Microstructure and Solute Segregation around the Melt-Pool Boundary of Orientation-Controlled 316L Austenitic Stainless Steel Produced by Laser Powder Bed Fusion
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- (1)
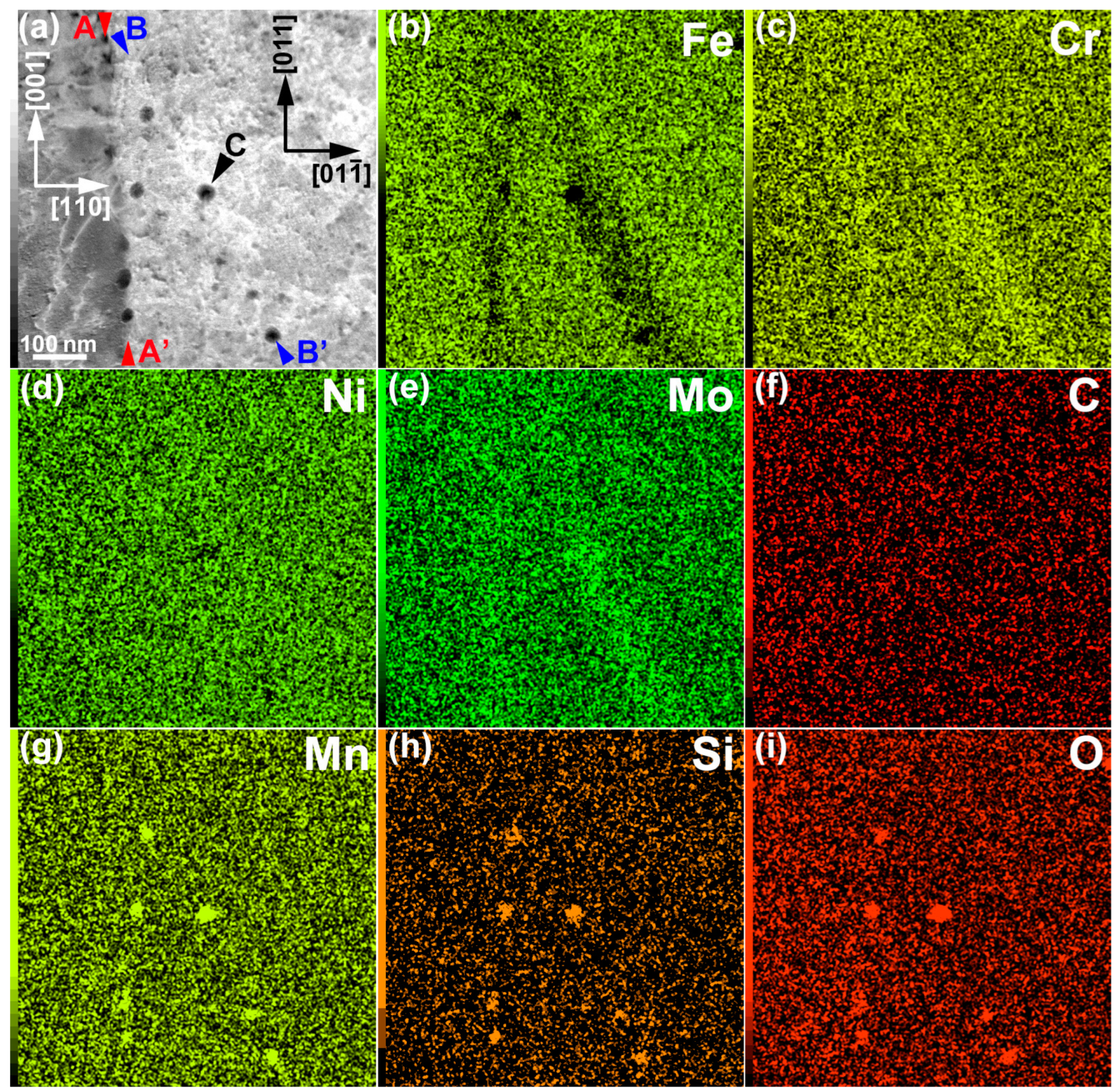
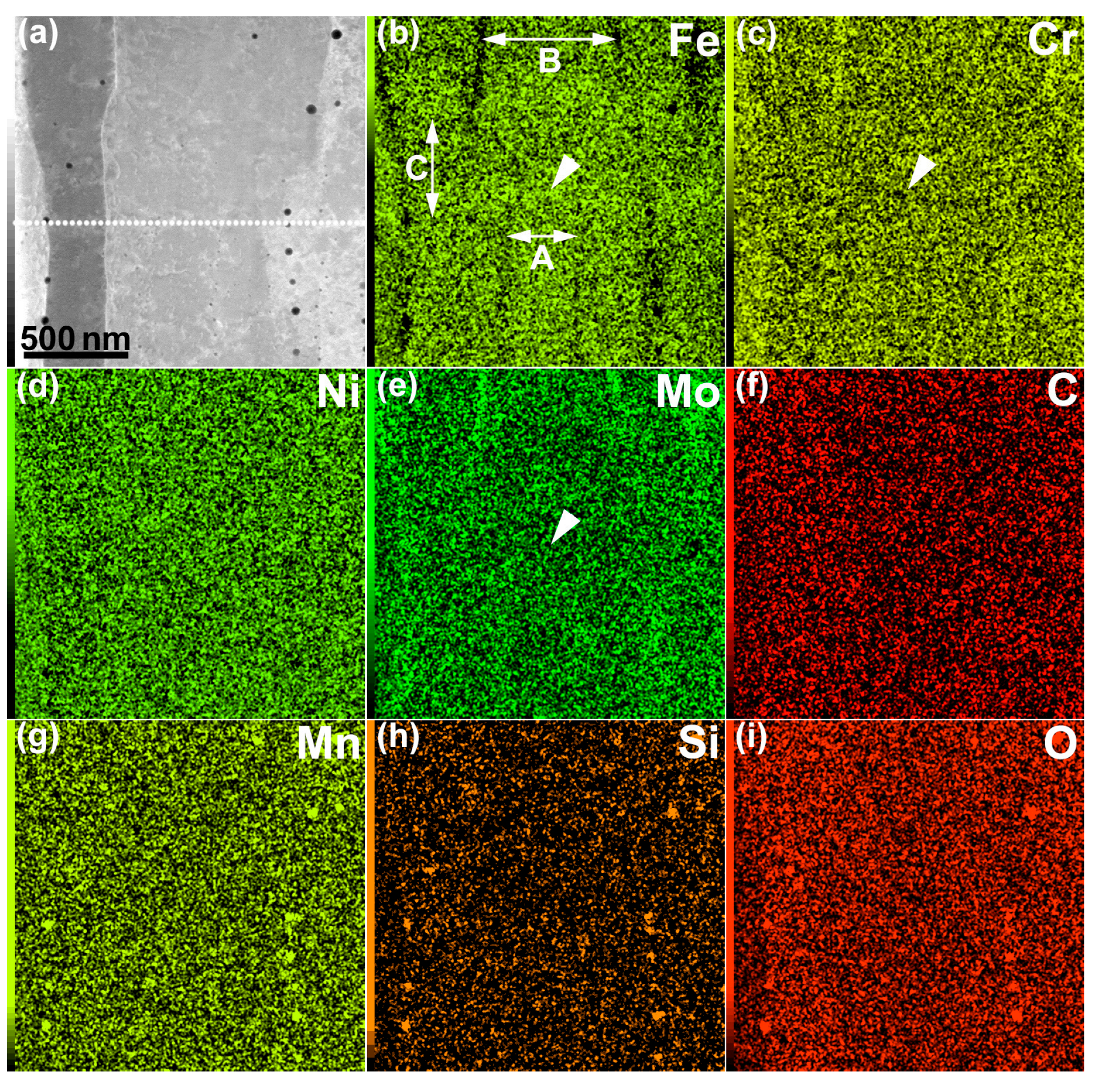
- Cr and Mo were enriched along the lamellar boundaries, as well as in the dislocation cell walls. The segregation of Cr and Mo was ~1 and 1–2 wt %, respectively.
- (2)
- The Cr and Mo contents slightly decreased (by ~0.5 wt %) at the melt-pool boundary.
- (3)
- The solidification cellular microstructures and melt-pool boundaries were visualized with the aid of solute segregation during crystal growth after laser melting.
- (4)
- Mn–Si–O inclusions (10–15 nm in diameter) were distributed along the lamellar boundary, as well as in the dislocation cell wall. It is considered that the grain growth of inclusions can be effectively suppressed by rapid quenching during the LPBF process.
- (5)
- A thin region without cellular microstructures was observed at the melt-pool boundary. The cellular spacing widened near the bottom of the melt pool, owing to the decrease in the cooling rate.
- (6)
- Atomic-structure analysis of the lamellar boundary by HRTEM revealed a local interfacial structure, which is complementary to the SEM-EBSD results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- MacDonald, E.; Wicker, R. Multiprocess 3D printing for increasing component functionality. Science 2016, 353, 1512. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, Y.; Okugawa, M. Digital twin science of metal powder bed fusion additive manufacturing: A selective review of simulations for integrated computational materials engineering and science. ISIJ Int. 2022, 62, 2183–2196. [Google Scholar] [CrossRef]
- Khairallah, S.A.; Anderson, A.T.; Rubenchik, A.; King, W.E. Laser powder-bed fusion additive manufacturing: Physics of complex melt flow and formation mechanisms of pores, spatter, and denudation zones. Acta Mater. 2016, 108, 36–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Aoyagi, K.; Yamanaka, K.; Chiba, A. Role of operating and environmental conditions in determining molten pool dynamics during electron beam melting and selective laser melting. Addt. Manuf. 2020, 36, 101559. [Google Scholar] [CrossRef]
- Voisin, T.; Forien, J.-B.; Perron, A.; Aubry, S.; Bertin, N.; Samanta, A.; Baker, A.; Wang, Y.M. New insights on cellular structures strengthening mechanisms and thermal stability of an austenitic stainless steel fabricated by laser powder-bed-fusion. Acta Mater. 2021, 203, 116476. [Google Scholar] [CrossRef]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Zeng, Z.; Zhang, Y.; Chen, W.; Roehling, T.T.; et al. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2018, 17, 63–73. [Google Scholar] [CrossRef]
- Godec, M.; Zaefferer, S.; Podgornik, B.; Sinko, M.; Tchernychova, E. Quantitative multiscale correlative microstructure analysis of additive manufacturing of stainless steel 316L by selective laser melting. Mater. Charact. 2020, 160, 110074. [Google Scholar] [CrossRef]
- Bajaj, P.; Hariharan, A.; Kini, A.; Kürnsteiner, P.; Raabe, D.; Jägle, E.A. Steels in additive manufacturing: A review of their microstructure and properties. Mat. Sci. Eng. A 2020, 772, 138633. [Google Scholar] [CrossRef]
- Sun, S.-H.; Ishimoto, T.; Hagihara, K.; Tsustumi, Y.; Hanawa, T.; Nakano, T. Excellent mechanical and corrosion properties of austenitic stainless steel with a unique crystallographic lamellar microstructure via selective laser melting. Scripta Mater. 2019, 159, 89–93. [Google Scholar] [CrossRef]
- Ishimoto, T.; Wu, S.; Ito, Y.; Sun, S.-H.; Amano, H.; Nakano, T. Crystallographic orientation control of 316L austenitic stainless steel via selective laser melting. ISIJ Int. 2020, 60, 1758–1764. [Google Scholar] [CrossRef]
- Krakhmalev, P.; Fredriksson, G.; Svensson, K.; Yadroitsev, I.; Yadroitsava, I.; Thuvander, M.; Peng, R. Microstructure, solidification texture, and thermal stability of 316L stainless steel manufactured by laser powder bed fusion. Metals 2018, 8, 643. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Ishimoto, T.; Oishi, T.; Manaka, T.; Chen, P.; Ashida, M.; Doi, K.; Katayama, H.; Hanawa, T.; Nakano, T. Crystallographic texture- and grain boundary density-independent improvement of corrosion resistance in austenitic 316L stainless steel fabricated via laser powder bed fusion. Addit. Manuf. 2021, 45, 102066. [Google Scholar] [CrossRef]
- Sato, K.; Matsumoto, H.; Kodaira, K.; Konno, T.J.; Chiba, A. Phase transformation and age-hardening of hexagonal α’ martensite in Ti-12mass%V-2mass%Al alloys studied by transmission electron microscopy. J. Alloys Compd. 2010, 506, 607–614. [Google Scholar] [CrossRef]
- Sato, K.; Tashiro, S.; Matsunaga, S.; Yamaguchi, Y.; Kiguchi, T.; Konno, T.J. Evolution of long-period stacking order (LPSO) in Mg97Zn1Gd2 cast alloys viewed by HAADF-STEM multi-scale electron tomography. Phil. Mag. 2018, 98, 1945–1960. [Google Scholar] [CrossRef]
- Cliff, G.; Lorimer, G.W. The quantitative analysis of thin specimens. J. Microsc. 1975, 103, 203–207. [Google Scholar] [CrossRef]
- Okugawa, M. (Arminia Bielefeld, Bielefeld, Germany); Koizumi, Y. (Osaka University, Osaka, Japan). Private Communications, 2022.
- Depinoy, S.; Sennour, M.; Ferhat, L.; Colin, C. Experimental determination of solute redistribution behavior during solidification of additively manufactured 316L. Scr. Mater. 2021, 194, 113663. [Google Scholar] [CrossRef]
- Yan, F.; Xiong, W.; Faierson, E.J. Grain structure control of additively manufactured metallic materials. Materials 2017, 10, 1260. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Qi, Y.; Chen, L.; Chen, C. Mechanism of (Mg, Al, Ca)-oxide inclusion-induced pitting corrosion in 316L stainless steel exposed to sulphur environments containing chloride ion. Corros. Sci. 2013, 67, 20–31. [Google Scholar] [CrossRef]
- Sato, K.; Semboshi, S.; Konno, T.J. Three-dimensional imaging of dislocations in a Ti-35mass%Nb alloy by electron tomography. Materials 2015, 8, 1924–1933. [Google Scholar] [CrossRef]
- Schubert, T.; Löser, W.; Schinnerling, S.; Bächer, I. Alternative phase transformation in thin strip casting of stainless steels. Mat. Sci. Tech. 1995, 11, 181–185. [Google Scholar] [CrossRef]
- Vrancken, B.; Thijs, L.; Kruth, J.-P.; Van Humbeeck, J. Microstructure and mechanical properties of a novel β titanium metallic composite by selective laser melting. Acta Mater. 2014, 68, 150–158. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, K.; Takagi, S.; Ichikawa, S.; Ishimoto, T.; Nakano, T. Microstructure and Solute Segregation around the Melt-Pool Boundary of Orientation-Controlled 316L Austenitic Stainless Steel Produced by Laser Powder Bed Fusion. Materials 2023, 16, 218. https://doi.org/10.3390/ma16010218
Sato K, Takagi S, Ichikawa S, Ishimoto T, Nakano T. Microstructure and Solute Segregation around the Melt-Pool Boundary of Orientation-Controlled 316L Austenitic Stainless Steel Produced by Laser Powder Bed Fusion. Materials. 2023; 16(1):218. https://doi.org/10.3390/ma16010218
Chicago/Turabian StyleSato, Kazuhisa, Shunya Takagi, Satoshi Ichikawa, Takuya Ishimoto, and Takayoshi Nakano. 2023. "Microstructure and Solute Segregation around the Melt-Pool Boundary of Orientation-Controlled 316L Austenitic Stainless Steel Produced by Laser Powder Bed Fusion" Materials 16, no. 1: 218. https://doi.org/10.3390/ma16010218
APA StyleSato, K., Takagi, S., Ichikawa, S., Ishimoto, T., & Nakano, T. (2023). Microstructure and Solute Segregation around the Melt-Pool Boundary of Orientation-Controlled 316L Austenitic Stainless Steel Produced by Laser Powder Bed Fusion. Materials, 16(1), 218. https://doi.org/10.3390/ma16010218






