A.V.; Alvino, J.; Lenahan, C.E.; Khodakov, D.A.; Metha, G.F.; Andersson, G.G. Molecular Structure of 3-Aminopropyltriethoxysilane Layers Formed on Silanol-Terminated Silicon Surfaces. J. Phys. Chem. C 2012, 116, 6289–6297. [Google Scholar] [CrossRef]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P.; et al. Antibiofilm Activity of a Monolayer of Silver Nanoparticles Anchored to an Amino-Silanized Glass Surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Ashur, I.; Jones, A.K. Immobilization of Azurin with Retention of Its Native Electrochemical Properties at Alkylsilane Self-Assembled Monolayer Modified Indium Tin Oxide. Electrochim. Acta 2012, 85, 169–174. [Google Scholar] [CrossRef]
- Sanli, S.; Ghorbani-Zamani, F.; Moulahoum, H.; Gumus, Z.P.; Coskunol, H.; Odaci Demirkol, D.; Timur, S. Application of Biofunctionalized Magnetic Nanoparticles Based-Sensing in Abused Drugs Diagnostics. Anal. Chem. 2020, 92, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Venkata Jagadeesh, R.; Lakshminarayanan, V. Electrochemical Investigation on Adsorption Kinetics of Long Chain Alkylsilanes and Influence of Solvents on Their Self-Assembly and Electron Transfer Behavior on Indium Tin Oxide. J. Appl. Electrochem. 2020, 50, 1129–1138. [Google Scholar] [CrossRef]
- Li, H.; Su, T.A.; Camarasa-Gómez, M.; Hernangómez-Pérez, D.; Henn, S.E.; Pokorný, V.; Caniglia, C.D.; Inkpen, M.S.; Korytár, R.; Steigerwald, M.L.; et al. Silver Makes Better Electrical Contacts to Thiol-Terminated Silanes than Gold. Angew. Chem. Int. Ed. 2017, 56, 14145–14148. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.-T. Transparent, Conductive and Superhydrophobic Cellulose Films for Flexible Electrode Application. RSC Adv. 2021, 11, 36607–36616. [Google Scholar] [CrossRef]
- Moses, P.R.; Wier, L.M.; Lennox, J.C.; Finklea, H.O.; Lenhard, J.R.; Murray, R.W. X-ray Photoelectron Spectroscopy of Alkylaminesilanes Bound to Metal Oxide Electrodes. Anal. Chem. 1978, 50, 576–585. [Google Scholar] [CrossRef]
- Zhu, M.; Lerum, M.Z.; Chen, W. How To Prepare Reproducible, Homogeneous, and Hydrolytically Stable Aminosilane-Derived Layers on Silica. Langmuir 2012, 28, 416–423. [Google Scholar] [CrossRef]
- Cirocka, A.; Zarzeczańska, D.; Wcisło, A. Good Choice of Electrode Material as the Key to Creating Electrochemical Sensors—Characteristics of Carbon Materials and Transparent Conductive Oxides (TCO). Materials 2021, 14, 4743. [Google Scholar] [CrossRef]
- Kern, W. Others Handbook of Semiconductor Wafer Cleaning Technology; Noyes Publication: Park Ridge, NJ, USA, 1993; pp. 111–196. [Google Scholar]
- Cirocka, A.; Zarzeczańska, D.; Wcisło, A.; Ryl, J.; Bogdanowicz, R.; Finke, B.; Ossowski, T. Tuning of the Electrochemical Properties of Transparent Fluorine-Doped Tin Oxide Electrodes by Microwave Pulsed-Plasma Polymerized Allylamine. Electrochim. Acta 2019, 313, 432–440. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact Angle Measurement and Contact Angle Interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact Angle Measurements and Interpretation: Wetting Behavior and Solid Surface Tensions for Poly(Alkyl Methacrylate) Polymers. J. Adhes. Sci. Technol. 2000, 14, 719–743. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Gietzelt, T.; Grundke, K.; Jacobasch, H.-J.; Neumann, A.W. Contact Angle Measurements and Contact Angle Interpretation. 1. Contact Angle Measurements by Axisymmetric Drop Shape Analysis and a Goniometer Sessile Drop Technique. Langmuir 1997, 13, 2880–2894. [Google Scholar] [CrossRef]
- Orazem, M.E.; Pébère, N.; Tribollet, B. Enhanced Graphical Representation of Electrochemical Impedance Data. J. Electrochem. Soc. 2006, 153, B129. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer-Verlag: New York, NY, USA, 2014; ISBN 978-1-4614-8932-0. [Google Scholar]
- Song, X.; Zhai, J.; Wang, Y.; Jiang, L. Self-Assembly of Amino-Functionalized Monolayers on Silicon Surfaces and Preparation of Superhydrophobic Surfaces Based on Alkanoic Acid Dual Layers and Surface Roughening. J. Colloid Interface Sci. 2006, 298, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Ogale, S.B.; Vijayamohanan, K.P. Tuning the Hydrophobic Properties of Silica Particles by Surface Silanization Using Mixed Self-Assembled Monolayers. J. Colloid Interface Sci. 2008, 318, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xu, G.; Gao, Y.; An, Y. Surface Wettability of (3-Aminopropyl)Triethoxysilane Self-Assembled Monolayers. J. Phys. Chem. B 2011, 115, 450–454. [Google Scholar] [CrossRef]
- Siqueira Petri, D.F.; Wenz, G.; Schunk, P.; Schimmel, T. An Improved Method for the Assembly of Amino-Terminated Monolayers on SiO2 and the Vapor Deposition of Gold Layers. Langmuir 1999, 15, 4520–4523. [Google Scholar] [CrossRef]


| Silane | Acronym | Molecular Structure |
|---|---|---|
| 3-aminopropyltrimethoxysilane | APTMS | 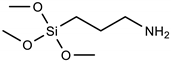 |
| Trimethoxy(propyl)silane | PTMS | 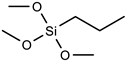 |
| trimethoxy(octyl)silane | OTMS |  |
| Silane | Reaction Conditions (Temperature, Time) | Electrode | Second Reaction Conditions (Temperature, Time) | Electrode |
|---|---|---|---|---|
| APTMS | 23 °C; 16 h | FTO/APTMS (16 h) | 23 °C; 16 h | FTO/APTMS/OTMS (16 h) |
| 23 °C; 72 h | FTO/APTMS (72 h) | 23 °C; 72 h | FTO/APTMS/OTMS (72 h) | |
| 50 °C; 0.5 h | FTO/APTMS (0.5 h) | |||
| PTMS | 50 °C; 0.5 h | FTO/PTMS (0.5 h) | ||
| OTMS | 23 °C; 16 h | FTO/OTMS (16 h) | 23 °C; 16 h | FTO/OTMS/APTMS (16 h) |
| 23 °C; 72 h | FTO/OTMS (72 h) | 23 °C; 72 h | FTO/OTMS/APTMS (72 h) | |
| 50 °C; 0.5 h | FTO/OTMS (0.5 h) | |||
| APTMS and OTMS | 23 °C; 16 h | FTO/APTMS_OTMS (16 h) | ||
| 23 °C; 72 h | FTO/APTMS_OTMS (72 h) |
| Electrode | Rct [Ω] | Q [µF] | n | W [µSs½] | Chi2 | Ea [V] | Ek [V] | ΔE [V] | Ia [µA] | Ik [µA] |
|---|---|---|---|---|---|---|---|---|---|---|
| FTO | 57.87 | 6.65 | 0.91 | 0.012 | 3.27 × 10−4 | 0.233 | −0.026 | 0.259 | 683.3 | −583.2 |
| FTO/APTMS (16 h) * | 48.46 | 8.28 | 0.88 | 0.012 | 6.19 × 10−4 | 0.226 | −0.012 | 0.238 | 676.3 | −594.2 |
| FTO/APTMS (72 h) * | 30.74 | 11.23 | 0.79 | 0.012 | 2.69 × 10−4 | 0.233 | 0.016 | 0.217 | 632.3 | −600.9 |
| FTO/OTMS (16 h) * | 426.40 | 5.69 | 0.89 | 0.015 | 7.31 × 10−4 | 0.384 | −0.209 | 0.593 | 399.2 | −376.9 |
| FTO/OTMS (72 h) * | 1545.00 | 3.52 | 0.84 | 0.004 | 3.47 × 10−4 | 0.521 | −0.311 | 0.832 | 298.5 | −326.8 |
| FTO/APTMS_ OTMS (16 h) * | 55.05 | 8.03 | 0.88 | 0.012 | 5.85 × 10−4 | 0.244 | −0.016 | 0.260 | 609.1 | −591.4 |
| FTO/APTMS_ OTMS (72 h) ** | 22,450.00 | 0.25 | 0.86 | - | 8.04 × 10−4 | 0.676 | −0.514 | 1.190 | 180.3 | −195.1 |
| FTO/APTMS/ OTMS (16 h) ** | 336.20 | 6.35 | 0.87 | - | 1.05 × 10−3 | 0.346 | −0.167 | 0.513 | 478.2 | −429.3 |
| FTO/APTMS/ OTMS (72 h) * | 1664.00 | 1.36 | 0.89 | 0.007 | 2.27 × 10−4 | 0.546 | −0.423 | 0.969 | 262.6 | −288.1 |
| FTO/OTMS/ APTMS (16 h) * | 439.30 | 4.39 | 0.89 | 0.009 | 4.91 × 10−4 | 0.388 | −0.219 | 0.607 | 403.4 | −406.6 |
| FTO/OTMS/ APTMS (72 h) * | 98.19 | 6.86 | 0.88 | 0.010 | 8.54 × 10−4 | 0.293 | −0.047 | 0.340 | 506.6 | −433.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domaros, A.; Zarzeczańska, D.; Ossowski, T.; Wcisło, A. Controlled Silanization of Transparent Conductive Oxides as a Precursor of Molecular Recognition Systems. Materials 2023, 16, 309. https://doi.org/10.3390/ma16010309
Domaros A, Zarzeczańska D, Ossowski T, Wcisło A. Controlled Silanization of Transparent Conductive Oxides as a Precursor of Molecular Recognition Systems. Materials. 2023; 16(1):309. https://doi.org/10.3390/ma16010309
Chicago/Turabian StyleDomaros, Anna, Dorota Zarzeczańska, Tadeusz Ossowski, and Anna Wcisło. 2023. "Controlled Silanization of Transparent Conductive Oxides as a Precursor of Molecular Recognition Systems" Materials 16, no. 1: 309. https://doi.org/10.3390/ma16010309
APA StyleDomaros, A., Zarzeczańska, D., Ossowski, T., & Wcisło, A. (2023). Controlled Silanization of Transparent Conductive Oxides as a Precursor of Molecular Recognition Systems. Materials, 16(1), 309. https://doi.org/10.3390/ma16010309






