Effect of CO2 on the Desulfurization of Sintering Flue Gas with Hydrated Lime
Abstract
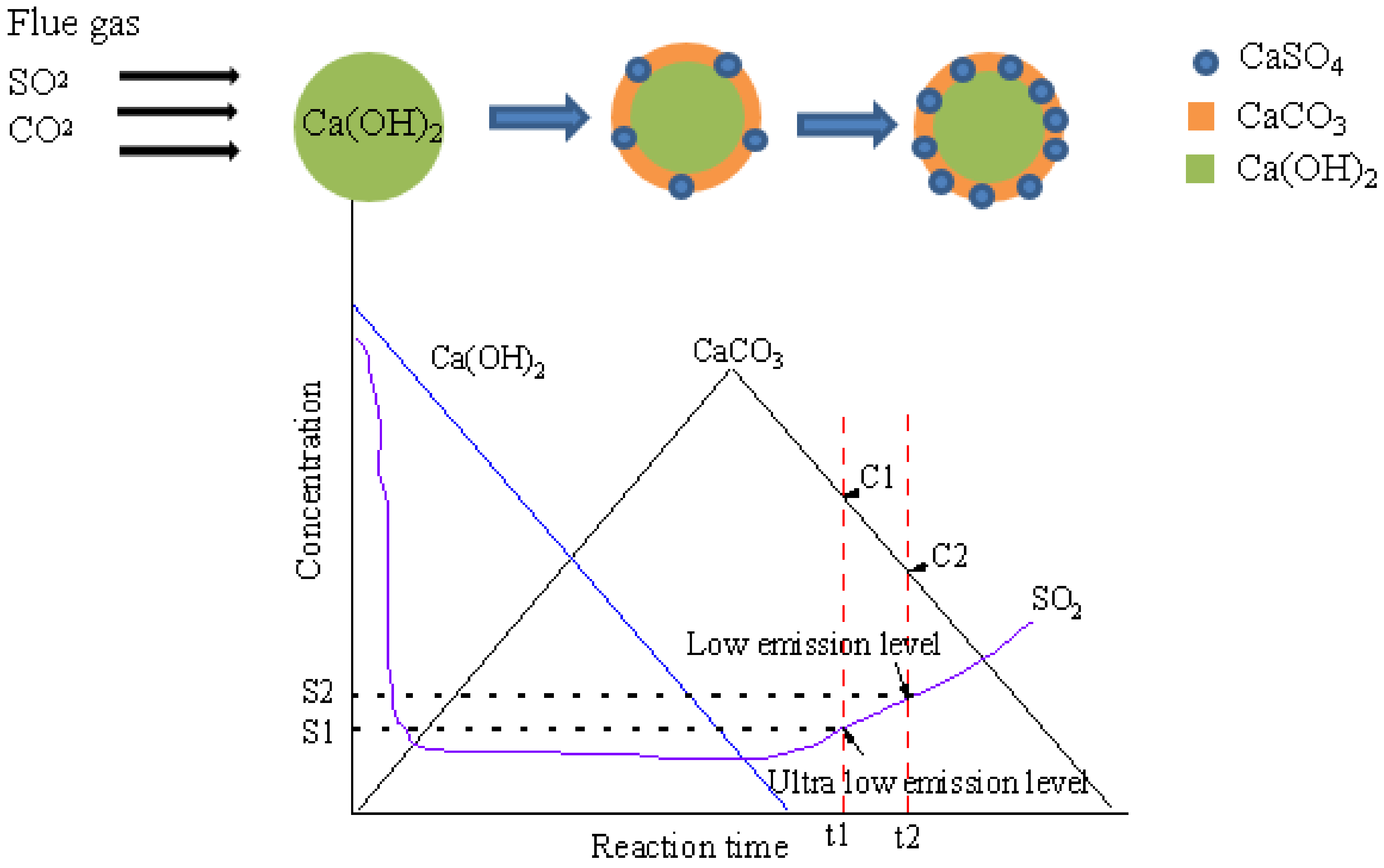
1. Introduction
2. Materials and Methods
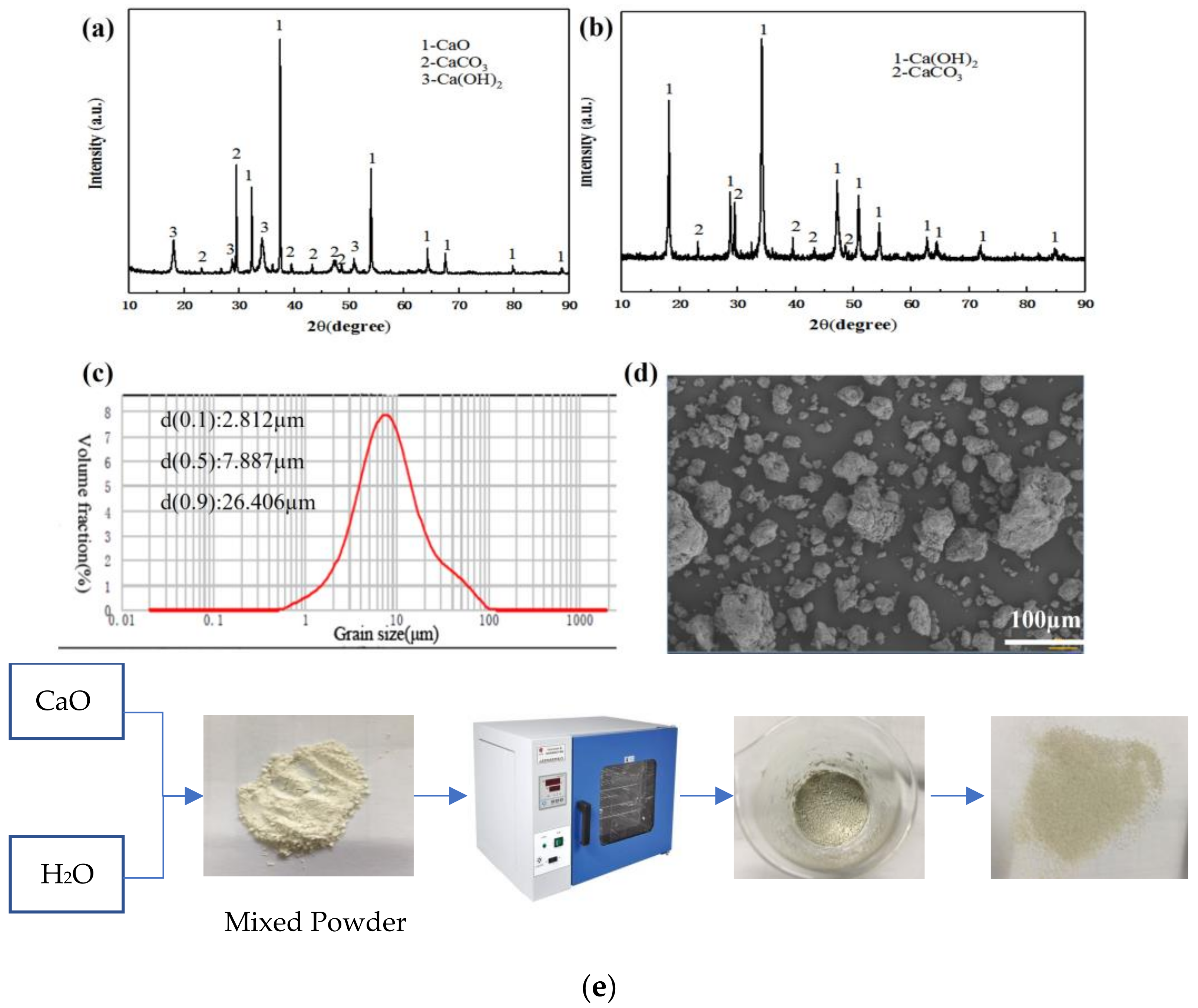
2.1. Experimental Materials
2.2. Experimental Methods
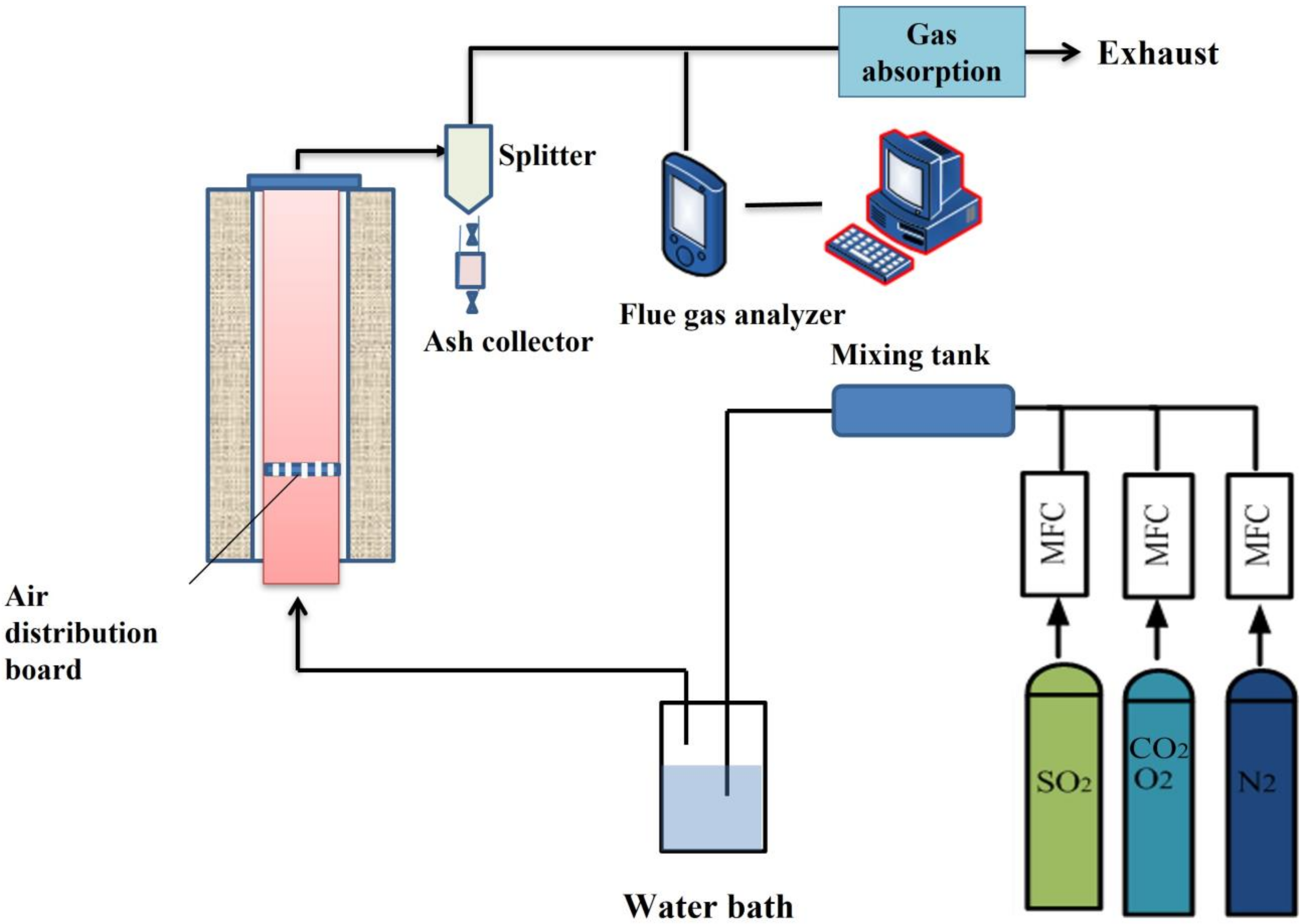
2.2.1. Experimental Device
2.2.2. Experimental Methods
- 1.
- Relative humidity calculation
- 2.
- Desulfurization ash composition analysis
2.2.3. Evaluations
- 1.
- Desulfurization efficiency
- 2.
- Penetration time
- 3.
- Penetration sulfur capacity
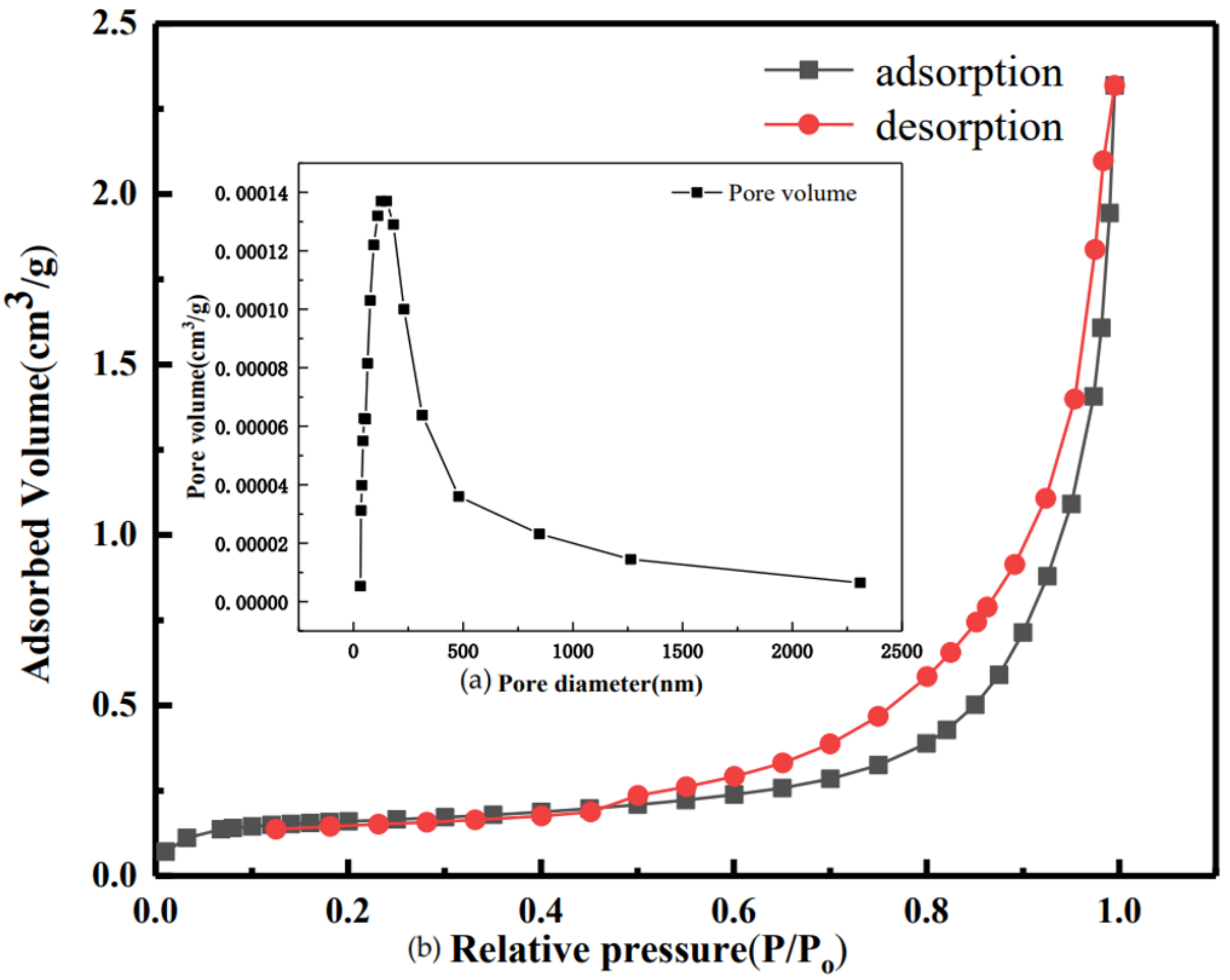
2.2.4. Characterization Methods
3. Results and Discussion
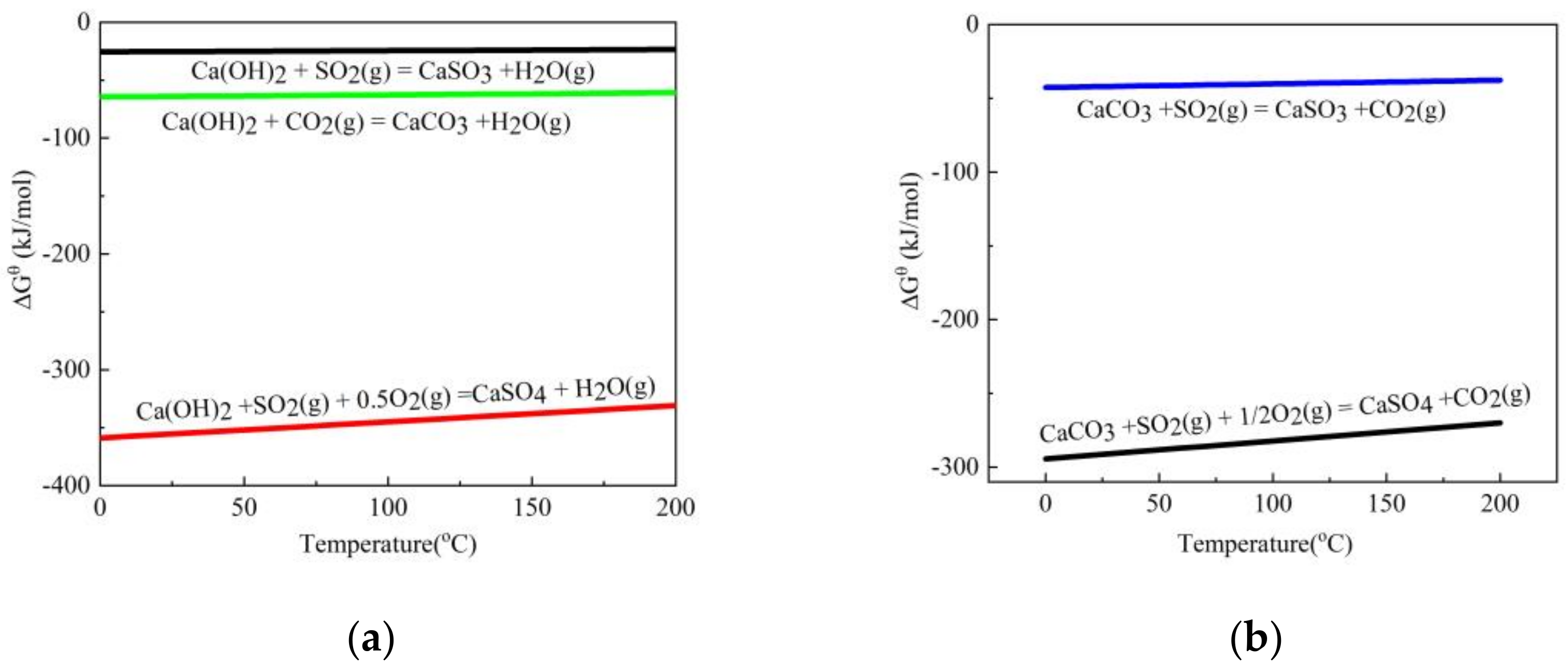
3.1. Thermodynamic Calculation
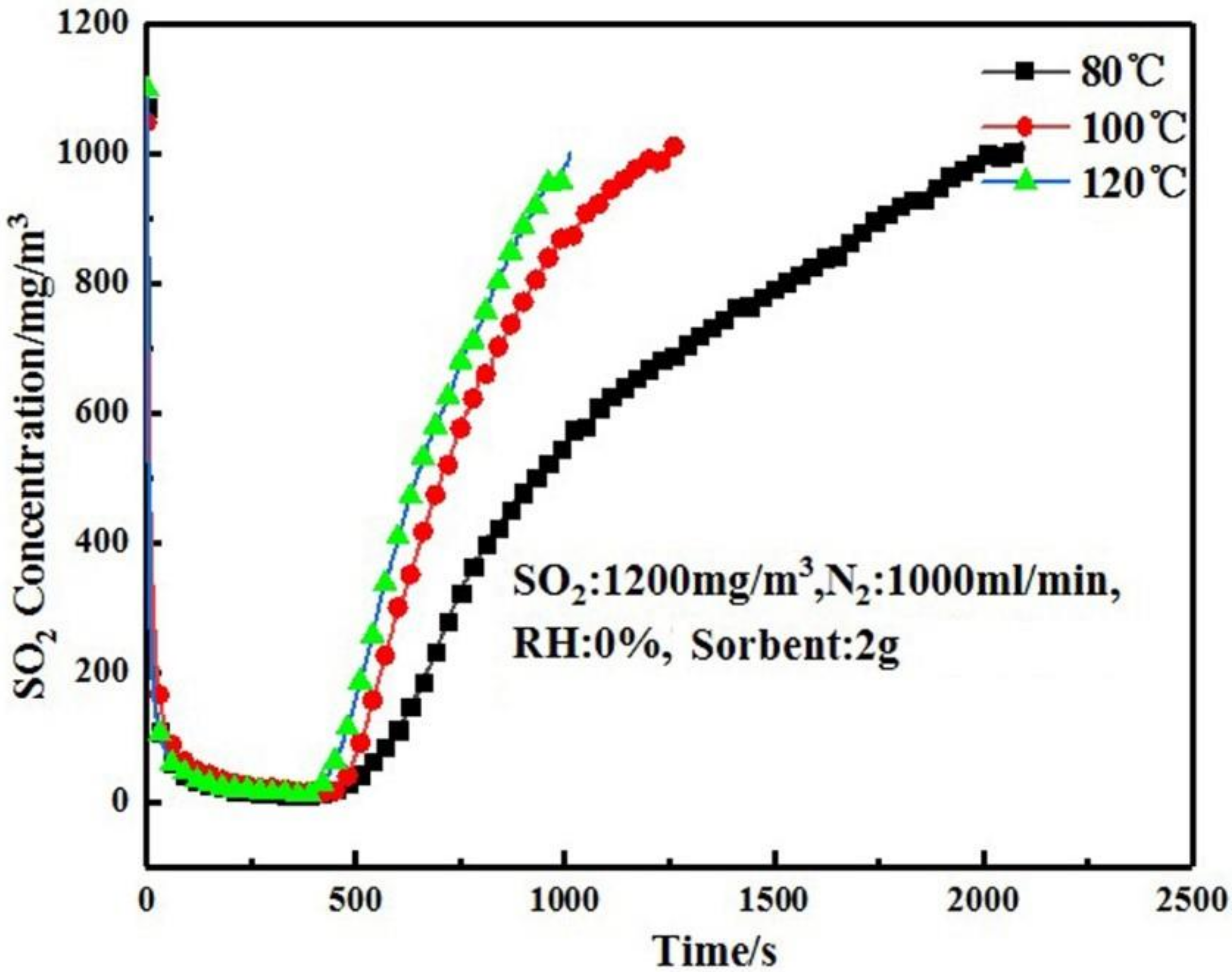
3.2. Effect of Temperature on Desulfurization Performance of Adsorbent
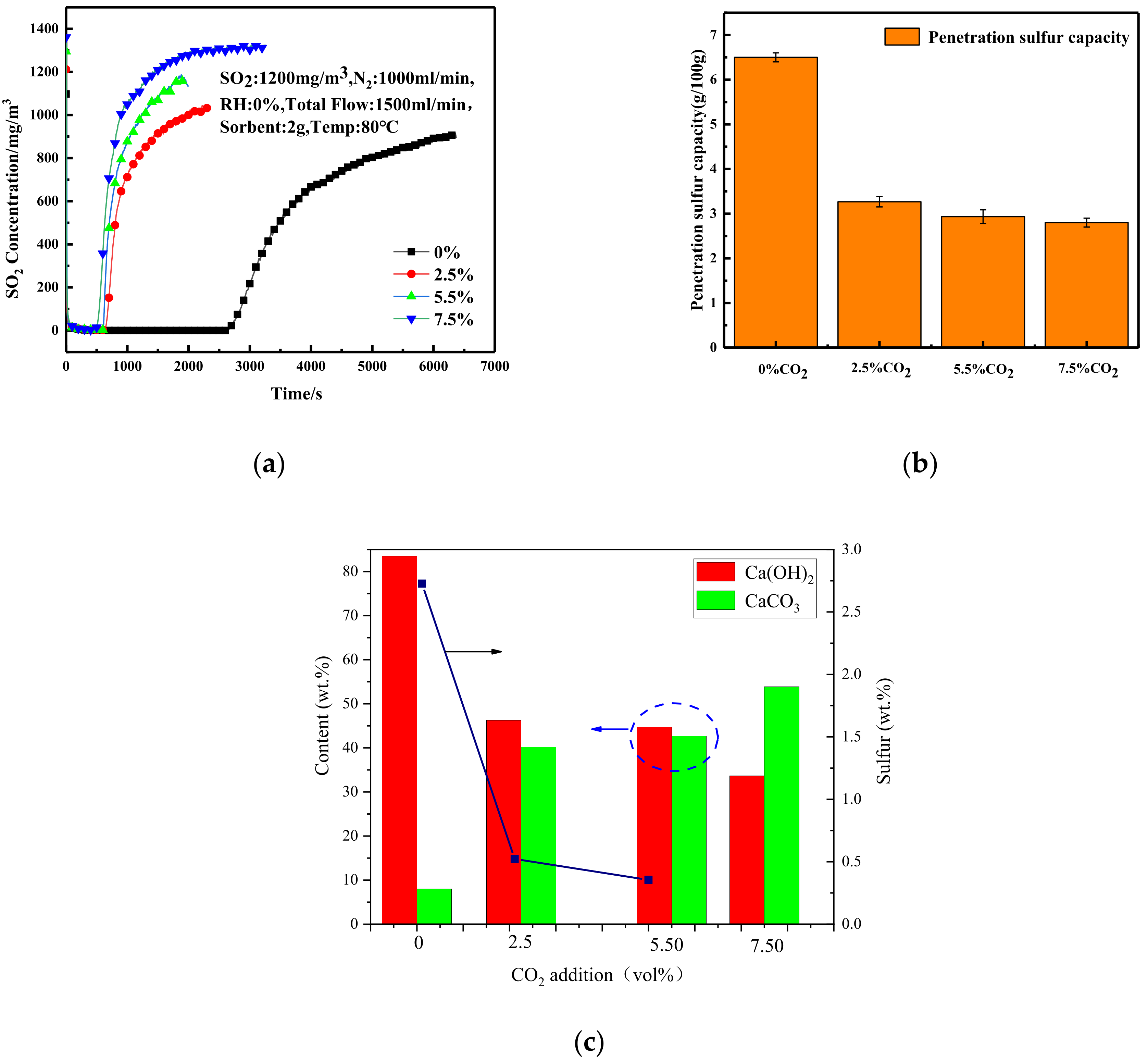
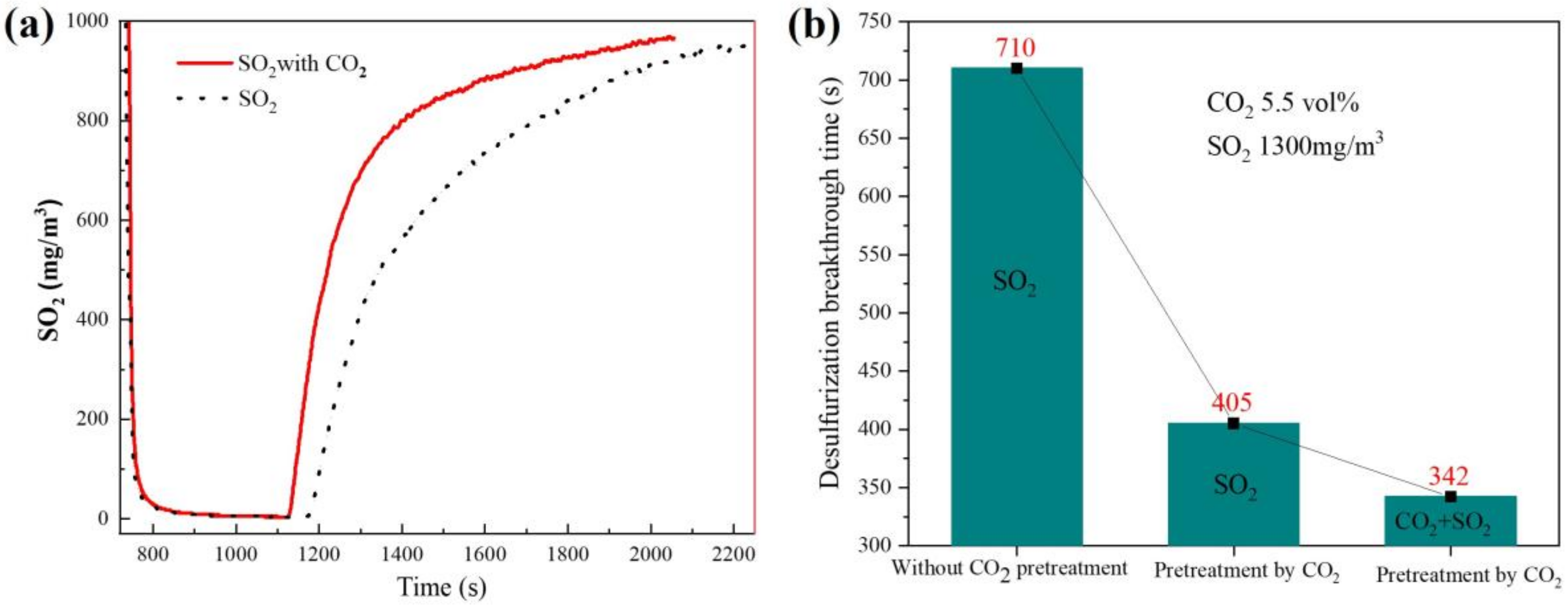
3.3. Effect of CO2 Concentration on Desulfurization Performance of Adsorbent
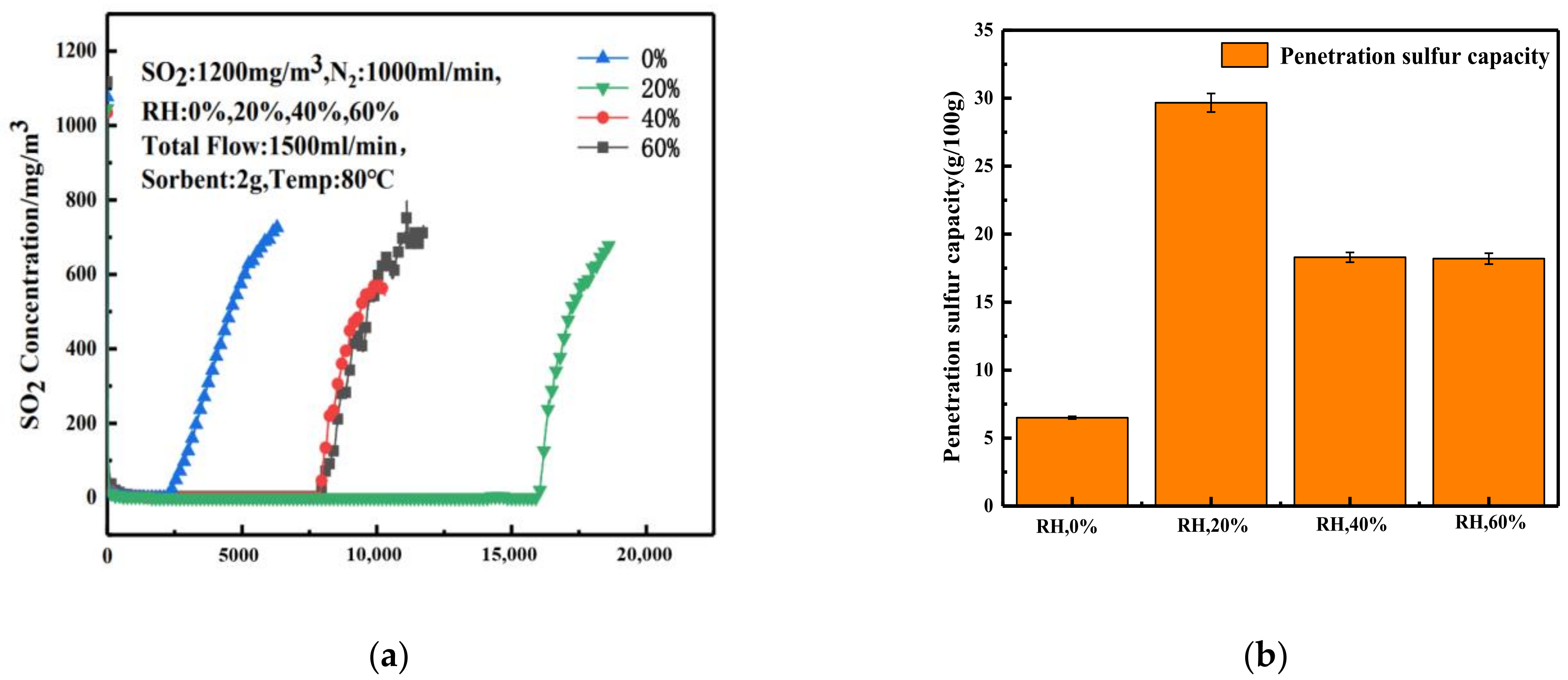
3.4. Effect of Relative Humidity on Desulfurization Performance of Adsorbent
3.4.1. Desulfurization Performance of Adsorbent
3.4.2. Morphology and Pore Structure of Desulfurized Ash
3.4.3. Composition Analysis of Desulfurization Ash
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, T.; Liu, Q.; Li, Y.; Yan, X.; Qi, F.; Ye, M. Emission Characteristics of Multiple Pollutants from Iron- Steel Sintering Flue Gas and Review of Control Technologies. Sci. Technol. Rev. 2014, 32, 51–56. [Google Scholar]
- Ke, X.W.; Cai, R.X.; Lyu, J.F.; Zhang, M.; Wu, Y.; Yang, H.R.; Zhang, H. Research Progress of the Effects of Ca-Based Sorbents on the NO_x Reaction in Circulating Fluidized Bed Boilers. Clean Coal Technol. 2019, 25, 1–11. [Google Scholar]
- Hao, R.; Zhang, Y.; Wang, Z.; Li, Y.; Yuan, B.; Mao, X.; Zhao, Y. An Advanced Wet Method for Simultaneous Removal of SO2 and NO from Coal-Fired Flue Gas by Utilizing a Complex Absorbent. Chem. Eng. J. 2017, 307, 562–571. [Google Scholar] [CrossRef]
- Du, J.; Cao, S. Research Status and Progress of Wet Flue Gas Desulfurization Technology. Appl. Chem. Ind. 2019, 48, 1495–1500. [Google Scholar]
- Ahlbeck, J.; Engman, T.; Fältén, S.; Vihma, M. A Method for Measuring the Reactivity of Absorbents for Wet Flue Gas Desulfurization. Chem. Eng. Sci. 1993, 48, 3479–3484. [Google Scholar] [CrossRef]
- Mohammed, M.Y.; Albayati, T.M.; Ali, A.M. Imidazolium-Based Ionic Liquids for Extraction of Sulfur Compounds from Real Heavy Crude Oil. Chem. Afr. 2022, 5, 1715–1722. [Google Scholar] [CrossRef]
- Mohammed, M.Y.; Ali, A.M.; Albayati, T.M. Choline Chloride-Based Deep Eutectic Solvents for Ultrasonic-Assisted Oxidative Desulfurization of Actual Heavy Crude Oil. Chem. Eng. Res. Des. 2022, 182, 659–666. [Google Scholar] [CrossRef]
- Khadim, A.T.; Albayati, T.M.; Cata Saady, N.M. Removal of Sulfur Compounds from Real Diesel Fuel Employing the Encapsulated Mesoporous Material Adsorbent Co/MCM-41 in a Fixed-Bed Column. Micropor. Mesopor. Mat. 2022, 341, 112020. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Liu, Y.; Dou, Z.; Zhang, T. Summary of Research Progress on Industrial Flue Gas Desulfurization Technology. Sep. Purif. Technol. 2022, 281, 119849. [Google Scholar] [CrossRef]
- Sun, L.; Li, K.; Tang, L.; Liu, N.; Ning, P.; Sun, X.; Zhang, X. Research Progress of Common Metal Oxides for Flue Gas Desulfurization. Chem. Ind. Eng. Prog. 2017, 36, 181–188. [Google Scholar] [CrossRef]
- Liu, F.; Cai, M.; Liu, X.; Zhu, T.; Zou, Y. O3 Oxidation Combined with Semi-Dry Method for Simultaneous Desulfurization and Denitrification of Sintering/Pelletizing Flue Gas. J. Environ. Sci. 2021, 104, 253–263. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, Y.; Song, F.; Zhang, S.; Zhong, Q. Active Sites Assembly Effect on CeO2-WO3-TiO2 Catalysts for Selective Catalytic Reduction of NO with NH3. Mol. Catal. 2019, 479, 110549. [Google Scholar] [CrossRef]
- Han, K.; Lu, C. Kinetic Model and Simulation of Promoted Selective Non-Catalytic Reduction by Sodium Carbonate. Chin. J. Chem. Eng. 2007, 15, 512–519. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Zhu, T.; Ye, M. Effects of Concentration and Adsorption Product on the Adsorption of SO2 and NO on Activated Carbon. Energy Fuels 2013, 27, 360–366. [Google Scholar] [CrossRef]
- Li, B.; Wu, H.; Liu, X.; Zhu, T.; Liu, F.; Zhao, X. Simultaneous Removal of SO2 and NO Using a Novel Method with Red Mud as Absorbent Combined with O3 Oxidation. J. Hazard. Mater. 2020, 392, 122270. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Z.; Lin, F.; Kuang, M.; Whiddon, R.; He, Y.; Liu, J. Characteristics of O3 Oxidation for Simultaneous Desulfurization and Denitration with Limestone–Gypsum Wet Scrubbing: Application in a Carbon Black Drying Kiln Furnace. Energy Fuels 2016, 30, 2302–2308. [Google Scholar] [CrossRef]
- Liu, C.; Shih, S. A Surface Coverage Model for the Reaction of Ca(OH)2 with SO2 at Low Temperatures. J. Chin. Inst. Chem. Eng. 2002, 33, 407–413. [Google Scholar]
- Lin, R.; Shih, S.; Liu, C. Characteristics and Reactivities of Ca(OH)2/Silica Fume Sorbents for Low-Temperature Flue Gas Desulfurization. Chem. Eng. Sci. 2003, 58, 3659–3668. [Google Scholar] [CrossRef]
- Liu, D.; Kuriyama, A. Influence between Desulfurization and Defluorination Reactions Using Ca(OH)2 in the Simulated Coal-Briquettes. J. Jpn. Inst. Energy 2019, 98, 44–51. [Google Scholar] [CrossRef]
- Lin, R.; Shih, S.; Liu, C. Structural Properties and Reactivities of Ca(OH)2/Fly Ash Sorbents for Flue Gas Desulfurization. Ind. Eng. Chem. Res. 2003, 42, 1350–1356. [Google Scholar] [CrossRef]
- Renedo, M.; Gonzalez, F.; Pesquera, C.; Fernandez, J. Study of Sorbents Prepared from Clays and CaO or Ca(OH)2 for SO2 Removal at Low Temperature. Ind. Eng. Chem. Res. 2006, 45, 3752–3757. [Google Scholar] [CrossRef]
- Wang, W.; Hu, M.; Dong, Y.; Ma, C. Study on the Effect of CO2 on the Consumption of Desulfurizing Agent Ca(OH)2 in Flue Gas Desulfurization. Ind. Eng. Chem. Res. 2010, 49, 1444–1449. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, H.; Ye, S.; Xie, Y.; Chen, Y. Ca-Based Sorbents Modified with Humic Acid for Flue Gas Desulfurization. Ind. Eng. Chem. Res. 2006, 45, 7120–7125. [Google Scholar] [CrossRef]
- Ghosh-Dastidar, A.; Mahuli, S.; Agnihotri, R.; Fan, L.-S. Ultrafast Calcination and Sintering of Ca(OH)2 Powder: Experimental and Modeling. Chem. Eng. Sci. 1995, 50, 2029–2040. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Yue, S.; Wang, C. Microstructure Evolution of Procedural Products during Limestone Simultaneous Calcination/Sulfation. CIESC J. 2018, 69, 2149–2157. [Google Scholar]
- Anthony, E.J.; Granatstein, D.L. Sulfation Phenomena in Fluidized Bed Combustion Systems. Prog. Energy Combust. Sci. 2001, 27, 215–236. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Ollero, P. A Realistic Approach to Modeling an In-Duct Desulfurization Process Based on an Experimental Pilot Plant Study. Chem. Eng. J. 2008, 141, 141–150. [Google Scholar] [CrossRef]
- Lyu, J.; Huang, L.; Teng, D.; Fu, H.; Li, T.; Chen, H. Effect of Relative Humidity on Dry Flue Gas Desulfurization by Calcium-Based Desulfurizer. Chin. J. Environ. Eng. 2020, 14, 1563–1569. [Google Scholar]




| Elements | Ca | Mg | Fe | Al | Si | K |
|---|---|---|---|---|---|---|
| Wt.% | 88.26 | 0.83 | 0.62 | 0.47 | 0.35 | 0.23 |
| Gas Type | Mixed Gas | SO2 | N2 |
|---|---|---|---|
| Concentration | 10%CO2 + 5%O2 + surplus N2 | 4200 ppm | 99.99% |
| Samples | BET Specific Surface Area/m2∙g−1 | Average Pore Size/nm | Pore Volume/cm3∙g−1 |
|---|---|---|---|
| Original adsorbent | 11.74 | 166.14 | 0.049 |
| 0% CO2 | 10.87 | 126.18 | 0.036 |
| 2.5% CO2 | 7.56 | 163.12 | 0.033 |
| 5.5% CO2 | 8.91 | 137.21 | 0.033 |
| 7.5% CO2 | 7.63 | 136.30 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Zou, X.; Qin, Z.; Zhou, B.; Geng, S.; Zhang, Y.; Zou, X.; Lu, X. Effect of CO2 on the Desulfurization of Sintering Flue Gas with Hydrated Lime. Materials 2023, 16, 303. https://doi.org/10.3390/ma16010303
Hong J, Zou X, Qin Z, Zhou B, Geng S, Zhang Y, Zou X, Lu X. Effect of CO2 on the Desulfurization of Sintering Flue Gas with Hydrated Lime. Materials. 2023; 16(1):303. https://doi.org/10.3390/ma16010303
Chicago/Turabian StyleHong, Jianguo, Xinqing Zou, Ziqiang Qin, Bin Zhou, Shuhua Geng, Yuwen Zhang, Xingli Zou, and Xionggang Lu. 2023. "Effect of CO2 on the Desulfurization of Sintering Flue Gas with Hydrated Lime" Materials 16, no. 1: 303. https://doi.org/10.3390/ma16010303
APA StyleHong, J., Zou, X., Qin, Z., Zhou, B., Geng, S., Zhang, Y., Zou, X., & Lu, X. (2023). Effect of CO2 on the Desulfurization of Sintering Flue Gas with Hydrated Lime. Materials, 16(1), 303. https://doi.org/10.3390/ma16010303







